Abstract
A novel approach to potentially resolve serious thrombosis issues associated with kidney dialysis (hemodialysis) therapies is described. New water-soluble polymeric nitric oxide (NO) donors, based on the diazeniumdiolated branched poly(ethylenimine)s and their derivatives, are prepared and characterized. These macromolecular NO donors (with up to 4.15 μmol/mg of total NO release) are utilized as additives to the dialysate solution of model dialysis filters. The presence of these species can create a localized increase in NO levels at the high surface area dialysis fiber/blood interface within the hemodialyzers. Nitric oxide is a naturally occurring and potent anti-platelet agent, and is expected to greatly decrease the risk of thrombosis in the dialysis units.
Introduction
Healthy kidneys perform their life-sustaining job of removing toxic waste products and excess fluid from the body, maintaining a stable balance of body chemicals. When renal failure occurs in end-stage kidney disease patients, dialysis (i.e., hemodialysis) must be performed to sustain life. Generally, hollow dialysis fibers, the key component of hemodialyzers, use cellulose or polysulfone membranes in place of the phospholipid-bilayer membranes in real kidneys to separate the components of blood. Such semi-permeable membranes, with a given molecular weight cut-off (approx. 10–50 kDa), allow small waste molecules to pass through them into a flowing recipient solution (dialysate solution) and be removed from the blood. However, due to the highly porous surface structure of the dialysis fibers and their direct contact with blood, systemic or localized anticoagulation (e.g., with heparin) is usually required to reduce the risk of clot formation during such clinical procedures.1 The systemic administration of heparin can produce hemorrhagic complications in patients at high risk of bleeding, including intracranial hemorrhage.2, 3 To reduce these bleeding risks, alternative dialysis therapies have been developed without systemically heparinizing the patients.4 One of the most widely used clinical methods is to locally anticoagulate the blood within the dialysis system by lowering ionic calcium concentration via the continuous addition of citrate to the blood.5, 6 Although this approach reduces the increased risk of bleeding, it requires continuous re-introduction of calcium into the blood stream prior to re-entry of the blood into the patient and hence frequent measurements of ionized calcium levels to ensure proper physiological levels.
A potentially less risky but more convenient solution to the problem of thrombogenicity of the polymeric membranous fiber surface may now be realized via the addition of a water-soluble polymeric nitric oxide (NO) releasing additive to the dialysate solution, which is capable of spontaneously releasing a controlled level of NO through the porous membranous fibers at the polymer/blood interface. Due to the size of the molecule, ideally only NO would pass through the fibers, while the NO donor molecule would remain within the dialysate solution because of its high molecular weight/large hydrodynamic volume (see Figure 1). Indeed, NO is a naturally occurring, potent anti-platelet agent produced by endothelial cells that line the inner walls of all blood vessels, with a typical NO flux from the endothelial layer of 0.5–4×10−10 mol · cm−2 · min−1.7–10 Polymeric coatings that release NO with similar fluxes have already been shown to greatly decrease the risk of thrombosis in various blood contacting medical devices.11–18 It is envisioned that, with this novel approach to improve the blood compatibility of dialysis filters, heparin- and citrate-free dialysis therapy may be performed without the concern of either clotting or excess bleeding. Moreover, the use of such an NO-release dialysis approach would be safe and convenient method to efficiently deliver NO to the point-of-care (compared to the use of a high-pressure NO gas cylinder), and only a small amount of such an additive would need to be stored/used for each dialysis treatment. Indeed, this scheme would not require major modification of the basic hemodialyzer design that is currently used in the clinical arena.
Figure 1.
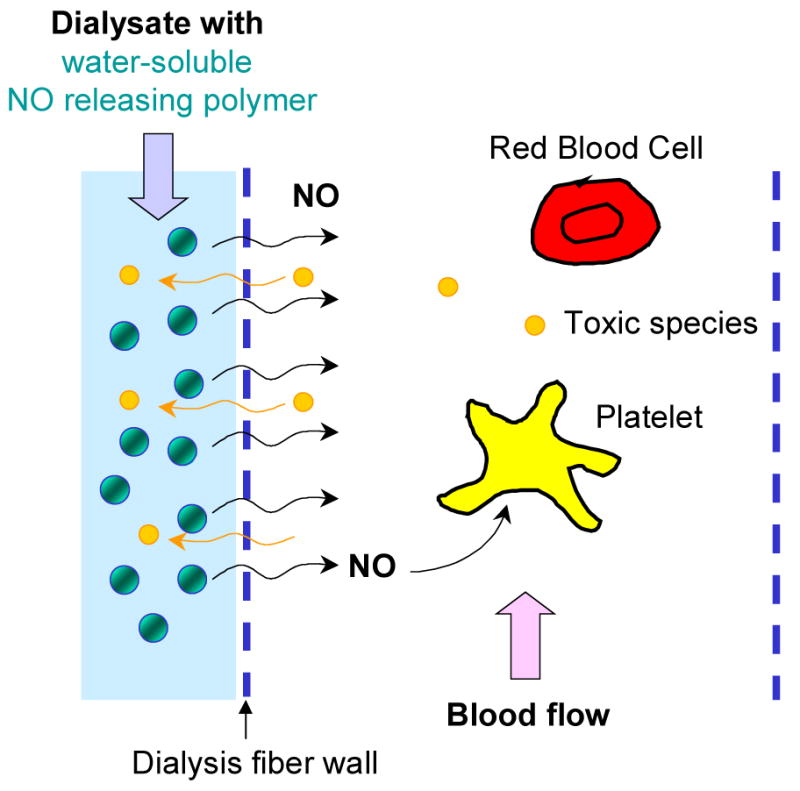
A schematic diagram illustrating the concept of improving the blood compatibility of hemodialyzer fibers via NO-release from a polymeric NO releasing agent added to the flowing dialysate solution.
N-Diazeniumdiolates are ideal NO donor candidates to realize the above concept. Their proton-mediated NO release property19 has been widely utilized in developing localized NO delivery prodrugs for clinical applications (i.e., small molecule diazeniumdiolated proline as injectable ultrafast NO donor20, 21) as well as NO releasing hydrophobic polymer coatings17, 18, 22–27 and particles as filler materials15, 28, 29 (i.e., NO releasing crosslinked PEI microsphere—a water-insoluble particle for modifying vascular grafts29) for biomedical applications. However, no such NO donors have been reported to date that possess all the three unique characteristics necessary to carry out dialysis therapy without anticoagulation. These characteristics are: (1) polymer-based molecules that prevent leaching; (2) molecules which are highly water-soluble for convenient use; and (3) rapid NO release rates for high efficiency. To this end, we designed, synthesized and investigated three new types of branched poly(ethylenimine) (PEI)-based NO releasing agents (utilizing sodium-stabilized N-diazeniumdiolates). Further, the first data from conceptual experiments related to artificial kidney dialysis (hemodialysis) therapies using such water-soluble polymer-based NO donors as dialysate additives are reported herein.
Experimental Section
Materials
Water-free low molecular weight branched PEI (LMwPEI, Mw=800, PDI=1.3, molar ratio of primary/secondary/tertiary amine=approx. 1/2/1) and high molecular weight branched PEI (HMwPEI, Mw=25,000, PDI=2.5, molar ratio of primary/secondary/tertiary amine=approx. 1/1.2/0.76) were purchased from Aldrich (Milwaukee, WI). HMwPEI was pre-treated via dialysis against DI water using Spectra/Pro 7 RC dialysis tubing (MWCO: 50 kDa, Spectrum Laboratories, Rancho Dominguez, CA) for ca. one week. The large molecular weight cut-off tubing was used to facilitate the dialysis process and the remaining high molecular weight fraction of the HMwPEI in the dialysis tubing was then lyophilized prior to further reactions. Methanol was dried over 4 Å molecular sieves and tetrahydrofuran (THF) was distilled over sodium/benzophenone ketyl under nitrogen prior to use. Boc-L-proline hydroxysuccinimide ester and the sodium salt of poly(acrylic acid) (PAANa, Mw=5,100) were used as received from Fluka (Ronkonkoma, NY), and succinic anhydride, sulfanilamide and N-(naphthyl)ethylenediamine dihydrochloride were purchased from Aldrich. 3-(2-Furoyl)quinoline-2-carboxaldehyde (FQCA) was obtained from Molecular Probes (Eugene, OR). Two types of hemodialyzers were used in the dialysis experiments: a Baxter™ Cellulose Triacetate Hollow Fiber Dialyzer—Model CT·110G (Baxter Healthcare Corporation, Deerfield, IL) and a Minifilter™ Plus Hemofilter (Minntech Corporation, Minneapolis, MN).
Measurements
1H NMR spectra were obtained on a Varian 400 MHz spectrometer (solvent=D2O). FT-IR spectra were collected on a Perkin Elmer spectrum BX FT-IR system. UV absorbance spectra were recorded using a Perkin Elmer Lambda 35 spectrometer. Fluorescence measurements were conducted using a Shimadzu RF-1501 spectrofluorometer. All NO measurements were performed using a Sievers-280™ chemiluminescence-based Nitric Oxide Analyzer (CL NOA, Boulder, CO). The instrument was calibrated before each experiment using an internal two-point calibration (zero gas and 45 ppm NO(g)). The flow rate was set to 200 mL/min with a cell pressure of 5.4 torr and an oxygen pressure of 6.0 psi. Nitric oxide released from all the diazeniumdiolated polymers was continuously swept from the headspace of the sample vessel, and purged from the test solution with a nitrogen sweep gas and bubbler into the CL NOA. To quantify the total amount of NO released, all the measurements were carried out for 2–3 d in 100 mM phosphate buffer at 37 °C with the addition of a diluted acid at the end of each experiment to completely decompose any residual intact NO donor within the polymer structure. Thermogravimetric analysis (TGA) was performed on a Perkin-Elmer TGA 7 under nitrogen. The TGAs were heated at a rate of 20 °C/min, and decomposition temperatures were reported at a 10 % weight loss.
Synthesis of Diazeniumdiolated Branched PEIs (3–6) (Scheme 1)
Scheme 1.
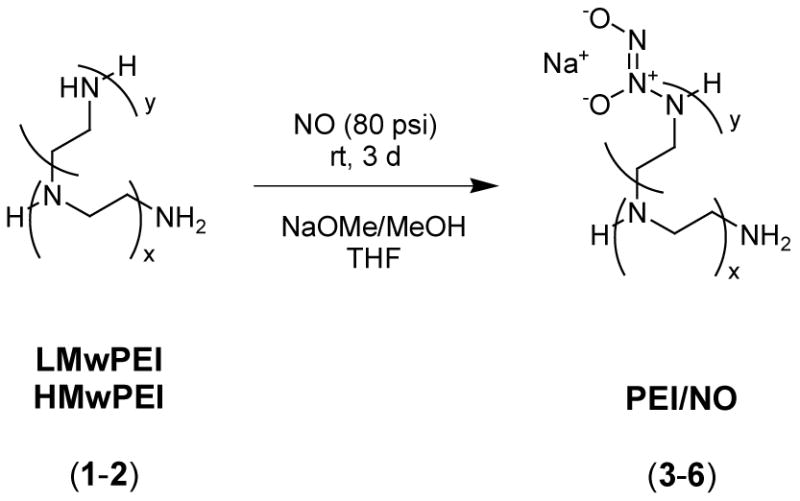
Synthesis of diazeniumdiolated branched PEIs.
Generally, a solution of 4 g of each PEI (low or high molecular weight, 1 or 2) in 20 mL of dry methanol and 60 mL of dry THF was prepared and placed in a high-pressure reactor (Parr bottle) with a stir bar. After flushing with argon for 10 min, sodium methoxide (0.2 or 1 eq with respect to the total amine sites) in methanol was added and the reactor was then closed. The Parr bottle was purged with argon thoroughly to remove residual oxygen and subsequently charged with NO(g) at 80 psi. The reaction mixture was stirred at room temperature for 72 h and then the NO was vented and the reactor was flushed with argon before each product was precipitated with dry THF/ether. The solvent was quickly removed by filtration, and the product was washed with dry ether and then vacuum dried to yield a light yellow powder (3–6) (Yield=49.2–74.8 %). The diazeniumdiolated products were stored in sealed vials charged with argon in the freezer for long-term stability. TGA, 10 % weight loss T(3)=105 °C, T(5)=108 °C.
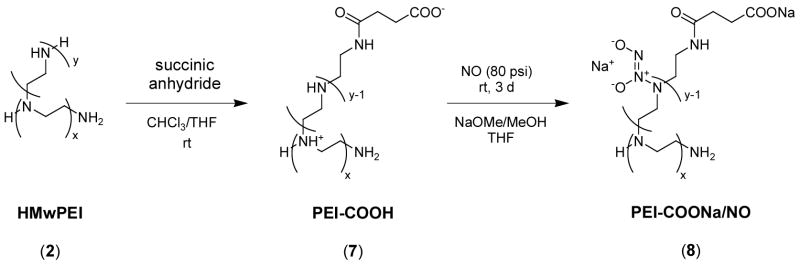
Synthesis of Carboxylated-PEI (7) and its NO Adduct (8) (Scheme 2)
Scheme 2.
Synthesis of diazeniumdiolated carboxylated-PEI.
To a solution of 5 g of HMwPEI (2) in 100 mL of chloroform was added a solution of 1.16 g (11.6 mmol, 0.1 eq) of succinic anhydride in 50 mL of chloroform and 20 mL of THF. The reaction mixture was stirred at room temperature under argon for 6 h and then a white precipitate was collected by filtration, washed with acetone/ether, and then vacuum dried to provide 2.3 g (38.0 %) of carboxylated-PEI (7). TGA, 10 % weight loss T=200 °C.
To a suspension of 1.1 g of (7) in 60 mL of dry methanol placed in a NO reactor under argon flow was added 4.7 mL of NaOMe/MeOH solution (4.37 M) followed by the addition of 30 mL of dry THF. After the general NO loading and workup procedures as described above for PEI/NOs, 1.56 g (69.7 %) of white powder (8) was obtained. The diazeniumdiolated product was stored in the freezer as described above. TGA, 10 % weight loss T=178 °C.
Synthesis of Boc-L-Proline-Incorporated PEI (9), and the Corresponding Deprotection (10) and NO Addition (11) Products (Scheme 3)
Scheme 3.
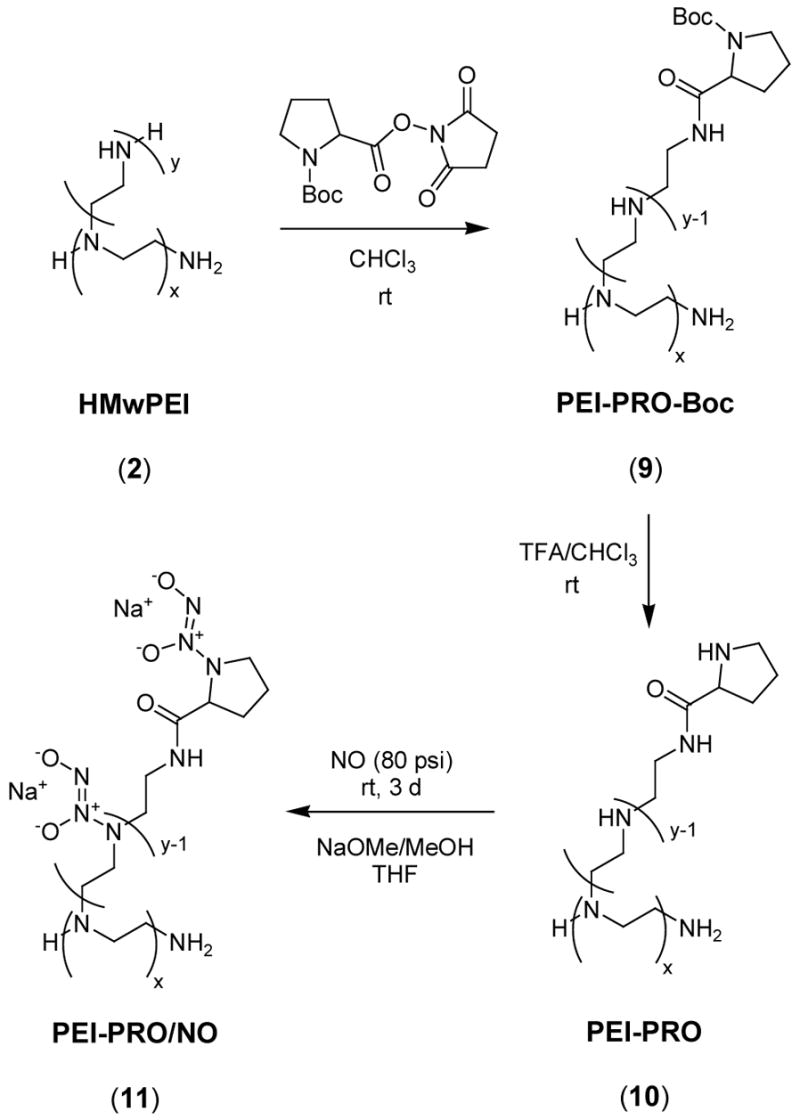
Synthesis of diazeniumdiolated L-proline-incorporated PEI.
Three g of HMwPEI (2) and 3 g of Boc-L-proline hydroxysuccinimide ester were dissolved in 200 mL of borate buffer (50 mM, pH 8.4) to form a foggy solution. After stirring at room temperature for 1 d, the resulting clear solution was dialyzed against DI water using dialysis tubing (MWCO: 50 kDa), and then lyophilized to provide 3.87 g (79.0 %) of white powder (9). TGA, 10 % weight loss T=233 °C.
To deprotect (9), 20 mL of trifluroacetic acid (TFA) was added slowly to a solution of 2.47 g of (9) in 70 mL of chloroform. A sticky precipitate formed during the addition and the stirring was stopped. The reaction mixture was then sonicated for 2 h and stirring was resumed at room temperature for another 2 h. After removal of solvent in vacuo, the residual was dissolved in 20 mL of water and neutralized with sodium bicarbonate solution. The resulting solution was dialyzed against DI water using dialysis tubing (MWCO: 50 kDa), and finally was lyophilized to quantitatively yield 2.09 g of off-white powder (10). TGA, 10 % weight loss T=208 °C.
To form the diazeniumdiolate of (10), a solution of 1 g of (10) in 40 mL of dry methanol and of 80 mL dry THF was first prepared, and 3 mL of NaOMe/MeOH solution (4.37 M) was then added under argon. The NO reactor was closed and followed by the general NO loading and workup procedures as described above to provide 1.3 g (61.2 %) of light yellow powder (11). The diazeniumdiolated product was stored as described previously. TGA, 10 % weight loss T=169 °C.
Nitrite-Release Measurements via Griess Assay
Twenty mg of each polymer (5, 8 or 11) was dissolved in 20 mL of 10 mM deoxygenated NaOH solution (pH 11.4) and placed in a dialysis tubing with a molecular weight cut-off of 15 kDa. The NO donor solution was dialyzed against 1 L of 10 mM NaOH with continuous stirring and nitrogen bubbling at room temperature. At selected time intervals (i.e., each hour for the first four hours and every day for three days), an aliquot of the NaOH dialysis solution was collected, neutralized, and the concentration of nitrite ion released from the polymer was measured via the Griess assay.30 Briefly, 100-μL samples were pipetted into a 96-well microtitier plate, neutralized with 0.5 M HCl and chilled to 4 ºC. Then 40 μL of a 1:1 mixture of 6 M HCl and 12.5 mM sulfanilamide was added to each sample. After the samples were chilled for an additional 10 min at 4 ºC, 20 μL of 12.5 mM N-(naphthyl)ethylenediamine dihydrochloride (NEDA) was then added to form an azo compound whose concentration is directly proportional to the concentration of nitrite. After 15-min incubation at room temperature, the concentration of the azo compound can be determined by its maximum absorbance at 540 nm as measured via a Labsystems Multiskan RC 96-well microplate reader.
Dialysis Experiments
A Baxter™ dialyzer (for adults, surface area=1.1 m2) and a Minifilter™ (for infants, surface area=0.08 m2) were employed in the model dialysis system as shown in Figure 2. The porous membranous fibers (blood pathway) within the dialyzer were in contact with a large volume of dialysate solution flowing continuously in the dialysate pathway. A stream of basic (high pH) water-soluble polymeric NO donor stock solution (1 mg/mL, prepared in pH>8 buffer) was infused into a stream of deoxygenated phosphate-buffered saline (PBS, a dialysate substitute containing 138 mM NaCl and 2.7 mM KCl, pH 7.4) and completely mixed in a 3.6 m mixing loop prior to entering the dialyzer. The flow of PBS buffer was controlled at the rate of 15 or 33 mL/min, mimicking the rate of the dialysate solution used with the Baxter™ dialyzer or Minifilter™ in clinical treatments. The water-soluble polymeric NO donor solution was infused at a varying rate in the range of 0.5–4 mL/min. The NO gas that is generated in the dialysate solution, which diffuses through the dialysis fiber walls to the blood pathway, was flushed with a nitrogen flow to a chemiluminescence-based NO analyzer. After the experiments, the NO levels were plotted vs. time with Excel.
Figure 2.
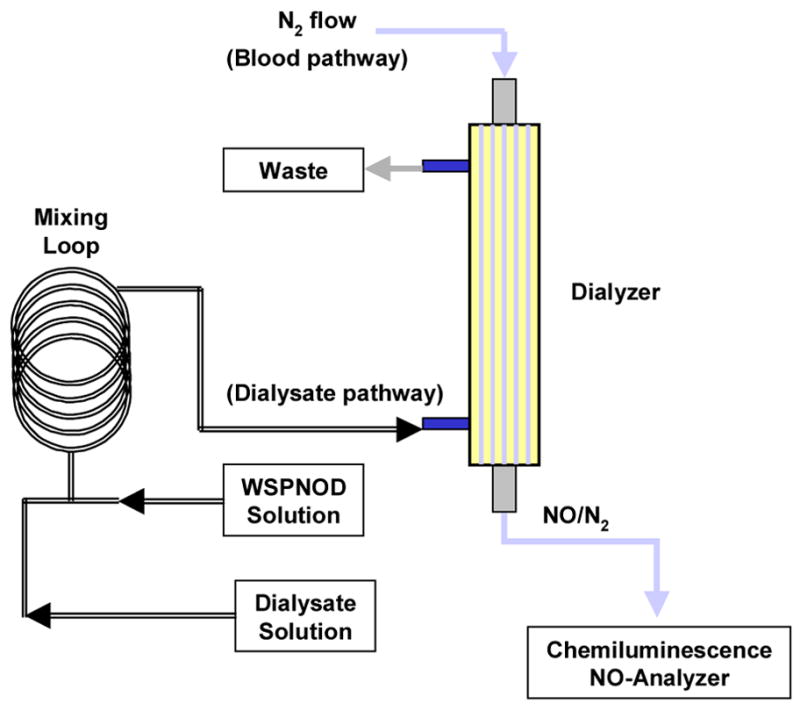
A schematic diagram of the NO-release dialysis set-up (WSPNOD: water-soluble polymeric NO donor).
Leaching Experiments
Nitric oxide adduct (HMwPEI/NO, 5) was chosen as a representative polymer for leaching studies using a hemodialyzer. The degree of leaching of any nitrite side-products and the polyamine decomposition products through the membranous fiber to the blood pathway was evaluated via a similar set-up as shown in Figure 2. Instead of using a nitrogen flow, a deoxygenated PBS buffer solution (10 mM, pH 7.4), maintained at 37 °C and a fixed volume of 150 mL, was continuously circulated through the blood pathway at a rate of 12 mL/min, equilibrating with a countercurrent flow of dialysate solution (rate=5.5 mL/min) containing HMwPEI/NO (0.1 mg/mL). An aliquot of the circulated solution from the blood pathway was collected every hour for four hours, bubbled with nitrogen for 30 min and then stored at 4 °C for further testing. The concentration of the nitrite in these solutions was determined by the Griess assay as described above. The degree of the polyamine leachables were evaluated with a fluorescence-based method for primary amine detection.28 Briefly, an aliquot of the sample solution was incubated in darkness at room temperature with a fluorescent precursor (FQCA) in the presence of KCN for 1 h. The resulting products are fluorescent FQ-polyamines. Fluorescence measurements were made with an excitation wavelength of 494 nm and emission spectra were recorded from 500 to 600 nm using a spectrofluorometer equipped with a 500 nm-yellow filter.
Results and Discussion
Synthesis and Characterization of NO Releasing Polymers
Three different types of water-soluble polymeric NO donors, based on diazeniumdiolated branched PEIs and their derivatives, were synthesized and characterized (see Schemes 1–3 and Table 1).
Table 1.
Yields and chemiluminescence (CL) characterization of the NO addition products of PEIs and their derivatives.
| Compound | NaOMe a | Yield (%) | Total NO release b (μmol/mg) c (D%)d |
|---|---|---|---|
| LMwPEI/NO (3) | 0.2 eq | 49.2 | 2.71 ± 0.28 (18.8 %) |
| LMwPEI/NO (4) | 1.0 eq | 74.8 | 2.76 ± 0.06 (19.2 %) |
| HMwPEI/NO (5) | 0.2 eq | 50.1 | 3.74 ± 0.41 (24.9 %) |
| HMwPEI/NO (6) | 1.0 eq | 68.0 | 3.84 ± 0.20 (26.8 %) |
| PEI-COONa/NO (8) | 1.3 eq | 69.7 | 2.92 ± 0.05 (24.5 %) |
| PEI-PRO/NO (11) | 1.3 eq | 61.2 | 4.15 ± 0.16 (32.2 %) |
Sodium methoxide used for NO addition with respect with total amine/carboxylate sites;
fresh samples measured in 100 mM phosphate buffer at 37 °C via the CL NOA (with acid addition);
Mean ± SD (n ≥ 3);
D%: percentage of diazeniumdiolation, based on the total primary and secondary amine sites; this value obtained herein is primarily contributed from the stable secondary amine diazeniumdiolates, given primary amine diazeniumdiolates are very unstable and may have undergone decomposition before NOA measurements.31
Diazeniumdiolation reactions were first directly carried out on two branched PEI starting materials in the presence of varying amounts of sodium methoxide. LMwPEI (1) was used only for studying the basic NO loading/releasing chemistry of its NO adducts, not for dialysis purposes. HMwPEI (2) was pre-dialyzed with a molecular weight cut-off of 50 kDa dialysis tubing in order to remove any low molecular weight fractions of the PEI, and therefore to potentially prevent leaching problems in the future dialysis experiments. All the resulting NO adducts (3–6), bearing ionic sodium-stabilized diazeniumdiolate functional groups, maintained their high water-solubility after NO addition. As shown in Table 1, the direct NO addition reactions afforded 49–75 % yields for the final products, with a notably increased yield (see the third column of the table) when higher amounts of sodium methoxide was used. The increased amount of base did not result in significant increases in the percentage of diazeniumdiolation (see the last column in Table 1) for both LMwPEI and HMwPEI. It is likely that with the increased amount of base, most primary amine sites will not be able to form stable diazeniumdiolates31 while the available number of secondary amine sites in each structure that can form stable diazeniumdiolates may be limited by steric effects.
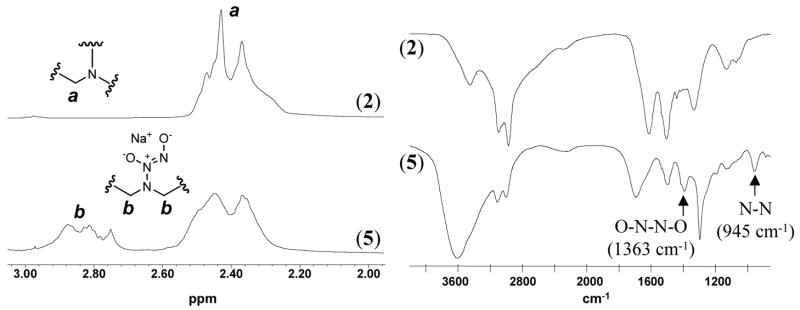
The UV-vis absorption spectra of all the diazeniumdiolated PEIs (3–6) show the characteristic absorption band for diazeniumdiolates at ca. 250 nm as measured in 10 mM deoxygenated PBS buffer at 26 °C.32 Using reaction conditions described in the experimental section, no detectable N-nitrosamine species (with UV absorbance bands in the range of 330–360 nm33) were found to form within the final polymers. The structural characterization of the representative product (5) using 1H NMR and IR is shown in Figure 3. Upon diazeniumdiolation, a portion of the proton signals (a) at 2.4 ppm (methylene groups adjacent to amine site in HMwPEI) are downfield shifted to (b) at approx. 2.8 ppm, due to the electron withdrawing effect of the diazeniumdiolate group. The integration of the shifted proton signals compared to the total proton integration of the compound (5) suggests 27 % of the theoretical diazeniumdiolation (not considering other side reactions). This agrees well with the 24.9 % diazeniumdiolation achieved for the same polymer as determined via the CL NOA measurements. The formation of the diazeniumdiolate group was also confirmed by the appearance of two distinct IR bands at 1363 and 945 cm−1 that were reported to be characteristic for the O-N-N-O asymmetric stretch and in-plane N2 symmetric stretch of the N2O2− group, respectively,34 as well as by the appearance of a large OH band around 3600 cm−1 that was from the absorbed moisture due to the hydroscopic nature of the diazeniumdiolated polymer. The former two bands decreased with time as the IR sample of polymer (5) was kept in an oven at 150 °C (nearly moisture-free condition) until they totally disappeared after two days, indicating the complete loss of all the diazeniumdiolate groups via thermal dissociation (spectra not shown here). However, such compounds are reasonably stable at room temperature (i.e., with approx. 8% of total NO loss after 5-day storage under ambient conditions for polymer (5)) and their thermal dissociation can be eliminated completely by storage of diazeniumdiolated polymers in argon or nitrogen under −20 °C for extended periods of time. This was proven by a long-term stability test of the NO donors after a 6-month storage under the above conditions (approx. 3% loss of NO over 6 months at −20 °C). After this period, the total NO release from the representative compound (5) was found to be 3.62 ± 0.35 μmol/mg, not statistically different from that measured with the fresh samples (3.74 ± 0.41 μmol/mg).
Figure 3.
Typical 1H NMR (solvent=D2O) and FT-IR spectra of HMwPEI (2) and its NO addition product HMwPEI/NO (5).
To obtain a PEI-based NO donor with a relatively fast NO release rate (discussed below), carboxylate sites (as inherent additive sites) were introduced to the polyamine substrate via the amidation reaction of HMwPEI (2) with succinic anhydride (0.1 eq in respect with the total amine sites). The appearance of two new IR bands at 1653 and 1405 cm−1 in the resulting product (7) confirms the C=O and C-N stretches of the incorporated NC(O)CH2CH2COO− moieties (see Figure 2s in the Supporting Information). Surprisingly, this compound was not soluble in water nor was it soluble in most organic solvents tested, making the selection of NO addition solvents difficult; however, upon addition of sodium methoxide prior to NO loading in THF/CH3OH, a clear solution was obtained. It is likely that sodium methoxide breaks the COO−/NH+R2 ion-pairs that may crosslink the polymer chains and decrease solubility. Final NO addition to (7) afforded nearly 70 % of a highly water-soluble product (8) with both characteristic UV absorption band and IR bands of the N2O2− group (see Figure 2s in the Supporting Information). Although the introduction of carboxylate sites caused a decrease in the total NO loading of the final polymer (8) compared to the direct NO addition product (6) (2.92 vs. 3.84 μmol/mg), the resulting NO release rate of PEI-COONa/NO was quite different than that of diazeniumdiolated PEIs without the appended carboxylate groups (see below).
The naturally occurring amino acid (L-proline) was previously reported to form ultrafast NO releasing species (PROLI/NO) after the diazeniumdiolation reaction. PROLI/NO has a half-life of less than 2 s as measured under physiological conditions.20, 21 Tethering PROLI/NO onto polymer backbones would potentially result in a new type of polymeric NO donors with rapid NO release rates. To achieve this goal, PROLI/NO-incorporated PEI (11) was prepared by first anchoring Boc-protected L-proline onto the HMwPEI (2) structure, followed by Boc-deprotection to release free proline secondary amine sites for subsequent NO addition. This reaction was carried out in a borate buffer, pH 8.4, where Boc-L-proline hydroxysuccinimide ester has its optimal reactivity with PEI amine sites. Subsequently, the resulting small molecule N-hydroxysuccinimide product and the remaining buffer salts, as well as possible hydrolytic products of Boc-L-proline hydroxysuccinimide ester, were completely removed via extensive dialysis, yielding 79 % of Boc-L-proline-incorporated PEI (9). The typical proton NMR signals of the Boc-group at 1.2–1.3 ppm were used as a probe to quantify the degree of the Boc-protected L-proline incorporated within (9). It was found that 11.5 % (theoretically 13.6 %, based on the initial feed ratio) of the total amine sites reacted with Boc-L-proline hydroxysuccinimide ester forming stable amide bonds (see Figure 3s in the Supporting Information). The appearance of two overlapped C=O bands (amide=1696 cm−1, Boc=1667 cm−1) in the IR spectrum confirms the formation of the desired structure (see Figure 4s in the Supporting Information). The band at 1667 cm−1 disappeared after the deprotection reaction using TFA. This observation, in combination with the disappearance of the Boc signal in the NMR spectrum of (11), indicates complete Boc-deprotection. Diazeniumdiolation of this polymer (yield=61.2 %) was successful, with stable diazeniumdiolates formed on the free secondary amine sites in the proline ring structure as well as on the free PEI secondary amine sites. Again, the final polymer (11) also shows both characteristic UV absorbance and IR bands of the N2O2− group (see Figure 4s in the Supporting Information). It should be noted that this polymer provides the highest NO release (4.15 μmol/mg) among all the polymeric NO donors prepared in this study. It is very likely that the incorporation of the proline units converts a number of PEI primary amines, which do not readily form stable diazeniumdiolates, into an equivalent amount of secondary amine sites, thereby increasing the number of potential diazeniumdiolation sites.
Decomposition and NO Release of Diazeniumdiolated Polymers
N-Diazeniumdiolates have been shown to decompose and release NO by two mechanisms, proton-driven19 and thermal14 dissociation. In the IR study discussed above, it was demonstrated that when the diazeniumdiolated polymer (HMwPEI/NO) was heated to 150 °C under the moisture-free conditions, only thermal dissociation occurred. When exposed to proton sources (i.e., PBS buffer) at 37 °C, both thermal and proton-drive dissociations can occur. However, given the fact (data not shown here) that the NO donors studied herein undergo very insignificant decomposition in dry N2 at physiological temperature (only thermal dissociation), it is assumed that their dissociation in deoxygenated buffer solution to release NO is primarily proton-mediated. Thus, the decomposition of water-soluble polymeric NO donors to release NO in aqueous solution was carefully investigated in this work under various conditions (pH, additive, temperature, etc.) using UV and chemiluminescence-based NO analyzer (NOA). Such studies revealed the basic properties of the different donors and provided important information for the formulation of such dialysate additives as well as for the design and optimization of the dialysis system using the proposed NO-release strategy.
It was found that HMwPEI/NO (5) exhibited substantially different NO release patterns in phosphate buffer at different concentrations, as shown in Figure 4. A low and sustained level of NO release was observed from (5) when measured in 10 mM PBS buffer while dramatically faster NO release was found when measured in a 100 mM phosphate buffer. This effect is not due to a difference in the ionic strength of the two buffers, since adding additional NaCl up to 198 mM to the 10 mM PBS did not change the rate of NO release observed with this buffer system. However, upon addition of an acid, the retained NO was spontaneously and completely released from (5) in 10 mM PBS buffer. The pHs of the HMwPEI/NO test solutions made with 10 mM PBS and 100 mM phosphate buffer (1 mg/mL, originally pH 7.4) were found to be 9.2 and 7.5, respectively. This suggests that the basic polyamine structure within the PEI and any residual sodium methoxide can greatly affect the pH of a buffer solution with a relatively weak buffer capacity; therefore, this affects the NO release rate from the diazeniumdiolates within such a buffer, which is a highly pH-dependent process.19 Indeed, this pH effect can be applied as the principle of NO release at the site of application (dialysis fibers) for the new NO-release dialysis system. The water-soluble polymeric NO donor is initially dissolved in a high pH buffer where its NO release should be greatly inhibited; however, dramatic NO release is stimulated spontaneously once this donor solution merges with a large amount of lower pH dialysis fluid in the mixing loop/dialyzer, creating a localized increase in NO level at the high surface area dialysis fiber/blood interface within the dialysis filters.
Figure 4.
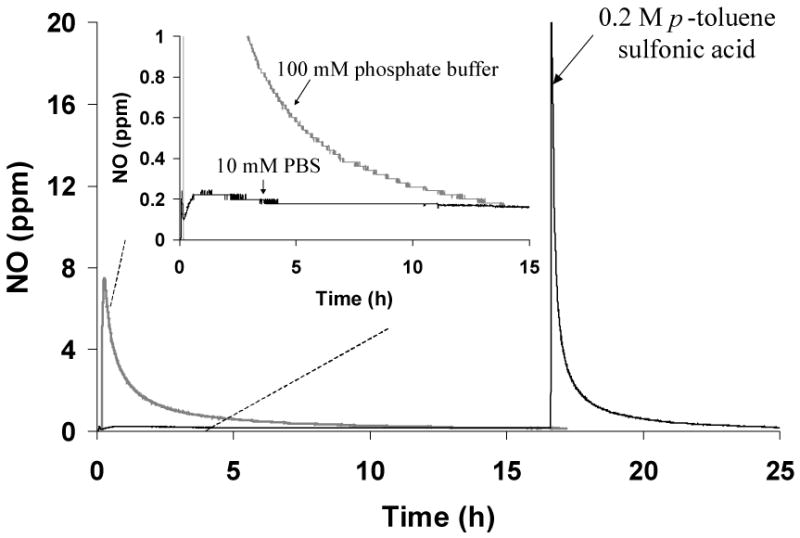
NO release from HMwPEI/NO (5) in phosphate buffers with various buffer capacities. The NO levels detected were normalized as a value per 1 mg of HMwPEI/NO. The measurements were performed in deoxygenated buffers at 37 °C via the CL NOA.
The decomposition kinetics of (5) was studied using UV spectroscopy by monitoring the decrease of absorbance at 250 nm with time (see Figure 5). It was found that the decomposition of (5) in the physiological pH (7.4) at room temperature exhibits near-first-order behavior (plot of log[diazeniumdiolate concentration] vs. time yielded a straight line) with a rate constant of 3.9×10−5 s−1 and a half-life of 5.0 h (300 min, see Figure 5, inset). This t1/2 value indicates that HMwPEI/NO (5) has a dramatically faster NO release rate than the previously reported NO releasing crosslinked PEI microsphere (PEIX/NO) with a calculated half-life of 66.2 h under the same conditions.29 The previous water-insoluble PEIX/NO was actually developed as a filler material for vascular grafts, which requires minimal leaching (maximal water-insolubility) and slow NO release rate for long-term applications.
Figure 5.
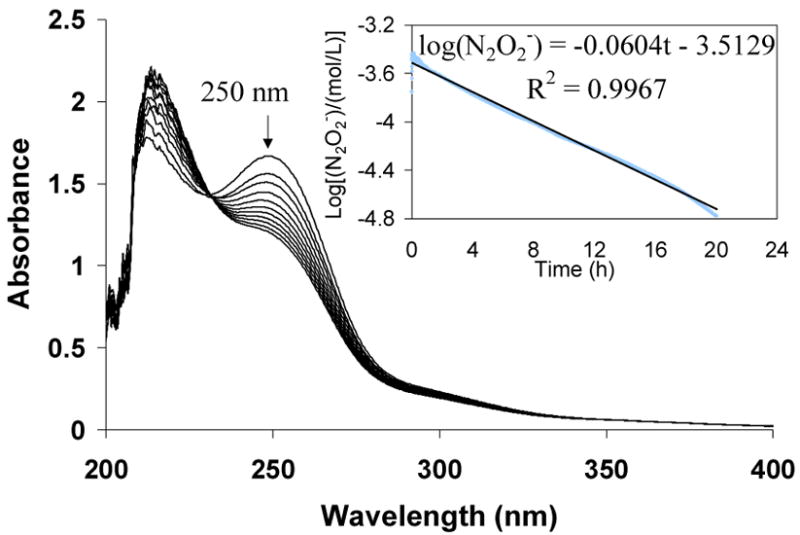
Decomposition kinetic study of diazeniumdiolates within polymer HMwPEI/NO (5) as measured via UV in 100 mM deoxygenated phosphate buffer (0.1 mg/mL, pH 7.4) at 26 °C; each spectrum was collected at a 10 min interval. Inset: Plot used to determine a near-first-order rate constant of kD=3.9×10−5 s−1 (each absorbance data was collect at λ=250 nm with an interval of 15 s).
Chemiluminescence-based NOA was also employed to study the dissociation kinetics of the representative water-soluble diazeniumdiolated polymers—HMwPEI/NO (5), PEI-COONa/NO (8) and PEI-PRO/NO (11)—but at physiological temperature (37 °C). The resulting kinetic data and NO release profiles of the polymers are compared in Table 2 and shown in Figure 6.
Table 2.
Decomposition parameters of the water-soluble diazeniumdiolated polymers.
| Compound | aqueous media | kD (×10−5 s−1) | t1/2a,c (min) |
|---|---|---|---|
| HMwPEI/NO (5) | 100 mM phosphate buffer | 6.0 | 192.0 ± 1.7 |
| HMwPEI/NO (5) | PAANa solution b | 16.1 | 72.0 ± 3.0 |
| PEI-COONa/NO (8) | 100 mM phosphate buffer | 19.4 | 59.4 ± 0.8 |
| PEI-PRO/NO (11) | 100 mM phosphate buffer | 24.1 | 48.0 ± 2.5 |
measured at 37 °C via the CL NOA;
1 mg/mL of sodium salt of poly(acrylic acid) in 100 mM phosphate buffer (pH 7.4);
Mean ± SD (n ≥ 3).
Figure 6.
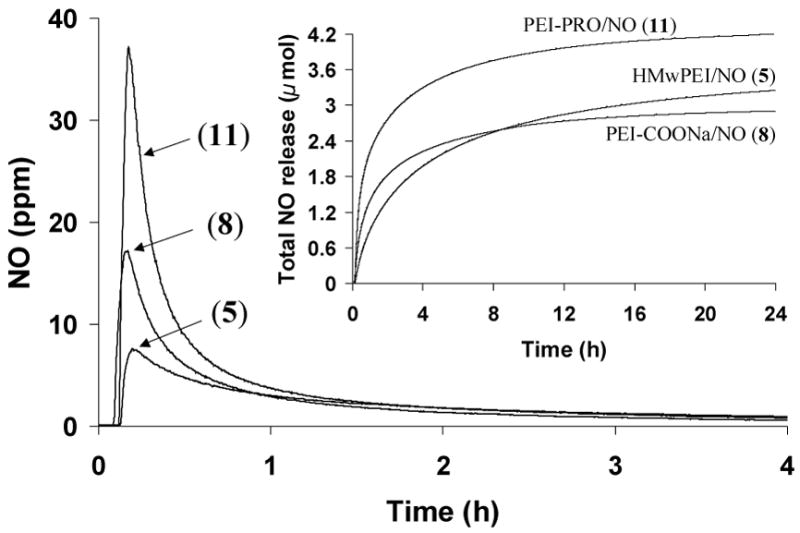
NO release from HMwPEI/NO (5), PEI-COONa/NO (8) and PEI-PRO/NO (11) as measured in deoxygenated phosphate buffer (pH 7.4, 100 mM) at 37 °C via the CL NOA; Inset: Total NO release of the three compounds. All the NO amounts detected were normalized as a value per 1 mg of donor.
The decomposition of the polymeric NO donors was found to be temperature dependent; for example, compound (5) was observed to have a much faster NO release rate at 37 °C than at 26 °C, with a rate constant determined to be 6.0×10−5 s−1 and a t1/2 value of 192 min. Interestingly, it was also found that the dissociation of (5) was greatly accelerated in the presence of the sodium salt of poly(acrylic acid) (PAANa, 1mg/mL), a heparin mimic with antithrombin-activating properties;35, 36 the resulting decomposition reaction also follows near-first-order kinetics, but has an even greater rate constant and a much shorter half-life (k=1.6×10−4 s−1, t1/2= 72 min) in the presence of PAANa. Notably, the addition of a low concentration of PAANa did not affect the pH of the buffer at all, indicating that this was not a solution pH effect similar to that described above.
This finding inspired the design and synthesis of a faster NO release version of the original PEI material, PEI-COONa/NO (8), that contains pendant carboxylate sites within the same polymeric structure to speed NO release from the polymer. Indeed, PEI-COONa/NO was found to exhibit much faster NO release kinetics (t1/2= 59.4 min) than HMwPEI/NO (5). This rate is similar to the NO release of HMwPEI/NO in the presence of the PAANa additive. It is speculated that the carboxylate sites within PEI-COONa/NO serve to promote the decomposition speed of the surrounding diazeniumdiolate groups to release NO, similar to the function of the exogenous PAANa additive. As suggested in Scheme 4, it is likely that in the aqueous media, the carboxylate groups can form more stable acid-base ion-pairs with the neighboring amine sites that are created after the NO release reaction, thereby forcing the equilibrium toward the decomposition of the inter/intramolecular diazeniumdiolate groups and speeding NO release. It is also possible that substitution of carboxyl sites for amines on the polymer could decrease any stabilizing interaction between the pendant diazeniumdiolates and adjacent amines and/or the presence of the adjacent amines could contribute to stabilization by increasing the local pH near the diazeniumdiolate species. The latter is a more likely alternate to the mechanism suggested in Scheme 4; however, even the carboxylated PEI-COONa/NO donors have considerable amount of unmodified tertiary amine sites in the vicinity of the diazeniumdiolate groups, and hence one would think that these stronger base sites would likely control any microenvironmental pH effects that would control NO release rates. In the case of increased rates of NO release when PAANa is added separately to the test solution containing the PEI/NO species, it is possible that the PAANa complexes with the positively charged PEI/NO (owing the excess primary and tertiary amine sites still present on such polymers), and this interaction provides a local pH microenvironment from residual protons on the PAANa structure that increase the rate of reaction. Sorting these various possibilities out will require further studies.
Scheme 4.
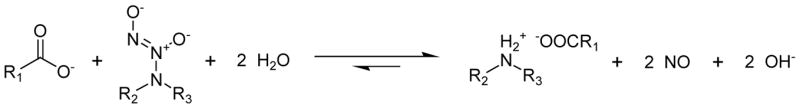
Proposed mechanism by which use of carboxylate site speeds NO release.
Among the three water-soluble PEI-based NO releasing materials, diazeniumdiolated L-proline-incorporated PEI (11) was found to be the most efficient NO donor (fastest and largest amount of total NO release, as shown in Figure 6) with a half-life of only 48 min (37 °C, pH 7.4). The decomposition of this candidate polymer was extensively studied at two other temperatures (26 and 45 °C) and the half-lives were found to be 151.8 and 24.2 min, respectively, showing the expected strong temperature-dependent NO release kinetics. This information is helpful for the future design of the NO-release dialysis system. The efficient NO release feature of (11) is mostly attributed to the covalently linked proline units within the PEI structure, which are loaded with NO. The diazeniumdiolated free proline (PROLI/NO) is the fastest NO donor reported to date.20 It should be noted that two types of diazeniumdiolate groups may exist within polymer (11): one on the proline amine sites (faster NO release) and the other on the PEI amine sites (slower NO release). Both ultimately contribute to the overall NO release speed of the compound. However, the NO release rate of PEI-PRO/NO (11) is substantially slower than that of its corresponding small molecule NO adduct PROLI/NO (t1/2: 48 min vs. 2 s, at 37 °C). It is likely that these significantly different half-lives can be attributed to the structural difference between the two species. Indeed, the PEI-based water-soluble polymeric NO donors contain a considerable amount of tertiary amines within the polymer structures, which cannot form diazeniumdiolates but may increase the microenvironment basicity, thereby enhancing the stability of the adjacent diazeniumdiolate groups.
The development of new NO donors based on polymer structures lacking tertiary amines is one possible approach to obtain faster versions of water-soluble polymeric NO donors for wider applications. Efforts to prepare such water-soluble NO donors by incorporating PROLI/NO onto high molecular weight poly(allylamine) backbone or linking mono-diazeniumdiolated piperazine onto carboxylmethyl cellulose structure are currently in progress in this laboratory.
It should be noted that the PEI materials used to create some of the water-soluble polymeric NO donors contain considerable levels of primary amines. Although diazeniumdiolates of primary amine groups are much less stable with respect to yielding NO release moieties,31 Keefer et al. reported that diazeniumdiolation of primary amines can, in some instances, produce adducts that release nitroxyl (HNO).37 To test whether polymer (5) produced any measurable levels of HNO, along with NO, a fluorescence assay of total NO/HNO was employed38, 39 and results were compared to total NO only release, as measured by chemiluminescence. A pH 6.4 phosphate buffer was used for these measurements, to speed the NO/HNO release reactions. After 4 h of reaction at 37 °C, the total NO/HNO measured by fluorescence was 6.5 % greater than the moles of NO detected by chemiluminescence. This suggests that some small amount of HNO may, in fact, be released from polymer (5), but the level is much less than the total NO species that is produced by this new water-soluble polymeric NO donor agent.
NO Release Studies for Potential Hemodialysis in a Static Setting
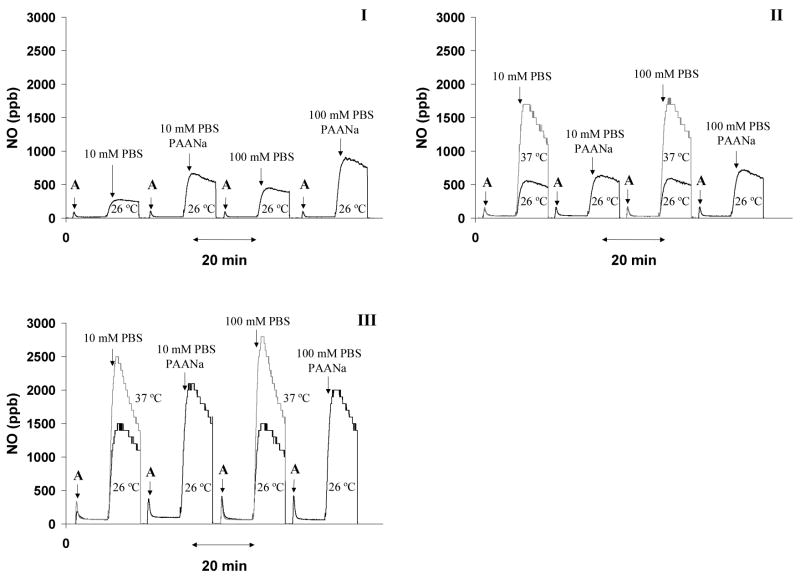
To demonstrate that dramatically increased NO release can be created spontaneously upon mixing of a basic water-soluble polymeric NO donor stock solution and a dialysate fluid (pH 7.4) at a given volume ratio, static mixing experiments were designed and conducted. Different factors that could influence the amount of NO release were also investigated. As expected, NO release was inhibited as each NO donor was kept in a high pH buffer solution (Figure 7, I–III). The initial levels of NO detected from 100 μL of the NO donor stock solutions were highly dependent on the stability (half-life) of the water-soluble polymeric NO donors; for example, 18 ppb for HMwPEI/NO but 72 ppb for PEIPRO/NO. Upon addition of 3300 μL of buffer (10 mM PBS or 100 mM phosphate buffer, pH 7.4), a significantly increased level of NO (up to 2800 ppb, at 37 °C) was observed. This experiment proves that the potent NO release potential of all the polymeric donors can be retained until they are merged with a stream of lower pH dialysate solution immediately prior to entering the hemodialysis filter.
Figure 7.
Proton-stimulated spontaneous NO releases as measured at 26 and 37 °C via the CL NOA by mixing a 100 μL solution of PEI/NO (I), PEI-COONa/NO (II) or PEIPRO/NO (III) (A=addition of an NO donor solution: 1 mg/mL in phosphate buffer, pH 8.0) with a 3300 μL of 10 mM PBS or 100 mM phosphate buffer (pH 7.4) in the presence and absence of PAANa additive (30 μg/mL).
The addition of a low dose of PAANa to the solution phase (30 μg/mL, 5,100 Da PAANa used herein only to demonstrate the effect but not for dialysis purposes) was found to effectively enhance the NO release level for all the polymeric donors (see Figure 7). The effect was most significant in the case of HMwPEI/NO where addition of PAANa enhanced NO release by a factor of 2.5-fold for both buffers tested. Such additive effect was also observed for PEI-COONa/NO and PEI-PRO/NO (up to 20 and 40 % increase in NO level, respectively) though not as significant as for HMwPEI/NO. Furthermore, as shown in Figure 7, increasing the temperature plays an important role in generating higher levels of NO.
NO Release Studies using Hemodialysis Filters in a Flowing System
Finally, a commercial Baxter™ hemodialysis dialyzer (for adults, surface area=1.1 m2) and a Minntech™ mini-filter (for infants, surface area=0.08 m2) were employed in a flowing system, as shown in Figure 2, to demonstrate the concept of NO release for hemodialysis. Porous membranous fibers housed within the dialysis filters were continuously perfused with a large volume of flowing dialysate solution at a rate of 33 mL/min (for Baxter™ dialyzer) or 15 mL/min (for Minntech™ mini-filter). In real clinical treatments, the blood that is removed from the patient’s vein is pumped through these fibers in a countercurrent flow against the dialysate solution. The non-circulating dialysate solution is always removed as waste. The semi-permeable membranous fibers, which are typically 10–20 μm thick, have a large surface area, with pores that have desired molecular weight cut-offs. Generally, molecules with a molecular weight less than 5,000 pass through the membranous fibers fairly easily. In this manner, toxic small molecule wastes, such as urea, can be removed from the body via the two-phase dynamic equilibrium. In these preliminary experiments, a stream of basic water-soluble polymeric NO donor solution (1 mg/mL) was merged with a stream of PBS buffer (a dialysate substitute, pH 7.4) within a mixing loop prior to entering the dialyzer. The length and pre-heating temperature of the mixing loop can be adjusted to achieve maximal/optimal NO fluxes at the membranous fiber/blood interface within the dialysis filters.
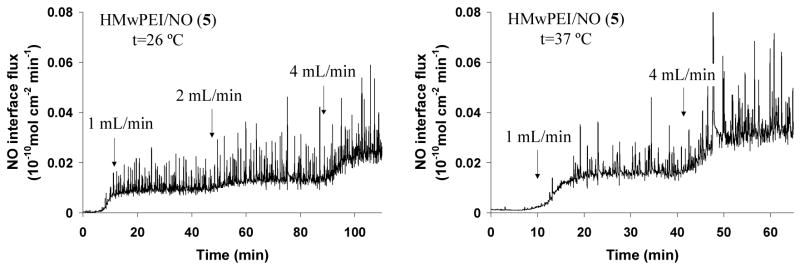
Figure 8 demonstrates that steady interfacial NO fluxes (up to 0.03×10−10 mol · cm−2 · min−1) can be generated and diffuse through the fiber walls of the Baxter™ dialyzer when using HMwPEI/NO as the dialysate additive. Such NO levels can be controlled by simply varying the experimental conditions, such as mixing rate of the NO donor stock solution and pre-heating temperature. It should be noted that during the dialysis experiments, some water/buffer droplets were observed to diffuse through the dialysis fiber walls to the “blood-side” (gas phase) due to permeation equilibria between two phases. Such discontinuous droplets containing dissolved NO gas can cause spikes that are superimposed onto the stable NO fluxes generated by gas diffusion only (shown in both Baxter™ dialyzer and Minntech™ mini-filter cases). These spikes varied in the different experimental conditions as well as when dialysis filters made of different fibers were used.
Figure 8.
Interfacial NO fluxes generated across the dialysis membranous fibers within a Baxter™ dialyzer at room/physiological pre-heating temperature as measure via the CL NOA. HMwPEI/NO (5): 1 mg/mL in phosphate buffer (pH 8) at a rate of 1–4 mL/min; dialysate solution: PBS buffer (pH 7.4) at a controlled rate of 33 mL/min.
The NO fluxes through the walls of the dialysis fibers can be greatly enhanced (by up to 2.5-fold) via the addition of PAANa to the dialysate solution at a low dose of 30 mg/L (data not shown here). Although for safety considerations, high molecular weight PAANa can be used to prevent possible leaching into the blood side, it is noteworthy that PAANa has been reported to possess equivalent/near equivalent anticoagulation activity as low-affinity-heparin and other polysaccharide species.35, 36
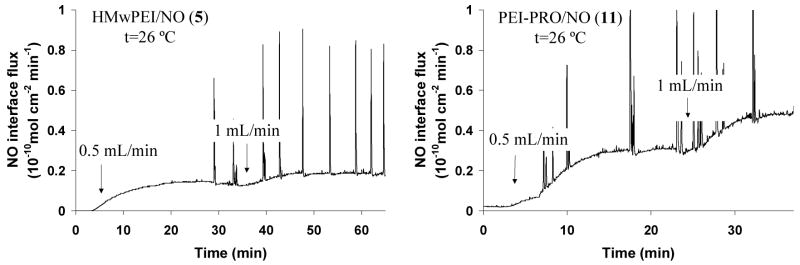
In a second experimental set-up using the Minntech™ mini-filter (with 7 % fiber surface area of the Baxter™ filter), steady-state NO interface fluxes (see Figure 9) were greatly increased by approx. 20-fold when HMwPEI/NO solution was mixed at a rate of 1 mL/min at 26 °C, compared to the same infusion conditions with Baxter™ filter (see Figure 8) (NO fluxes: 0.2 vs. 0.01×10−10 mol · cm−2 · min−1). Moreover, in combining the most efficient candidate PEI-PRO/NO with this dialysis set-up, even higher NO fluxes can be obtained (up to 0.5×10−10 mol · cm−2 · min−1), within the range of the physiological NO flux level (0.5–4×10−10 mol · cm−2 · min−1)7 from endothelial cells. The interfacial NO release levels as well as the NO release efficiency can be enhanced even further through optimization of the dialysis set-up/experiment conditions (i.e., fine-tune dialysate solution pH, increase length and temperature of the mixing loop and etc.). Indeed, this latter combination was chosen as the primary proof-of-concept candidate for the ongoing in vivo animal studies of the NO-release dialysis experiments at the University of Michigan Medical School.
Figure 9.
Interfacial NO fluxes generated across the dialysis membranous fibers within a Minntech™ mini-filter at room temperature as measured via the CL NOA. HMwPEI/NO (5) and PEI-PRO/NO (11): 1 mg/mL in phosphate buffer (pH 8) at a rate of 0.5–1 mL/min; dialysate solution: PBS buffer (pH 7.4) at a controlled rate of 15 mL/min.
It should be noted that the basic concept of releasing NO through the walls of the dialysis fibers used in hemodialyzers would not pose a risk of systematically decreasing the blood pressure of individuals who would potentially undergo dialysis using such an approach. Indeed, the low flux of NO that comes through the fibers from the dialysate solution (ideally equal to the normal fluxes that come from the endothelial cells that line the walls of all blood vessels) would immediately react with the far excess of hemoglobin in the blood. Hence, no free NO would exist to exert vasodilation effects downstream in the patient’s body after the blood passes through the dialysis unit.
Leaching Studies
In addition to the desired N-diazeniumdiolate moieties, a given amount of nitrite species also form within the NO adduct structures during synthesis and was found to be released into the surrounding bathing solution,15 and further diffuse through the membranous fibers to the blood side of the dialyzer. The initial nitrite (a minor side-product during NO addition and following workup15) released with time from three NO donors (5, 8 and 11) was measured using the Griess assay30 (see Figure 5s in the Supporting Information). Such nitrite measurements were conducted in basic and oxygen-free conditions where no (or minimal) NO release and subsequent oxidation can occur, as NO could be oxidized to nitrite, yielding false values for the inherent nitrite levels in the polymeric NO donor preparations. It was found that the three polymeric compounds have total nitrite release potentials of (5) 1.00 ± 0.02, (8) 0.67 ± 0.02 and (11) 0.58 ± 0.03 μmol/mg, respectively. These values are very small compared to the total NO release values of the polymers (see Table 1). At the 4-h point, each milligram of the compound can release an accumulated amount of nitrite of 0.77 ± 0.02, 0.21 ± 0.01 and 0.11 ± 0.01 μmol, respectively. In a 4-h dialysis leaching test experiment using (5), approx. 120 mg of NO donor was initially used with 1.32 L of dialysate solution, which should make the final nitrite concentration in the dialysate solution to be 70 μM (0.77 μmol/mg ×120 mg/1.32 L) at the end of the dialysis. In fact, on the blood side, the actual nitrite concentration in 150 mL of “blood” (deoxygenated PBS solution) at the end of the 4-h dialysis was found to be ca. 10 μM, only 14 % of the theoretical/possible leachable value. In the real NO-release hemodialysis experiment using the same set-up, the nitrite concentration may be higher due to the oxidation reaction of the released NO with the oxygen in the blood. Actually, nitrite ion is NO’s primary oxidation product at physiological pH and a normal constituent present in abundance in human physiological fluids (between 0.5 and 21 μM in the plasma of healthy human individuals40, 41). Indeed, recently, a growing body of evidence suggests that the nitrite anion may represent the largest intravascular and tissue storage form of NO.42
The potential leaching of the polyamines, hydrolytic products of the water-soluble polymeric NO donors through the dialysis fiber into the blood side, was also examined. It should be noted that in vitro testing conducted by the dialyzer manufacturer has already proved that macromolecules with small sieving coefficients (i.e., 0.030 for albumin, Mw=65,000) have much less chance of leaching than small molecules (i.e., 0.99 for urea, Mw=60). In a dialysis experiment using HMwPEI/NO (5) as the dialysate additive, the presence of polyamine leachables on the blood-side (10 mM PBS buffer, pH 7.4) was measured at intervals of 60 min for 4 h (targeted dialysis time). After treatments with FQCA, the fluorescence intensities of the PBS buffer at the selected intervals were found to be nearly the same as the control (blank PBS), indicating no evidence of detectable polymer leaching through the dialysis fiber walls.
Conclusions
Three different water-soluble poly(ethylenimine)-based NO releasing agents were designed, synthesized and characterized for potential use as NO release agents in hemodialysis systems. It has been shown that water-soluble polymers with up to 4.15 μmol/mg of total NO release potential can be prepared and the rates of NO release can be modulated by the specific structure of the polymer, as well as experimental factors such as pH, temperature and use of exogenous additives (e.g., the sodium salt of poly(acrylic acid)). When used as NO generating reagents in the dialysate fluid of commercial hemodialysis units, the new diazeniumdiolated polymers were found to provide a steady and controllable NO interfacial fluxes through the fibers of such devices. Use of the most efficient NO donor candidate, PEI-PRO/NO, provides physiological levels of NO flux (0.5×10−10 mol · cm−2 · min−1) at the membranous fiber/blood interface within the dialyzer. Thus, improved blood compatibility of dialysis fibers in the absence of other anticoagulants (i.e., heparin or citrate) during the dialysis treatment may be possible without concern of either clotting or excess bleeding. Although no significant evidence was found that the new PEI-based polymers crossed the dialysis fiber walls to the blood side, increasing the molecular weight of the NO donors would further ensure safe dialysis with the proposed method. At the same time, additional research is required to develop water-soluble polymeric NO donors with even faster release kinetics. Indeed, at present, given the short residence time of the water-soluble polymeric NO donors in the dialysis unit (minutes), a substantial fraction of the loaded NO is never used to potentially reduce thrombosis. Hence, creating macromolecular NO donors with t1/2 values on the order of 2–5 minutes would provide the ideal polymers for the dialysis application. Nevertheless, until such new polymers can be prepared, the water-soluble polymeric NO donors described here are being examined in animal experiments to further assess this NO-release dialysis concept in terms of blood compatibility of dialysis fibers and any toxicity issues associated with the use of such polymeric NO donors in dialysis therapies.
Supplementary Material
Typical 1H NMR and FT-IR spectra of representative HMwPEI (2), carboxylated-PEI (7) and its NO addition product (8); Boc-protected-L-proline-incorporated PEI (9) and the corresponding deprotection (10) and NO addition (11) products. Accumulated nitrite release from HMwPEI/NO (5), PEI-COONa/NO (8) and PEI-PRO/NO (11) under the basic and oxygen-free conditions at room temperature as measured via the Griess assay. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We gratefully acknowledge the NIH (EB-000783 and HD-015434) and Michigan Critical Care Consultants, Inc., for supporting this work.
References
- 1.Salzman EW, Merrill EW. In: Hemostasis and Thrombosis. Colman RW, Hirsh J, Marder VS, Salzman EW, editors. JB Lippincott; Philadelphia: 1987. pp. 1335–1347. [Google Scholar]
- 2.Kim YG. Nephrology. 2003;8:S23–S27. doi: 10.1046/j.1440-1797.8.s.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Janssen M, vanderMeulen J. Neth J Med. 1996;48:198–207. doi: 10.1016/0300-2977(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 4.Lohr JW, Schwab SJ. J Am Soc Nephrol. 1991;2:961–975. doi: 10.1681/ASN.V25961. [DOI] [PubMed] [Google Scholar]
- 5.Pinnick RV, Wiegmann TB, Diederich DA. N Engl J Med. 1983;308:258–261. doi: 10.1056/NEJM198302033080506. [DOI] [PubMed] [Google Scholar]
- 6.Faber LM, Devries P, Oe PL, Vandermeulen J, Donker AJM. Neth J Med. 1990;37:219–224. [PubMed] [Google Scholar]
- 7.Radomski MW, Palmer RMJ, Moncada S. Biochem Biophys Res Commun. 1987;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 8.Radomski MW, Palmer RMJ, Moncada S. Br J Pharmacol. 1987;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siney L, Lewis MJ. Eur J Pharmacol. 1992;229:223–226. doi: 10.1016/0014-2999(92)90559-m. [DOI] [PubMed] [Google Scholar]
- 10.Sneddon JM, Vane JR. Proc Natl Acad Sci U S A. 1988;85:2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, Meyerhoff ME, Bartlett RH. Crit Care Med. 2000;28:915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, Politis JK, Elmore J, Merz SI, Meyerhoff ME. J Med Chem. 2003;46:5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 13.Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM, Merz SI, Meyerhoff ME, Bendick PJ, Zelenock GB, Shanley CJ. J Vasc Surg. 2004;40:803–811. doi: 10.1016/j.jvs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HP, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME. Biomaterials. 2002;23:1485–1494. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HP, Annich GM, Miskulin J, Stankiewicz K, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME. J Am Chem Soc. 2003;125:5015–5024. doi: 10.1021/ja0291538. [DOI] [PubMed] [Google Scholar]
- 16.Frost MC, Rudich SM, Zhang HP, Maraschio MA, Meyerhoff ME. Anal Chem. 2003;75:1037–1037. doi: 10.1021/ac025944g. [DOI] [PubMed] [Google Scholar]
- 17.Frost MC, Reynolds MM, Meyerhoff ME. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MM, Frost MC, Meyerhoff ME. Free Radic Biol Med. 2004;37:926–936. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Davies KM, Wink DA, Saavedra JE, Keefer LK. J Am Chem Soc. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra JE, Southan GJ, Davies KM, Lundell A, Markou C, Hanson SR, Adrie C, Hurford WE, Zapol WM, Keefer LK. J Med Chem. 1996;39:4361–4365. doi: 10.1021/jm960616s. [DOI] [PubMed] [Google Scholar]
- 21.Waterhouse DJ, Saavedra JE, Davies KM, Citro ML, Xu X, Powell DA, Grimes GJ, Potti GK, Keefer LK. J Pharm Sci. 2006;95:108–115. doi: 10.1002/jps.20486. [DOI] [PubMed] [Google Scholar]
- 22.Smith DJ, Chakravarthy D, Pulfer S, Simmons ML, Hrabie JA, Citro ML, Saavedra JE, Davies KM, Hutsell TC, Mooradian DL, Hanson SR, Keefer LK. J Med Chem. 1996;39:1148–1156. doi: 10.1021/jm950652b. [DOI] [PubMed] [Google Scholar]
- 23.Mowery KA, Schoenfisch MH, Saavedra JE, Keefer LK, Meyerhoff ME. Biomaterials. 2000;21:9–21. doi: 10.1016/s0142-9612(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhou ZR, Meyerhoff ME. Biomaterials. 2005;26:6506–6517. doi: 10.1016/j.biomaterials.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Nablo BJ, Prichard HL, Butler RD, Klitzman B, Schoenfisch MH. Biomaterials. 2005;26:6984–6990. doi: 10.1016/j.biomaterials.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Nablo BJ, Rothrock AR, Schoenfisch MH. Biomaterials. 2005;26:917–924. doi: 10.1016/j.biomaterials.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Marxer SM, Rothrock AR, Nablo BJ, Robbins ME, Schoenfisch MH. Chem Mater. 2003;15:4193–4199. [Google Scholar]
- 28.Frost MC, Meyerhoff ME. Journal of Biomedical Materials Research Part A. 2005;72A:409–419. doi: 10.1002/jbm.a.30275. [DOI] [PubMed] [Google Scholar]
- 29.Pulfer SK, Ott D, Smith DJ. J Biomed Mater Res. 1997;37:182–189. doi: 10.1002/(sici)1097-4636(199711)37:2<182::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt HHHW, Kelm M. In: Methods in Nitric Oxide Research. Feelisch M, Stamler JS, editors. John Wiley & Sons; West Sussex: 1996. pp. 491–497. [Google Scholar]
- 31.Drago RS, Karstett Br. J Am Chem Soc. 1961;83:1819–1822. [Google Scholar]
- 32.Zhou ZR, Meyerhoff ME. Biomacromolecules. 2005;6:780–789. doi: 10.1021/bm049462l. [DOI] [PubMed] [Google Scholar]
- 33.Kostyukovskii YL, Melamed DB. Usp Khim. 1988;57:625–655. [Google Scholar]
- 34.Keefer LK, Flippen-Anderson JL, George C, Shanklin AP, Dunams TA, Christodoulou D, Saavedra JE, Sagan ES, Bohle DS. Nitric Oxide: Biol Chem. 2001;5:377–394. doi: 10.1006/niox.2001.0359. [DOI] [PubMed] [Google Scholar]
- 35.Monien BH, Cheang KI, Desai UR. J Med Chem. 2005;48:5360–5368. doi: 10.1021/jm0503648. [DOI] [PubMed] [Google Scholar]
- 36.Monien BH, Desai UR. J Med Chem. 2005;48:1269–1273. doi: 10.1021/jm0492960. [DOI] [PubMed] [Google Scholar]
- 37.Miranda KM, Katori T, de Holding CLT, Thomas L, Ridnour LA, MeLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, Tocchetti CG, Saavedra JE, Keefer LK, Houk KN, Fukuto JM, Kass DA, Paolocci N, Wink DA. J Med Chem. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 38.Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 39.Espey MG, Miranda KM, Thomas DD, Wink DA. Free Radic Biol Med. 2002;33:827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 40.Leone AM, Francis PL, Rhodes P, Moncada S. Biochem Biophys Res Commun. 1994;200:951–957. doi: 10.1006/bbrc.1994.1542. [DOI] [PubMed] [Google Scholar]
- 41.Ueda T, Maekawa T, Sadamitsu D, Oshita S, Ogino K, Nakamura K. Electrophoresis. 1995;16:1002–1004. doi: 10.1002/elps.11501601167. [DOI] [PubMed] [Google Scholar]
- 42.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Feelisch M, Lundberg JO. Nature Chemical Biology. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Typical 1H NMR and FT-IR spectra of representative HMwPEI (2), carboxylated-PEI (7) and its NO addition product (8); Boc-protected-L-proline-incorporated PEI (9) and the corresponding deprotection (10) and NO addition (11) products. Accumulated nitrite release from HMwPEI/NO (5), PEI-COONa/NO (8) and PEI-PRO/NO (11) under the basic and oxygen-free conditions at room temperature as measured via the Griess assay. This material is available free of charge via the Internet at http://pubs.acs.org.