Abstract
Background
Although obesity is strongly associated with diabetes, the greater prevalence of diabetes in persons of African ancestry than in those of other ancestries cannot be explained simply by differences in total or central adiposity.
Objective
We examined whether skeletal muscle composition is associated with diabetes in 1249 men of African ancestry aged ≥40 y.
Design
Anthropometry and fasting serum glucose were measured, and lower-leg skeletal muscle composition was assessed with peripheral quantitative computerized tomography (pQCT).
Results
The prevalence of diabetes in this population was high (21%). We observed an age-associated adipose tissue remodeling in skeletal muscle and greater intermuscular (IMAT) and lesser subcutaneous (SAT) adipose tissue area with advancing age (P < 0.0001). Multivariate stepwise logistic regression identified more IMAT and less SAT to be significantly associated with a greater prevalence of diabetes. Even among normal-weight men [body mass index (BMI; in kg/m2) <25], diabetic men had significantly (P = 0.01) more IMAT than did those without diabetes. Greater IMAT was also associated with a greater prevalence of hyperglycemia in men with a family history of diabetes than in those without such history (P for interaction = 0.02).
Conclusions
These findings underscore the independent associations of subcutaneous and intermuscular fat among men of African ancestry, an effect that may be modified by a family history of diabetes. Further studies are needed to identify the genetic and physiologic mechanisms that influence the distribution and remodeling of adipose tissue in skeletal muscle with aging.
INTRODUCTION
Obesity and diabetes disproportionately affect populations of African background (1, 2). Although obesity is strongly associated with insulin resistance and type 2 diabetes, the greater prevalence of diabetes among persons of African ancestry than among persons of other races-ethnicities cannot be explained simply by differences in body weight, total body fat, or waist circumference (3, 4). Even among normal-weight persons, those of African ancestry have higher concentrations of fasting glucose and are more insulin-resistant than are those of other racial-ethnic groups (3, 4). Moreover, differences in abdominal visceral and subcutaneous adipose tissue (SAT) do not explain racial-ethnic differences in hyperglycemia and insulin sensitivity (5–11). In addition, body mass index (BMI; in kg/m2) does not reflect changes in fat mass or muscle mass with aging.
More recently, research has focused on adipose tissue within the fascia surrounding skeletal muscle [intermuscular adipose tissue (IMAT)] as an independent risk factor for insulin resistance and diabetes (12–14). Compared with whites, African Americans have greater IMAT despite comparable total body fat (6, 14, 15). The main objective in the present study was to assess the relation between total adipose tissue (TAT), SAT, and IMAT in the lower leg and diabetes in a large cohort of Afro-Caribbean men. We tested the hypothesis that intermuscular fat is associated with diabetes independent of total and subcutaneous fat.
SUBJECTS AND METHODS
Study sample
The Tobago Health Study (THS), a population-based, longitudinal study of 3200 men ≥40 y old on the Caribbean island of Tobago, began in 1997. Men were recruited without regard to their health status from the 7 parishes on the island, and participation was representative of the parish census. Subjects were recruited primarily by word of mouth. The population of Tobago is predominantly of West African origin. Ancestry-informative molecular markers have estimated 94% West African, 4.6% European, and 1.4% Native American ancestry in this population (16). Men who participated in the THS were contacted again, beginning in late 2004, for a follow-up clinic examination. The present analysis is limited to Afro-Caribbean men at the follow-up visit who had comprehensive data on anthropometric measures, demographic information, medical history, lifestyle, fasting glucose, and pQCT measures of lower-leg skeletal muscle composition (n = 1249).
Written informed consent was obtained from all subjects, using forms and procedures approved by the Tobago Ministry of Health and Social Services and the institutional review board of the University of Pittsburgh.
Data collection
Height was measured to the nearest 0.1 cm by using a wall-mounted stadiometer. Weight was recorded to the nearest 0.1 kg on a balance-beam scale while the subject was shoeless. BMI was calculated. Information on lifestyle habits, demographics, medical conditions, family history, and medication use also was assessed by using standardized interviewer-administered questionnaires that were reviewed with the participant in the clinic. Alcohol consumption was self-reported in previously defined categories (none or <1, 1–3, 4–7, 8–14, 15–21, 22–27, or ≥28 drinks/wk). Hours of television watching also were self-reported in previously defined categories (0, 1–6, 7–13, 14–20, 21–27, or ≥28 h/wk). We arbitrarily created 2 categories of television viewing (≤6 and ≥7 h/wk) and 2 categories of alcohol use (<1 and ≥1 drink/wk). We also recorded information on self-reported walking, because walking is the predominant form of physical activity on the island. Information on family history of diabetes was collected for first-degree relatives (parents, siblings, and offspring).
Quantitative computerized tomography measures
A lower-leg pQCT scan was performed by using a Stratec XCT-2000 scanner (Orthometrix Inc, White Plains, NY) to evaluate the total, muscle, and fat cross-sectional areas of the calf. Scans were obtained at 66% of the tibial length, proximal to the terminal end of the tibia. This site was chosen because it is the region with the largest calf circumference, and there is little variability across persons (17). Images of cross-sectional areas of the muscle and fat tissue were analyzed by using STRATEC software (version 5.5D; Orthometrix Inc). To maintain consistency, all images were analyzed by a single investigator who was blinded to the subject’s diabetes status. With the use of edge detection and thresholding steps, the pQCT image can be segmented into bone and soft tissue measures. For this project, we selected measures of the calf TAT, SAT, IMAT, and total muscle cross-sectional areas (all expressed in mm2).
Blood sample collection, glucose measurement, and classification of diabetes
Blood samples were obtained by venipuncture in the morning after a 12-h fast. Whole blood was drawn into sterile red-top (serum) tubes, which were left standing at room temperature for a minimum of 20 min to clot before centrifugation. Then the serum was aliquoted into 1.0-mL cryovials. The aliquots of frozen serum were shipped on ice packs by express courier and stored in −80 °C freezers at the University of Pittsburgh. Serum glucose was measured by using an enzymatic procedure (18). Diabetes was defined as a fasting serum glucose concentration ≥126 mg/dL (19) or current use of antidiabetes medication.
Statistical analysis
Analysis of covariance (ANCOVA) was conducted by using the general linear model procedure (GLM) to test for differences in adjusted mean characteristics of participants by age group and by diabetes status among normal-weight men. The percentage difference between younger and older age groups was calculated as [(trait mean ≥70 y age group − trait mean 40–49 y age group)/trait mean 40–49 y age group] × 100%. Logistic regression was used to examine the contributions of age, anthropometric measures, pQCT measures, lifestyle factors, and family history of diabetes to the risk of diabetes. The odds ratios (ORs) and 95% CIs were presented by a 1-SD increase for continuous variables. Variables with P < 0.10 were further considered for inclusion in the multivariate stepwise regression analysis to determine the independent correlates of diabetes. We examined multicollinearity before the stepwise regression analysis by assessing the variance inflation factor (VIF). A VIF > 2.5 for the logistic regression model was used as an indicator of multicollinearity (20). By replacing different fat-related variables one at a time, we included another anthropometric obesity measure (eg, BMI, weight, and waist) with no multicollinearity along with SAT and IMAT in the stepwise logistic regression model. The model with the best goodness-of-fit is presented as the final model. We used SAS software (version 9.1; SAS Institute, Cary, NC) for statistical analyses.
RESULTS
Demographic, anthropometric, pQCT, and lifestyle characteristics and the medical conditions of the 1249 subjects are presented in Table 1. Their mean age was 59 y (range: 40–91 y). Almost 46% of the men were overweight, 24% were obese, and 21% had diabetes. Nearly half of all participants reported a family history of diabetes. Of the 262 participants with diabetes, 178 (67.9%) were currently taking glucose-lowering pharmacologic medication, insulin, or both.
TABLE 1.
Characteristics of the study population1
| Age (y) | 59.5 ± 10.42 |
| Anthropometric characteristics | |
| Weight (kg) | 84.0 ± 16.1 |
| Height (cm) | 174.7 ± 6.8 |
| Waist circumference (cm) | 93.1 ± 11.1 |
| BMI (kg/m2) | 27.5 ± 4.9 |
| pQCT lower-leg skeletal muscle composition | |
| TAT (mm2) | 1765.1 ± 754.5 |
| IMAT (mm2) | 262.1 3 ± 40.0 |
| SAT (mm2) | 1323.0 ± 644.6 |
| Total muscle area (mm2) | 7313.1 ± 1308.3 |
| Lifestyle characteristics | |
| Ever smoked (%) | 31.6 |
| Current smoking (%) | 9.9 |
| Walking (h/wk) | 3.0 ± 4.4 |
| TV watching ≥7 h/wk (%) | 76.5 |
| Alcohol intake ≥1 drink/wk (%) | 18.2 |
| Medical conditions | |
| Overweight (%)3 | 45.8 |
| Obesity (%)4 | 23.9 |
| Diabetes (%)5 | 21.0 |
| Family history of diabetes (%) | 48.3 |
n = 1249. pQCT, peripheral quantitative computerized tomography; TAT, total adipose tissue cross-sectional area; IMAT, intermuscular adipose tissue cross-sectional area; SAT, subcutaneous adipose tissue cross-sectional area; TV, television.
x̄ ± SD (all such values).
BMI (in kg/m2) = 25–29.9.
BMI ≥30.
Fasting glucose of ≥126 mg/dL or use of insulin or hypoglycemic medications, or both.
First, we examined mean values of pQCT traits by 10-y age groups (Figure 1). TAT showed no differences across the age groups. In contrast, total muscle area decreased (P < 0.0001 after adjustment for height), IMAT increased, and SAT decreased with advancing age (P < 0.0001 after adjustment for height, total fat and total muscle area). IMAT was 84% greater in men aged ≥70 y than in men aged 40–49 y. In contrast, SAT was 15% lower in men aged ≥70 y than in those aged 40–49 y.
FIGURE 1.
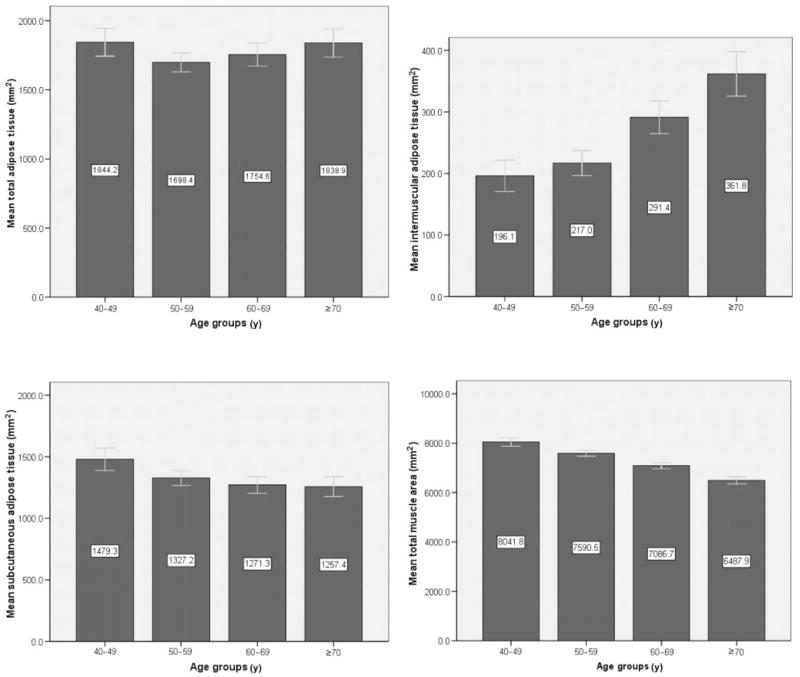
Unadjusted mean (± 2SE) lower-leg peripheral quantitative computerized tomography (pQCT) skeletal muscle composition measures across 10-y age groups (the numerical values are shown in white boxes inside the bars). n = 214, 444, 348, and 243 for the 40–49, 50–59, 60–69, and ≥70 y age groups, respectively. ANCOVA was used to assess age group differences in pQCT measures after adjustment for height (total adipose tissue and total muscle area) or height, total adipose tissue, and total muscle area (intermuscular and subcutaneous adipose tissue). Differences were significant (P < 0.0001) across age groups for all pQCT measures except total adipose tissue.
Traditional lifestyle-related risk factors for diabetes (eg, physical inactivity, smoking, and alcohol intake) were not significantly associated with diabetes in age-adjusted analyses in this population (Table 2). Most of the obesity-related measures (eg, weight, waist circumference, BMI, and intermuscular fat) and family history of diabetes were positively and subcutaneous fat was inversely associated with diabetes, independent of age. We further tested the independent associations of these factors with diabetes in a stepwise multivariate logistic regression analysis. The model with the best goodness-of-fit is presented as the final model in Table 2. Age, BMI, intermuscular fat, and family history of diabetes remained positively correlated with diabetes, but subcutaneous fat was negatively correlated with diabetes (Table 2).
TABLE 2.
Odds ratios (ORs) (and 95% CI) for diabetes in the total study population1
| Age-adjusted2 | Multivariate-adjusted3 | |
|---|---|---|
| Age (y) | 1.52 (1.32, 1.73)4 | 1.57 (1.33, 1.84)4 |
| Weight (kg) | 1.29 (1.13, 1.48)4 | — |
| Height (cm) | 0.96 (0.82, 1.11) | — |
| Waist circumference (cm) | 1.42 (1.23, 1.63)4 | — |
| BMI (kg/m2) | 1.32 (1.16, 1.51)4 | 1.70 (1.38, 2.10)4 |
| TAT (cm2) | 0.91 (0.79, 1.04) | — |
| IMAT (mm2) | 1.24 (1.11, 1.41)4 | 1.17 (1.03, 1.37)4 |
| SAT (mm2) | 0.84 (0.74, 0.97)4 | 0.60 (0.49, 0.72)4 |
| Total muscle area (mm2) | 0.93 (0.90, 1.09) | — |
| Walking (h/wk) | 0.99 (0.96, 1.03) | — |
| Ever smoked | 1.18 (0.88, 1.58) | — |
| Current smoking | 0.80 (0.48, 1.32) | — |
| TV watching ≥7 h/wk | 0.95 (0.66, 1.25) | — |
| Alcohol intake ≥1 drink/wk | 0.68 (0.45, 1.01) | — |
| Family history of diabetes | 3.10 (2.22, 4.10)4 | 2.85 (2.10, 3.90)4 |
n = 1249. TAT, total adipose tissue; IMAT, intermuscular adipose tissue; SAT, subcutaneous adipose tissue; TV, television. All values are OR; 95% CI in parentheses. For continuous variables, OR was presented by a 1-SD increase in predictor variable.
Logistic regression analysis was used to obtain the age-adjusted ORs. The model including age was unadjusted.
Multivariate stepwise regression analysis was used to obtain the multivariate ORs (multivariate model included 1149 participants).
Significant OR.
Taking into consideration our findings from the multivariate analysis, we were also interested in whether there were any differences in lower-leg fat distribution by diabetes status in men with normal BMI (18.5–24.9) and similar waist circumference. It is interesting that, after adjustment for age, height, total fat, and total muscle area, lean diabetic men, compared with lean nondiabetic men, had significantly greater intermuscular fat (adjusted x̄ ± SEM: 230.4 ± 20.6 and 180.3 ± 9.2 mm2, respectively; P = 0.01) but similar subcutaneous fat (adjusted x̄ ± SEM: 865.7 ± 22.6 and 895.8 ± 10.1 mm2, respectively; P = 0.39).
Finally, we tested the association between tertiles of intermuscular and subcutaneous fat and fasting serum glucose concentrations, stratified by family history of diabetes, in the total sample. The positive association between intermuscular fat and fasting plasma glucose concentrations was stronger in men who had a family history of diabetes than in those without such history (P = 0.02 for interaction after adjustment for age, height, total fat, and total muscle area). This interaction effect remained significant after the inclusion of BMI or waist circumference as a covariate (P < 0.05 for both). No family history of diabetes × glucose interaction was observed for subcutaneous fat (P = 0.59 for interaction after adjustment for age, height, total fat, and total muscle area).
DISCUSSION
In the present study, we determined the prevalence and risk factors for diabetes in a large, well-characterized population cohort of Afro-Caribbean men. Although comparison of prevalence estimates among different populations can be difficult, the prevalence of diabetes reported among Afro-Caribbean men of comparable age in Barbados was 14.8% (21). The prevalence of diabetes among the more admixed African American men of similar age in the third National Health and Nutrition Examination Survey (NHANES III) was 13.1% (22). Thus, the prevalence of diabetes in this Afro-Caribbean male population of Tobago appears to be very high (21%).
The current study supports the hypothesis that remodeling of fat distribution in skeletal muscle occurs with advancing age. Although TAT was similar across age groups, we found a decrease in subcutaneous fat and increase in intermuscular fat across age groups. This observation is in agreement with previous studies, which were conducted in African American women (23, 24). Moreover, greater accumulation of intermuscular fat and, in contrast, lower accumulation of subcutaneous fat was associated with diabetes, independent of other risk factors. Among normal-weight men, greater IMAT was associated with diabetes independent of age, BMI, and total fat and muscle areas. On the other hand, subcutaneous fat did not differ between lean men with or without diabetes.
The question of whether fat reduction or fat accumulation in certain anatomical depots is associated with diabetes remains to be answered. For example, a previous study by Goodpaster et al (12) showed that, among obese persons and persons with type 2 diabetes, intermuscular fat but not subcutaneous fat is associated with insulin resistance. That study also reported that older persons with normal body weight are at higher risk of diabetes if they have an excessive amount of intermuscular or visceral fat, but such findings have not been observed for subcutaneous fat. Still, subcutaneous fat may be necessary as a storage depot for excess fat. In animal models, low subcutaneous fat has been strongly associated with insulin resistance, and transplantation of subcutaneous fat was associated with the setback of insulin resistance and dyslipidemia and less accumulation of ectopic adipose tissue in the liver (25). In addition, patients with lipodystrophy have greater skeletal muscle adipose tissue accumulation. It was hypothesized that this may be to a defect in the ability of subcutaneous fat to store excess fatty acids and to its limited storage capacity, which leads to an overflow of fat into other depots, such as the intermuscular compartment (26).
We found significantly stronger associations between inter-muscular fat and fasting glucose in persons with a family history of diabetes than in those without such a history. This finding implies that certain persons of African ancestry may be genetically susceptible to diabetes in the presence of greater amounts of IMAT. We are unaware of other published reports of studies examining the relations between a family history of diabetes, glucose concentrations, and adipose tissue infiltration in skeletal muscle. Future genetic studies of the intermuscular fat phenotype may help to explain why some lean persons are at greater risk than are other lean persons for the development of insulin resistance and type 2 diabetes.
Previous studies reported that African Americans have more intermuscular fat than do whites and Asians, even after adjustment for differences in total adiposity, skeletal muscle mass, and other potential covariates (13, 14). The mechanisms underlying black-white differences in ectopic fat accumulation are unknown, but some studies have found less fat oxidation (27) and less lipolysis (28) in African American women than in matched white women. Other studies suggest that ectopic deposition of lipids may be higher in obese African American women than in obese white women because of the former group’s higher rate of fatty acid uptake and higher expression of fatty acid–transporting proteins (29). Further studies are needed to better understand the potential causes of greater accumulation of fat in the skeletal muscle of persons of African ancestry.
Defects in mitochondrial function also are believed to contribute to impaired fat oxidation and to greater adipose tissue accumulation in skeletal muscle (30). Moreover, animal models showed that overexpression of uncoupling proteins (UCPs) in skeletal muscle, leads to better glucose tolerance, possibly by conversion of intramyocellular fat into thermal energy and by causing a lower accumulation of fat in skeletal muscle (31, 32). Although the mechanisms linking accumulation of fat in skeletal muscle and insulin resistance are unclear, several human and animal studies have shown that an excess of fatty acid disrupts the insulin-signaling cascade (33). Differences in the secretion of adipokines between intermuscular and subcutaneous fat also could be an explanation for the observed differences in fat depot associations with diabetes, because differences in adipose tissue function according to its anatomical location have been reported (34).
The present study has several limitations. First, we did not assess the burden of diabetes among children, adolescents, younger men, or women, which may limit the generalizability of our findings. Second, our study was cross-sectional in design, and our analyses may be subject to the limitations of cross-sectional studies, such as cohort effects and biases introduced by selective survivorship. A longitudinal study would better delineate the effects of aging on adipose tissue infiltration in the skeletal muscle. Other potential limitations are recall bias in reporting the presence of diabetes in family members and the possibility that the reported family history of diabetes included cases of type 1 diabetes. Furthermore, participants may have inaccurately reported that they are receiving diabetes medications. Participants in the THS were primarily self-referred, and therefore, there is the potential for a selection bias to inflate the estimated prevalence rates. However, the THS was originally designed to estimate the burden of prostate cancer, and thus we believe that self-selection bias would not affect the prevalence of diabetes in the population sample of the present study. Finally, most previous studies have used a single CT slice of the midthigh to assess fat infiltration in skeletal muscle (35–37). By obtaining a single slice in the calf muscle, we were able to measure a smaller depot of skeletal muscle adipose tissue than that available through CT measures of the midthigh. Nonetheless, previous studies have noted a relatively strong correlation between calf and midthigh muscle adipose tissue infiltration (38).
In conclusion, we observed in this Afro-Caribbean population a high prevalence of diabetes, adipose tissue remodeling in skeletal muscle with advancing age, and a positive association of intermuscular fat with diabetes, even among lean persons. We also observed a stronger association between intermuscular fat and glucose concentrations in persons who had a family history of diabetes than in those who did not. Our results illustrate the importance of separately measuring the subcutaneous and inter-muscular fat depots. Our study also illuminated a possible modulating effect of a family history of diabetes on the relation between intermuscular fat accumulation and diabetes. Further studies are warranted to identify the specific genetic and physiologic mechanisms that influence the distribution of adipose tissue in skeletal muscle. Such studies may provide new insights into the pathophysiology of and genetic susceptibility to diabetes and may suggest new therapeutic targets for the treatment and prevention of insulin resistance.
Acknowledgments
We thank the participants of the Tobago Health Study and all supporting staff.
The authors’ responsibilities were as follows—IM-G: project development, statistical analyses, and writing of the manuscript; CLG: analysis of all peripheral quantitative computerized tomography scans and data interpretation; BHG and LHK: assistance with data interpretation; CHB, ALP, and VWW, Co-Principal Investigators of the Tobago Health Study: study design and supervision of data collection; RWE, as head of a laboratory: responsibility for glucose measurements; JMZ, a Co-Principal Investigator of the Tobago Health Study: supervision of the progress of this project and guidance for project development, and statistical analyses; and all authors: significant suggestions, advice, and consultation on the preparation of the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Supported by grant no. R01AR049747 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by postdoctoral training grant no. F32 HL083641 from the National Heart, Lung, and Blood Institute (to IM-G).
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253–9. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 3.Palaniappan LP, Carnethon MR, Fortmann SP. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002;25(8):1351–7. doi: 10.2337/diacare.25.8.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.der Merwe MT, Panz VR, Crowther NJ, et al. Free fatty acids and insulin levels—relationship to leptin levels and body composition in various patient groups from South Africa. Int J Obes Relat Metab Disord. 1999;23(9):909–17. doi: 10.1038/sj.ijo.0800969. [DOI] [PubMed] [Google Scholar]
- 5.Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat. Metabolism. 1999;48(2):194–9. doi: 10.1016/s0026-0495(99)90033-1. [DOI] [PubMed] [Google Scholar]
- 6.Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res. 2002;10(5):336–44. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 7.Despres JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20(8):1932–8. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 8.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61(4):765–71. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 9.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69(3):381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 10.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45(9):1119–24. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 11.Ding J, Visser M, Kritchevsky SB, et al. The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hypertens. 2004;17(10):971–6. doi: 10.1016/j.amjhyper.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 13.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82(6):1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–10. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz J, Gower BA. Relationship between serum leptin concentration and low-density muscle in postmenopausal women. J Clin Endocrinol Metab. 2003;88(3):1157–61. doi: 10.1210/jc.2002-020959. [DOI] [PubMed] [Google Scholar]
- 16.Miljkovic-Gacic I, Ferrell RE, Patrick AL, Kammerer CM, Bunker CH. Estimates of African, Eur and Native American ancestry in Afro-Caribbean men on the island of Tobago. Hum Hered. 2005;60(3):129–33. doi: 10.1159/000089553. [DOI] [PubMed] [Google Scholar]
- 17.Simonsick EM, Maffeo CE, Rogers SK, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52(5):M264–74. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 18.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20(5):586–90. [PubMed] [Google Scholar]
- 19.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 20.Allison PD. Logistic regression using the SAS system—theory and application. Cary, NC: SAS Institute, Inc; 1999. [Google Scholar]
- 21.Hennis A, Wu SY, Nemesure B, Li X, Leske MC. Diabetes in a Caribbean population: epidemiological profile and implications. Int J Epidemiol. 2002;31(1):234–9. doi: 10.1093/ije/31.1.234. [DOI] [PubMed] [Google Scholar]
- 22.Okosun IS, Chandra KM, Choi S, Christman J, Dever GE, Prewitt TE. Hypertension and type 2 diabetes comorbidity in adults in the United States: risk of overall and regional adiposity. Obes Res. 2001;9(1):1–9. doi: 10.1038/oby.2001.1. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa S, Odamaki M, Ikegaya N, et al. Association of age with muscle mass, fat mass and fat distribution in non-diabetic haemodialysis patients. Nephrol Dial Transplant. 2005;20(5):945–51. doi: 10.1093/ndt/gfh643. [DOI] [PubMed] [Google Scholar]
- 24.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79(5):874–80. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 25.Colombo C, Cutson JJ, Yamauchi T, et al. Transplantation of adipose tissue lacking leptin is unable to reverse the metabolic abnormalities associated with lipoatrophy. Diabetes. 2002;51(9):2727–33. doi: 10.2337/diabetes.51.9.2727. [DOI] [PubMed] [Google Scholar]
- 26.Gan SK, Samaras K, Thompson CH, et al. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51(11):3163–9. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 27.Hickner RC, Privette J, McIver K, Barakat H. Fatty acid oxidation in African-American and Caucasian women during physical activity. J Appl Physiol. 2001;90(6):2319–24. doi: 10.1152/jappl.2001.90.6.2319. [DOI] [PubMed] [Google Scholar]
- 28.Barakat H, Hickner RC, Privette J, et al. Differences in the lipolytic function of adipose tissue preparations from Black American and Caucasian women. Metabolism. 2002;51(11):1514–8. doi: 10.1053/meta.2002.35589. [DOI] [PubMed] [Google Scholar]
- 29.Bower JF, Davis JM, Hao E, Barakat HA. Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am J Physiol Endocrinol Metab. 2006;290(1):E87–91. doi: 10.1152/ajpendo.00194.2005. [DOI] [PubMed] [Google Scholar]
- 30.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63(2):363–8. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Nolte LA, Ju JS, et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6(10):1115–20. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 32.Choi CS, Fillmore JJ, Kim JK, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117(7):1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulver MW, Dohm GL. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc. 2004;63(2):375–80. doi: 10.1079/pns2004351. [DOI] [PubMed] [Google Scholar]
- 34.van Harmelen V, Dicker A, Ryden M, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51(7):2029–36. doi: 10.2337/diabetes.51.7.2029. [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–83. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 37.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23(2):126–32. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 38.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14(1):73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]


