Abstract
Previous small studies have reported familial clustering of myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). We identified 6217 PV, 2838 ET, 1172 MF, and 812 MPN unclassifiable (NOS) patients diagnosed in Sweden, 43 550 controls, and first-degree relatives of cases (n = 24 577) and controls (n = 99 542). Using a marginal survival model, we calculated relative risks (RRs) and 95% confidence intervals as measures of familial aggregation. Relatives of MPN patients had significantly increased risks of PV (RR = 5.7; 3.5-9.1), ET (RR = 7.4; 3.7-14.8), and MPN NOS (RR = 7.5; 2.7-20.8). Analyses stratified by type of first-degree relative revealed consistently higher risks for siblings, compatible with a model of recessive genetic inheritance, which can be confirmed only by identifying the susceptibility gene(s). Mean age at MPN diagnosis was not different (P = .20) for affected relatives of cases (57.5 years) versus controls (60.6 years), and risk of MPN by age was not different for parents versus offspring of MPN cases (P = .10), providing no support for anticipation. Relatives of MPN patients had a borderline increased risk of chronic myeloid leukemia (CML; RR = 1.9; 0.9-3.8; P = .09). Our findings of 5- to 7-fold elevated risk of MPNs among first-degree relatives of MPN patients support the hypothesis that common, strong, shared susceptibility genes predispose to PV, ET, MF, and possibly CML.
Introduction
Chronic myeloproliferative disorders, including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (MF), are now called myeloproliferative neoplasms (MPNs) by the upcoming 4th edition of the World Health Organization classification of hematopoietic tumors.1 They are stem cell–derived clonal proliferative diseases whose shared and diverse phenotypic characteristics can be attributed to dysregulated signal transduction because of acquired somatic mutations.2 Approximately half of all MPN patients are reported to be asymptomatic at diagnosis.1 Among PV and ET patients, the major symptoms are related to hypertension or vascular abnormalities, including predilection to thrombosis and hemorrhage.3,4 In addition, patients with PV and ET have an excess risk of developing MF, or transformation to leukemia.3,4 Although available data are restricted, life expectancy of patients with ET has been reported to be similar to the general population; however, for PV patients, life expectancy has been observed to be reduced compared with the general population.5 In contrast, primary MF patients have an average survival of less than 5 years.6
The causes of MPNs are largely unknown. A mutation in the gene for Janus kinase 2, (JAK2)V617F, is present in all erythropoietin-independent erythroid colonies in PV and gives a proliferative advantage of these cells. The mutation is present in 95% of PV patients and in approximately 50% of ET and MF patients.3 However, because case studies have shown that only a minority of granulocytes affected by a chromosome 20q deletion also harbored JAK2V617F, other primary pathogenetic factors have been hypothesized.7 A role for genetic factors in the etiology of MPNs has also been suggested from case reports and smaller case series showing evidence of familial clustering of PV, ET, MF, and chronic myeloid leukemia (CML).8–12 Recently, anticipation in age of onset was reported in a single-center study using an interview-based approach, including 35 MPN families.13 In addition, primary familial congenital polycythemia and hereditary thrombocythemias, which are rare Mendelian disorders, have been found to be caused by mutations in the erythropoietin receptor gene and thrombopoetin gene, respectively.14 Nevertheless, mutations in these genes have not been detected in the more common MPNs.14 Further, the JAK2 mutation has recently been assessed in multiple affected MPN families but has been shown not to be an early germ line predisposing factor for MPN but rather a facilitator of pro-liferative advantages.8,12
To our knowledge, no previous population-based study has systematically investigated patterns of specific MPNs and related malignancies among first-degree relatives of MPN patients. We have conducted a large nationwide study including 6217 PV, 2838 ET, 1172 MF, and 812 MPN unclassifiable (MPN NOS) patients diagnosed in Sweden, 43 550 population-based matched controls, and first-degree relatives of cases (n = 24 577) and controls (n = 99 542). The aim of our population-based study was to quantify risks of various types of MPNs and related malignancies among relatives of PV, ET, MF, and MPN NOS patients.
Methods
Central registries, patients, controls, and first-degree relatives
All residents of Sweden are, on birth or immigration, assigned a unique national registration number that is used in government-maintained nationwide healthcare and population registers, whereby record linkage is possible with a high degree of accuracy. For each person, the date of death is centrally registered in the Swedish Cause of Death Registry.
Since the mid-1950s, Sweden has provided universal medical health care available for the entire population, currently approximately 9 million people. In contrast to many other countries, for example, the United States (where the majority of hematologic patients are primarily seen and treated by doctors outside hospitals in private practice), the Swedish healthcare system has a geographically defined referral structure for specialist assessments. Patients with hematologic disorders are primarily diagnosed, treated, and followed clinically by physicians at hospital-based hematology/oncology centers. These centers are centralized to 7 regional university hospitals, which also offer inpatient hospital care to a defined primary catchment area population in addition to being the hematology/oncology referral center for a whole healthcare region.
Since 1958, all physicians and pathologists/cytologists in Sweden are obliged by law to report each incident case of cancer they diagnose and/or treat to the centralized nationwide Swedish Cancer Registry. The Registry contains information on diagnosis, sex, date of birth, date of diagnosis, and region/hospital where the diagnosis was made.15 In a recent validation study focusing on lymphoproliferative hematologic tumors diagnosed in 1964 to 2003, we found the completeness and the diagnostic accuracy of the Registry to be more than 90% to 95%.16
In Sweden, in the mid-1970s to the late 1990s, PV and ET diagnostics were based on the Polycythemia Vera Study Group criteria, including frequent use of red cell mass determinations and bone marrow histology.17,18 From the late 1990s, the prevailing attitude regarding diagnostic and therapeutic procedures has changed among Swedish hematologists.19 In accord with established WHO criteria,20 spleen size determination, bone marrow histology, and serum erythropoietin measurement are often used, whereas direct determination of the red blood cell mass has become a less common procedure. With regard to diagnostic criteria for MF, they were initially based on the Polycythemia Vera Study Group guidelines.17 Similar to PV and ET, in more recent time, WHO criteria have been applied for MF diagnostics (including a leukoerythroblastic blood picture, splenomegaly, bone marrow fibrosis involving more than one-third of the sectional area of a bone marrow biopsy, and the absence of well-established diagnostic criteria for other MPNs).20
We identified all living incident patients diagnosed from 1958 to 2005 with a MPN from the nationwide Swedish Cancer Registry. In addition, we retrieved information on all living incident MPN patients through our national MPN network (the Swedish Myeloproliferative Disorder Study Group), which included all major hematology/oncology centers in Sweden. By taking this approach, we established a nationwide MPN cohort, which was used to identify and add MPN patients who were not reported to the Swedish Cancer Registry.
For each MPN patient, 4 population-based controls matched by sex, year of birth, and county of residence were chosen randomly from the Swedish Population database. All controls had to be alive at the time of MPN diagnosis for the corresponding case and free of cancer at the date of the corresponding case's diagnosis.
From the Swedish Multigenerational Registry,21 which includes information on parent-offspring relations for all Swedish citizens who were born 1932, and later we obtained information on all first-degree relatives (parents, siblings, and offspring) of cases and controls and linked them to the Swedish Cancer Registry to obtain information on living incident cancer cases.
Approval was obtained from the Karolinska Institutional Review Board for this study. Informed consent was waived because we had no contact with study subjects. An exemption from Institutional Review Board review was obtained from the National Institutes of Health Office of Human Subjects Research because we used existing data without personal identifiers.
Statistical analysis
The statistical approach was based on a model proposed by Liang22 and described in detail elsewhere.23 We classified relatives as “affected” if they had any primary cancer registration with the tumor of interest (examining up to 5 tumor registrations). Here, the age or age at onset of disease in a relative of a proband was modeled by a proportional hazards model. Familial aggregation for each condition was evaluated by testing the hazard ratio of being a relative of a case compared with a relative to a control. The model was fitted to the data using the PHREG procedure in SAS, version 9.1. We used relative risk (RR) to denote the hazard ratio defined herein. The robust sandwich covariance matrix accounts for the dependence of the family members. We tested separately for increased risk for PV, ET, MF, and MPN NOS, and we tested for increased risk of developing any MPN considered as a combined entity. We also assessed the risk of CML, acute myeloid leukemia (AML), and myelodysplastic syndromes (MDS) among relatives. Furthermore, in an exploratory analysis including all defined hematologic malignancies and solid tumor sites, we examined if there was evidence of increased risk of any malignancy among relatives to MPN patients. Because case and control probands were matched for risk factors thought to be important for MPN, the relatives should be generally well matched. However, because they cannot be individually matched, we adjusted for sex in all analyses.
The main effect of interest in this analysis is the increased risk associated with being a relative of a case compared with being a relative of a control. However, we were also interested in testing whether other factors, such as sex of case, sex of relative, type of relative, and age of disease onset in the case proband, affected case-control comparisons. Thus, we analyzed the data both by stratifying these factors and by testing them as interaction effects in one model. Age at diagnosis was stratified at less than 65 versus more than or equal to 65 years.
To test for anticipation, we computed Kaplan-Meier estimates of risk of MPN by age and tested for homogeneity of parent and offspring strata using the log-rank test (PROC LIFETEST, SAS, version 9.1).
Results
A total of 11 039 (6217 PV, 2838 ET, 1172 MF, and 812 MPN NOS) MPN patients diagnosed in Sweden in 1958 to 2005, 43 550 population-based matched controls, and first-degree relatives of cases (n = 24 577) and controls (n = 99 542) were included in the study. The characteristics of MPN patients and controls are shown in Table 1. The mean age at diagnosis for PV, ET, MF, and MPN NOS patients was 66, 66, 69, and 71 years, respectively. Approximately 90% of the patients were identified through the central Swedish Cancer Registry, with the remaining 10% of the cases captured in local hospitals via our nationwide Swedish Myeloproliferative Disorder Study Group (Table 1). A majority of the ET (∼85%) and MF (∼60%) cases were diagnosed between the mid-1980s and the end of the study period (2005). Offspring constitutes the largest group of relatives, as expected, given inherent characteristics of the applied database.
Table 1.
Characteristics of MPN patients, controls, and first-degree relatives
| Variable | MPN patients |
Controls | ||||
|---|---|---|---|---|---|---|
| PV | ET | MF | MPN NOS | Any MPN | ||
| No. (%) of patients | 6217 (100) | 2838 (100) | 1172 (100) | 812 (100) | 11 039 (100) | 43 550 (100) |
| Male, sex, n (%) | 3078 (49.5) | 1214 (42.8) | 644 (55.0) | 401 (49.4) | 5337 (48.4) | 21 152 (48.6) |
| Age at diagnosis, y [mean (range)] | 66 (3-97) | 66 (1-98) | 69 (16-93) | 71 (20-97) | 67 (1-90) | 67 (1-98) |
| Age group at diagnosis, no. (%) | ||||||
| Younger than 40 y | 208 (3.4) | 212 (7.5) | 22 (1.9) | 18 (2.2) | 460 (4.2) | 1840 (4.2) |
| 40-49 y | 443 (7.1) | 253 (8.9) | 57 (4.9) | 41 (5.1) | 794 (7.2) | 3174 (7.3) |
| 50-59 y | 1037 (16.7) | 385 (13.6) | 150 (12.8) | 74 (9.1) | 1646 (14.9) | 6572 (15.0) |
| 60-69 y | 1661 (26.7) | 580 (20.4) | 310 (26.4) | 177 (21.8) | 2728 (24.7) | 10 826 (24.9) |
| 70-79 y | 1968 (31.6) | 865 (30.5) | 431 (36.8) | 269 (33.1) | 3533 (32.0) | 13 834 (31.8) |
| Older than 80 y | 900 (14.5) | 543 (19.1) | 202 (17.2) | 233 (28.7) | 1878 (17.1) | 7304 (16.8) |
| Year of birth, mean (range) | 1917 (1872-1993) | 1926 (1875-1990) | 1919 (1879-1979) | 1927 (1901-1982) | 1920 (1872-1993) | 1921 (1872-1993) |
| Calendar year of diagnosis, no. (%) | ||||||
| 1958-1965 | 590 (9.5) | 48 (1.7) | 1 (0.1) | — | 639 (5.8) | 2556 (5.9) |
| 1966-1975 | 1172 (18.9) | 145 (5.1) | 124 (10.6) | — | 1441 (13.1) | 5747 (13.2) |
| 1976-1985 | 1364 (21.9) | 201 (7.1) | 328 (28.0) | — | 1893 (17.2) | 7443 (17.1) |
| 1986-1995 | 1527 (25.5) | 1542 (54.3) | 412 (35.2) | 159 (19.6) | 3640 (32.9) | 14 250 (32.7) |
| 1996-2005 | 1564 (25.2) | 902 (31.8) | 307 (26.2) | 653 (80.4) | 3426 (31.0) | 13 554 (31.1) |
| Data source, no. (%) | ||||||
| Cancer Registry | 5491 (88.3) | 2367 (83.4) | 1030 (87.9) | 812 (100) | 3998 (87.9) | 38 260 (87.8) |
| Local hospitals | 726 (11.7) | 471 (16.6) | 142 (12.1) | — | 1339 (12.1) | 5290 (12.2) |
| Relatives, no. (%) | ||||||
| Any relative | 12 374 (100) | 7499 (100) | 2471 (100) | 2233 (100) | 24 577 (100) | 99 542 (100) |
| Parents | 1880 (15.2) | 1557 (20.8) | 345 (14.0) | 408 (18.3) | 4190 (17.0) | 17 100 (17.2) |
| Siblings | 1450 (11.7) | 1237 (16.5) | 284 (11.5) | 345 (15.4) | 3316 (13.5) | 13 635 (13.7) |
| Offspring | 9044 (73.1) | 4705 (62.7) | 1842 (74.5) | 1480 (66.3) | 17 071 (69.5) | 68 807 (69.1) |
MPN indicates myeloproliferative neoplasm; PV, polycythemia vera; ET, essential thrombocythemia; MF, myelofibrosis; and NOS, unclassifiable.
As shown in Table 2, relatives of MPN patients had an increased risk of any MPN (RR = 5.6; 95% confidence interval [CI], 3.8-8.2). When we assessed the familial risk for specific MPNs, we found relatives of patients with any MPN to have an increased of PV (RR = 5.7; 95% CI, 3.5-9.1), ET (RR = 7.4; 95% CI, 3.7-14.8), and MPN NOS (RR = 7.5; 95% CI, 2.7-20.8). The risk of MF was not increased, but it was a rare outcome (in total 11 cases among case and control relatives).
Table 2.
Relative risk of MPN among first-degree relatives of MPN patients
| Proband | Any MPN |
PV |
ET |
MF |
MPN NOS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | |
| Any MPN | 95 | 69 | 5.6 (3.8-8.2) | 53 | 38 | 5.7 (3.5-9.1) | 29 | 16 | 7.4 (3.7-14.8) | 2 | 9 | 0.9 (0.2-4.2) | 11 | 6 | 7.5 (2.7-20.8) |
| PV | 53 | 44 | 4.9 (3.1-7.8) | 37 | 25 | 6.0 (3.3-11.0) | 12 | 9 | 5.4 (2.2-13.2) | 0 | 6 | NA | 4 | 4 | 4.1 (1.0-16.2) |
| ET | 29 | 17 | 6.8 (3.4-13.5) | 12 | 9 | 5.4 (2.2-13.1) | 12 | 6 | 8.0 (2.6-24.5) | 1 | 1 | 4.0 (0.2-61.7) | 4 | 1 | 16.6 (1.9-146.1) |
| MF | 2 | 3 | 2.7 (0.5-16.2) | 0 | 2 | NA | 0 | 1 | NA | 0 | 1 | N.A | 1 | 0 | NA |
| MPN NOS | 11 | 5 | 9.4 (3.2-28.0) | 4 | 2 | 8.4 (1.5-46.4) | 4 | 1 | 17.5 (1.9-16.3) | 1 | 1 | 4.1 (0.2-63.1) | 2 | 1 | 8.9 (0.6-140.1) |
All estimates were adjusted for sex of first-degree relative.
MPN indicates myeloproliferative neoplasm; PV, polycythemia vera; ET, essential thrombocythemia; MF, myelofibrosis; NOS, unclassifiable; RR, relative risk; CI, confidence interval; and NA, not applicable.
We also assessed risk of MPNs in models restricted to relatives of patients with PV, ET, MF, and MPN NOS only. Because numbers got smaller when we stratified by subtype of MPN, some of the estimates were less precise, which was reflected in wide confidence intervals. Among relatives of PV patients, we found increased risk of any MPN (RR = 4.9; 95% CI, 3.1-7.8), PV (RR = 6.0; 95% CI, 3.3-11.0), ET (RR = 5.4; 95% CI, 2.2-13.2), and MPN NOS (RR = 4.1; 95% CI, 1.0-16.2). Relatives of ET patients had elevated risk for any MPN (RR = 6.8; 95% CI, 3.4-13.5), PV (RR = 5.4; 95% CI, 2.2-13.1), ET (RR = 8.8; 95% CI, 2.6-24.5), MPN NOS (RR = 16.1; 95% CI, 1.9-146.1), and nonsignificantly increased risk for MF (RR = 4.0; 95% CI, 0.2-61.7). For relatives of MF patients, we found a nonsignificantly increased risk for any MPN (RR = 2.7; 95% CI, 0.5-16.2); however, numbers were too small for further detailed analyses (Table 2). When we defined risk estimates for relatives of MPN NOS patients, we found increased risk for any MPN (RR = 9.4; 95% CI, 3.2–28.0), PV (RR = 8.4; 95% CI, 1.5-46.4), ET (RR = 17.5; 95% CI, 1.9-16.3), and nonsignificantly increased risk for MPN NOS (RR = 8.9; 95% CI, 0.6-140.1).
We also conducted analyses by various types of first-degree relatives (parents, siblings, offspring). In these models, the risk estimates were consistently higher for siblings (Table 3). When we calculated risks by sex of the MPN patient and sex of the first-degree relative, respectively, with the exception of female relatives of MDP NOS patients, we found female sex (of proband as well as first-degree relative) to be consistently associated with a higher risk (Table 3), but the CIs clearly overlap. Similarly, relatives of younger probands have somewhat higher but nonsignificant risks.
Table 3.
Relative risk of MPN among first-degree relatives of MPN patients, by type of relative, sex, and age of proband
| Probands with any MPN | Any MPN |
PV |
ET |
MF |
MPN NOS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | |
| Type of relative1* | |||||||||||||||
| Parent | 42 | 35 | 5.0 (3.1-7.8) | 32 | 20 | 6.6 (3.8-11.6) | 8 | 8 | 4.1 (1.5-11.7) | 0 | 4 | NA | 2 | 3 | 2.7 (0.5-16.3) |
| Sibling | 12 | 5 | 10.3 (3.2-33.3) | 1 | 2 | 2.1 (0.2-22.7) | 5 | 2 | 10.7 (1.8-64.0) | 2 | 1 | 8.5 (0.8-88.9) | 4 | 0 | NA |
| Offspring | 41 | 29 | 5.6 (3.4-9.1) | 20 | 16 | 5.0 (2.6-9.6) | 16 | 6 | 10.5 (4.1-27.3) | 0 | 2 | NA | 5 | 3 | 6.6 (1.6-27.6) |
| Sex of proband1* | |||||||||||||||
| Male | 40 | 36 | 4.5 (2.8-7.4) | 22 | 20 | 4.5 (2.3-8.6) | 12 | 9 | 5.4 (2.3-12.8) | 1 | 4 | 1.0 (0.1-9.1) | 5 | 3 | 6.7 (1.6-28.0) |
| Female | 55 | 33 | 6.8 (4.2-11.1) | 31 | 18 | 7.0 (3.8-13.0) | 17 | 7 | 9.9 (3.7-26.3) | 1 | 5 | 0.8 (0.1-6.9) | 6 | 3 | 8.2 (2.1-32.9) |
| Sex of relative | |||||||||||||||
| Male | 41 | 35 | 4.8 (2.9-7.9) | 26 | 19 | 5.6 (3.0-10.5) | 7 | 9 | 3.2 (1.1-9.2) | 1 | 5 | 0.8 (0.1-6.9) | 7 | 2 | 14.5 (3.0-69.0) |
| Female | 54 | 34 | 6.5 (4.0-10.5) | 27 | 19 | 5.8 (3.0-10.9) | 22 | 7 | 12.7 (5.1-31.7) | 1 | 4 | 1.0 (0.1-9.0) | 4 | 4 | 4.1 (1.0-16.2) |
| Age at diagnosis1* | |||||||||||||||
| Younger than 65 y | 63 | 40 | 6.5 (4.1-10.3) | 38 | 22 | 7.1 (4.1-12.5) | 18 | 10 | 7.4 (3.0-18.5) | 1 | 5 | 0.8 (0.1-7.0) | 6 | 3 | 8.3 (2.1-32.9) |
| Older than 65 y | 32 | 29 | 4.4 (2.6-7.3) | 15 | 16 | 3.7 (1.8-7.6) | 11 | 6 | 7.3 (2.7-19.6) | 1 | 4 | 1.0 (0.1-8.8) | 5 | 3 | 6.6 (1.6-27.6) |
MPN indicates myeloproliferative neoplasm; PV, polycythemia vera; ET, essential thrombocythemia; MF, myelofibrosis; NOS, unclassifiable; RR, relative risk; CI, confidence interval; and NA, not applicable.
Estimates were adjusted for sex of first-degree relative.
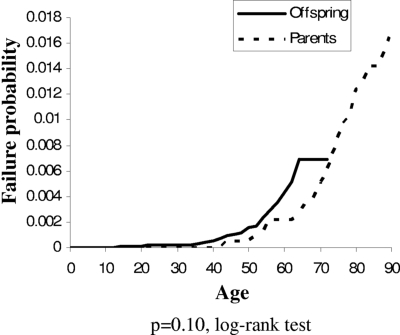
The mean age at diagnosis of MPN was not significantly (P = .20, t test) different for affected relatives of cases (57.5 years) compared with affected relatives of controls (60.6 years), indicating that familial MPN does not differ in age of onset from sporadic MPN. We also looked at differences in risk of MPN for parents compared with offspring of cases. As shown in Figure 1, the risk of MPN by age was not significantly different between them (P = .10, log-rank test), which does not support the anticipation hypothesis.
Figure 1.
Risk of MPN by age in parents versus offspring of MPN cases.
As shown in Table 4, we assessed risk of CML, AML, and MDS among relatives of MPN patients (vs controls). Among relatives of MPN patients we found a borderline increased risk of CML (RR = 1.9; 95% CI, 0.9-3.8; P = .09) but no increased risk of AML or MDS. However, we found a borderline increased risk of AML among relatives of ET patients (RR = 1.8; 95% CI, 0.9-3.8; P = .09). Among MPN families with a relative affected with AML/MDS (n = 34/n = 5), none of the corresponding MPN probands developed AML or MDS themselves.
Table 4.
Relative risk of CML, AML, and MDS among first-degree relatives of MPN patients
| Proband | CML |
AML |
MDS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | Patients | Controls | RR (95% CI) | |
| Any MPN | 11 | 24 | 1.9 (0.9-3.8) | 34 | 108 | 1.3 (0.9-1.9) | 5 | 13 | 1.6 (0.6-4.4) |
| PV | 7 | 13 | 2.2 (0.9-5.5) | 16 | 62 | 1.0 (0.6-1.8) | 3 | 5 | 2.4 (0.6-10.2) |
| ET | 1 | 4 | 1.0 (0.1-8.8) | 11 | 25 | 1.8 (0.9-3.8) | 0 | 7 | NA |
| MF | 2 | 3 | 2.7 (0.5-15.8) | 3 | 9 | 1.4 (0.3-5.3) | 1 | 0 | ND |
| MPN NOS | 1 | 4 | 1.0 (0.2-9.7) | 4 | 12 | 1.4 (0.5-4.3) | 1 | 1 | 4.6 (0.3-75.9) |
All estimates were adjusted for sex of first-degree relative.
CML indicates chronic myeloid leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; RR, relative risk; CI, confidence interval; MPN, myeloproliferative neoplasm; PV, polycythemia vera; ET, essential thrombocythemia; MF, myelofibrosis; NOS, unclassifiable; NA, not applicable; and ND, not done.
In an exploratory analysis, we assessed familial risks for all defined hematologic malignancies and solid tumors among relatives of MPN patients. We observed a significantly (RR = 1.6; 95% CI, 1.1-2.5) increased risk of chronic lymphocytic leukemia among family members of MPN patients. For solid tumors, we found significantly increased risks of malignant melanoma (RR = 1.4; 95% CI, 1.2-1.7) and cancer of the brain (RR = 1.3; 95% CI, 1.1-1.6). No other malignancies were significantly increased (data not shown).
Discussion
In this largest population-based case-control study to date, including more than 11 000 MPN patients and their almost 25 000 linkable first-degree relatives, we found 5- to 7-fold elevated risks of developing MPNs among first-degree relatives of MPN patients. Our results support the theory that there are common, strong, shared susceptibility genes that predispose to PV, ET, MF, and possibly CML.
Consistent with prior smaller studies and case reports, we found highly increased risk of all types of MPNs among first-degree relatives of MPN patients.8–12 The observed elevated risks of various types of MPNs were very similar among relatives of PV, ET, and MPN NOS patients. The lack of aggregation found for MF may be the result of the rarity of this outcome. Unlike with other tumor sites, there does not seem to be additional risk for the same MPN subtype in cases and relatives. There was no striking variation by age at diagnosis of the case. Interestingly, we found somewhat higher risk estimates among siblings, which is compatible with a model of recessive inheritance, although this can only be confirmed by identifying the actual susceptibility gene(s). Alternative explanations include the operation of some shared environmental influences possibly interacting with host (genetic) factors. When we assessed risk by sex of probands and family members, we found relatives of female probands and female relatives to be at higher risk than males, but this was not significant when these factors were added into the model as covariates (not shown). This might suggest there are some sex-specific underlying biologic pathways involved, or it could be a chance finding.
We also assessed risk of CML, AML, and MDS in various types of first-degree relatives. The risk of CML was borderline 2-fold increased (P = .09) among relatives of MPN patients; however, we found no significantly increased risks for AML or MDS among relatives of MPN patients overall. Neither did we see any aggregation of AML/MDS progression after MPN and de novo AML/MDS and in the same families. However, AML is a rare outcome so the power to detect associations is low. Interestingly, among relatives of ET patients, we found a borderline 2-fold increased risk (P = .09) for AML. This observation might be of interest given the fact that the risk of developing AML is increased among ET patients.5,24 Potentially, our finding of a borderline excess risk of AML among relatives to ET patients might indicate the operation of some shared germ line susceptibility genes in AML and ET. Future research is needed to confirm our findings and to define underlying mechanisms and assess potential gene-environment (including MPN therapy) interactions with regard to AML risk.
When we conducted an exploratory analysis, including all defined hematologic malignances and solid tumors, we found evidence of an increased risk of chronic lymphocytic leukemia, malignant melanoma, and brain cancer among relatives of MPN patients. At this time, we have no clear biologic explanation to these observations, and they might well be chance findings because of multiple comparisons.
Based on small numbers, the clinical presentation and outcome of familial MPN patients have been suggested to be different from sporadic MPN patients. For example, homozygosity for JAK2 (V617F) has been reported to identify a higher risk of disease evolution.8 However, a larger study of MPN patients did not find differences in clinical presentation between familial and sporadic cases.13 Our results showed no difference in age of onset of familial cases compared with sporadic cases MPN. Unlike the investigation by Rumi et al,13 we did not find any evidence for anticipation (Figure 1).
In our study, we used a register-based case-control design, which ruled out recall bias, ensured a population-based setting, and generalizability of our findings. By including all MPN cases diagnosed in Sweden in 1958 to 2005 with one or more linkable relatives, we were able to conduct a very large study on familial aggregation of MPN. Limitations include incomplete numbers of first-degree relatives, lack of information on potential confounders (although the matched design and analyses ensured adjustment for sex, age, and geography), lack of validation of the register-based data, and lack of clinical data. Other potential limitations of our study were the absence of a systematic validation of all MPN diagnoses. Because of the size of the study, we were not able to validate individual records. However, because we retrieved MPN patients both from local hospital registries (as part of our Swedish Myeloproliferative Disorder Study Group network) as well as the central Cancer Registry, we were able to compare descriptive characteristics of MPN patients from the 2 data sources. We found the cases to be very similar and familial risk estimates were virtually the same when we conducted subanalyses stratified by data source (data not shown). As shown in Table 1, the majority of ET and MF cases (∼60%-85%) were diagnosed between the mid-1980s to the end of the study period (2005). Presumably, these patterns reflect underascertainment of ET and MF in earlier years. However, because the current investigation is a case-control study, ascertainment of first-degree relatives and information regarding their present/absent MPN diagnoses should be nondifferential for case- and control-relatives. Inherently, because of the case-control design, any underascertainment of MPNs among relatives would lead to a conservative bias (ie, a null association). Finally, because we assessed familial aggregation using relatives of MPN cases and matched controls and they were obtained from the same registries, the validity of the diagnosis should be nondifferential and the relative risks should not be biased.
In conclusion, we found 5- to 7-fold elevated risks of developing MPNs among almost 25 000 first-degree relatives of more than 11 000 MPN patients. Our results support the theory that there are common, strong, shared susceptibility genes that predispose to PV, ET, MF, and possibly CML.25 This study provides novel information supporting the application of gene mapping and candidate gene approaches in high-risk families and case-control studies.
Acknowledgments
The authors thank Ms Shiva Ayobi (National Board of Health and Welfare, Stockholm, Sweden), Ms Susanne Dahllöf (Statistics Sweden, Orebro, Sweden) and Ms Emily Steplowski (Information Management Services, Silver Spring, MD), for important efforts in the development of this database.
This work was supported by grants from the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations, and the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: O.L., L.R.G., S.Y.K., and M.B. designed the study; O.L., J.S., S.Y.K., and M.B. obtained data; O.L. and L.R.G. analyzed data; O.L., L.R,G., S.Y.K., E.A.H., J.S., and M.B. were involved in the interpretation of the results; O.L. and M.B. initiated this work; O.L. wrote the report; and L.R.G., S.Y.K., E.A.H., J.S., and M.B. read the report, gave scientific input, and approved the final version of the report.
Conflict-of-interest disclosure: M.B. has received an unrestricted grant from Shire Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Ola Landgren, Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health & Human Services, 6120 Executive Boulevard, Bldg EPS, Room 7110, Bethesda, MD 20892-7236; e-mail: landgreo@mail.nih.gov.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle. 2007;6:550–566. doi: 10.4161/cc.6.5.3919. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33:313–320. doi: 10.1055/s-2007-976165. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Gangat N, Wolanskyj AP, et al. 20+ yr without leukemic or fibrotic transformation in essential thrombocythemia or polycythemia vera: predictors at diagnosis. Eur J Haematol. 2008;80:386–390. doi: 10.1111/j.1600-0609.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 5.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman R, Rondelli D. Biology and treatment of primary myelofibrosis. Hematology Am Soc Hematol Educ Program. 2007;2007:346–354. doi: 10.1182/asheducation-2007.1.346. [DOI] [PubMed] [Google Scholar]
- 7.Kralovics R, Teo SS, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 8.Bellanne-Chantelot C, Chaumarel I, Labopin M, et al. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood. 2006;108:346–352. doi: 10.1182/blood-2005-12-4852. [DOI] [PubMed] [Google Scholar]
- 9.Cario H, Goerttler PS, Steimle C, Levine RL, Pahl HL. The JAK2V617F mutation is acquired secondary to the predisposing alteration in familial polycythaemia vera. Br J Haematol. 2005;130:800–801. doi: 10.1111/j.1365-2141.2005.05683.x. [DOI] [PubMed] [Google Scholar]
- 10.Kralovics R, Stockton DW, Prchal JT. Clonal hematopoiesis in familial polycythemia vera suggests the involvement of multiple mutational events in the early pathogenesis of the disease. Blood. 2003;102:3793–3796. doi: 10.1182/blood-2003-03-0885. [DOI] [PubMed] [Google Scholar]
- 11.Pardanani A, Lasho T, McClure R, Lacy M, Tefferi A. Discordant distribution of JAK2V617F mutation in siblings with familial myeloproliferative disorders. Blood. 2006;107:4572–4573. doi: 10.1182/blood-2005-12-4988. [DOI] [PubMed] [Google Scholar]
- 12.Rumi E, Passamonti F, Pietra D, et al. JAK2 (V617F) as an acquired somatic mutation and a secondary genetic event associated with disease progression in familial myeloproliferative disorders. Cancer. 2006;107:2206–2211. doi: 10.1002/cncr.22240. [DOI] [PubMed] [Google Scholar]
- 13.Rumi E, Passamonti F, Della Porta MG, et al. Familial chronic myeloproliferative disorders: clinical phenotype and evidence of disease anticipation. J Clin Oncol. 2007;25:5630–5635. doi: 10.1200/JCO.2007.12.6896. [DOI] [PubMed] [Google Scholar]
- 14.Skoda R, Prchal JT. Lessons from familial myeloproliferative disorders. Semin Hematol. 2005;42:266–273. doi: 10.1053/j.seminhematol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Stockholm, Sweden: Centre for Epidemiology; 2003. Socialstyrelsen. Cancer incidence in Sweden 2001. [Google Scholar]
- 16.Turesson I, Linet MS, Bjorkholm M, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. Int J Cancer. 2007;121:2260–2266. doi: 10.1002/ijc.22912. [DOI] [PubMed] [Google Scholar]
- 17.Berlin NI. Diagnosis and classification of the polycythemias. Semin Haematol. 1975;12:339–351. [PubMed] [Google Scholar]
- 18.Kutti J, Wadenvik H. Diagnostic and differential criteria of essential thrombocythemia and reactive thrombocytosis. Leuk Lymphoma. 1996;22(suppl 1):41–45. doi: 10.3109/10428199609074359. [DOI] [PubMed] [Google Scholar]
- 19.Andreasson B, Lofvenberg E, Westin J. Management of patients with polycythaemia vera: results of a survey among Swedish haematologists. Eur J Haematol. 2005;74:489–495. doi: 10.1111/j.1600-0609.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Lyon, France: IARC Press; 2001. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 21.Skarle A. Stockholm, Sweden: Population Statistics; 2001. Flergenerationsregistret: Statistics Sweden. [Google Scholar]
- 22.Liang KY. Estimating effects of probands' characteristics on familial risk: I. Adjustment for censoring and correlated ages at onset. Genet Epidemiol. 1991;8:329–338. doi: 10.1002/gepi.1370080505. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer RM, Goldin LR, Chatterjee N, et al. Methods for testing familial aggregation of diseases in population-based samples: application to Hodgkin lymphoma in Swedish registry data. Ann Hum Genet. 2004;68:498–508. doi: 10.1046/j.1529-8817.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 24.Tefferi A, Fonseca R, Pereira DL, Hoagland HC. A long-term retrospective study of young women with essential thrombocythemia. Mayo Clin Proc. 2001;76:22–28. doi: 10.4065/76.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Pardanani A, Fridley BL, Lasho TL, Gilliland DG, Tefferi A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood. 2008;111:2785–2789. doi: 10.1182/blood-2007-06-095703. [DOI] [PubMed] [Google Scholar]