Abstract
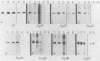
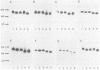
Glycoprotein D (gD) is an envelope component of herpes simplex virus types 1 (gD-1) and 2 (gD-2). The gD-1 polypeptide contains seven cysteine residues among its 369 amino acids; six are located on the N-terminal or luminal portion of the glycoprotein, and a seventh is located in the transmembrane region. Previous studies used a panel of monoclonal antibodies (MAbs) to define gD epitopes as continuous or discontinuous. Purified gD, denatured by reduction and alkylation, loses discontinuous epitopes, whereas continuous epitopes are retained. The contribution of disulfide bonds to maintenance of discontinuous epitopes is, therefore, significant. In the present study, our objective was to determine the contribution of individual cysteine residues to folding of gD-1 into its native conformation. Site-directed oligonucleotide mutagenesis was used to create seven mutants, each with a serine residue replacing a cysteine. The mutated genes were cloned into a eucaryotic expression vector and transfected into COS-1 cells, and the proteins were separated by nondenaturing polyacrylamide gel electrophoresis, followed by immunoblotting. Replacement of cysteine 7 (residue 333) had only a minimal effect on the antigenic properties of gD-1. In contrast, replacement of any one of the other six cysteine residues resulted in either a major reduction or a complete loss of binding of those MAbs that recognize discontinuous epitopes, with no effect on the binding of MAbs which recognize continuous epitopes. These mutations also had profound effects on the extent of oligosaccharide processing of gD-1. This was determined by digestion of the expressed proteins with various endoglycosidases, followed by electrophoresis and Western blotting (immunoblotting) to observe any mobility changes. Three mutant gD proteins which did not express discontinuous epitopes contained only high-mannose-type oligosaccharides, suggesting that processing had not proceeded beyond the precursor stage. Two mutant forms of gD exhibited reduced binding of MAbs to discontinuous epitopes. A small proportion of the molecules which accumulated at 48 h posttransfection contained complex oligosaccharides. One mutant exhibited reduced binding of MAbs to discontinuous epitopes, but was present at 48 h posttransfection only in the precursor form. The cysteine 7 mutant was processed to the same extent as wild-type gD. We conclude that the first six cysteine residues are critical to the correct folding, antigenic structure, and processing of gD-1, and we speculate that they form three disulfide-bonded pairs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Smith F. R. Effects of site-specific amino acid modification on protein interactions and biological function. Annu Rev Biochem. 1985;54:597–629. doi: 10.1146/annurev.bi.54.070185.003121. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carswell S., Resnick J., Alwine J. C. Construction and characterization of CV-1P cell lines which constitutively express the simian virus 40 agnoprotein: alteration of plaquing phenotype of viral agnogene mutants. J Virol. 1986 Nov;60(2):415–422. doi: 10.1128/jvi.60.2.415-422.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Matthews J. T., May M., Eisenberg R. Glycopeptides of the type-common glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1983 Jun;46(3):679–689. doi: 10.1128/jvi.46.3.679-689.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C., Sodora D. L., Long D., Levin J. Z., Eisenberg R. J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988 Jun;62(6):1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Cerini C. P., Heilman C. J., Joseph A. D., Dietzschold B., Golub E., Long D., Ponce de Leon M., Cohen G. H. Synthetic glycoprotein D-related peptides protect mice against herpes simplex virus challenge. J Virol. 1985 Dec;56(3):1014–1017. doi: 10.1128/jvi.56.3.1014-1017.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Hogue-Angeletti R., Cohen G. H. Amino-terminal sequence of glycoprotein D of herpes simplex virus types 1 and 2. J Virol. 1984 Jan;49(1):265–268. doi: 10.1128/jvi.49.1.265-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Ponce de Leon M., Matthews J. T., Spear P. G., Gibson M. G., Lasky L. A., Berman P., Golub E., Cohen G. H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985 Feb;53(2):634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Cohen G. H. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1980 Aug;35(2):428–435. doi: 10.1128/jvi.35.2.428-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Using recombinant DNA techniques to study protein targeting in the eucaryotic cell. Annu Rev Cell Biol. 1985;1:403–445. doi: 10.1146/annurev.cb.01.110185.002155. [DOI] [PubMed] [Google Scholar]
- Gibson M. G., Spear P. G. Insertion mutants of herpes simplex virus have a duplication of the glycoprotein D gene and express two different forms of glycoprotein D. J Virol. 1983 Nov;48(2):396–404. doi: 10.1128/jvi.48.2.396-404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese N. A., Robbins K. C., Aaronson S. A. The role of individual cysteine residues in the structure and function of the v-sis gene product. Science. 1987 Jun 5;236(4806):1315–1318. doi: 10.1126/science.3035718. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Dowbenko D. J. DNA sequence analysis of the type-common glycoprotein-D genes of herpes simplex virus types 1 and 2. DNA. 1984;3(1):23–29. doi: 10.1089/dna.1.1984.3.23. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark D. F., Lu S. D., Creasey A. A., Yamamoto R., Lin L. S. Site-specific mutagenesis of the human fibroblast interferon gene. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5662–5666. doi: 10.1073/pnas.81.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Cohen G. H., Eisenberg R. J. Synthesis and processing of glycoprotein D of herpes simplex virus types 1 and 2 in an in vitro system. J Virol. 1983 Nov;48(2):521–533. doi: 10.1128/jvi.48.2.521-533.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982 Oct 25;257(20):12277–12282. [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki S., Kobata A. Purification and characterization of an endo-beta-galactosidase produced by Diplococcus pneumoniae. J Biol Chem. 1976 Jun 25;251(12):3603–3609. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Watson R. J. DNA sequence of the Herpes simplex virus type 2 glycoprotein D gene. Gene. 1983 Dec;26(2-3):307–312. doi: 10.1016/0378-1119(83)90203-2. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Bacterial synthesis of herpes simplex virus types 1 and 2 glycoprotein D antigens. J Invest Dermatol. 1984 Jul;83(1 Suppl):102s–111s. doi: 10.1111/1523-1747.ep12281828. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gerhard W. Antigenic characterization of viruses by monoclonal antibodies. Annu Rev Microbiol. 1981;35:185–206. doi: 10.1146/annurev.mi.35.100181.001153. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]