Abstract
This prospective study aimed to develop reproducible diagnostic criteria for sporadic Burkitt lymphoma (BL), applicable to routine practice, and to evaluate the efficacy of dose-modified (dm) CODOX-M/IVAC in patients diagnosed using these criteria. The study was open to patients with an aggressive B-cell lymphoma with an MKI67 fraction approaching 100%. Immunophenotype and fluorescent in situ hybridization (FISH) were used to separate BL from other aggressive B-cell lymphomas. BL was characterized by the presence of a cMYC rearrangement as a sole cytogenetic abnormality occurring in patients with a germinal center phenotype with absence of BCL-2 expression and abnormal TP53 expression. A total of 128 patients were eligible for the study, of whom 58 were considered to have BL and 70 to have diffuse large B-cell lymphoma (DLBCL). There were 110 clinically fit patients who received dmCODOX-M (methotrexate, dose 3 g/m2) with or without IVAC according to risk group. The 2-year progression-free survival was 64% (95% confidence interval [CI] 51%-77%) for BL, 55% (95% CI 42%-66%) for DLBCL, 85% (95% CI 73%-97%) for low risk, and 49% (95% CI 38%-60%) for high-risk patients. The observed differences in outcome and other clinical features validate the proposed diagnostic criteria. Compared with the previous trial LY06 with full-dose methotrexate (6.7 g/m2), there was a reduction in toxicity with comparable outcomes. This study was registered at www.clinicaltrials.gov as NCT00040690.
Introduction
Sporadic Burkitt lymphoma (BL) is a rare, non-HIV–related, and highly curable B-cell lymphoma which predominantly occurs in younger adults.1,2 BL is characterized by the presence of a t(8;14) or variant translocation, resulting in cMYC rearrangement and overexpression.1–3 Cells with deregulated cMYC expression have a very high cell-cycle fraction as defined by expression of the nuclear protein MKI67. Cytogenetic analysis of these cases has, until recently, required fresh tissue or leukemic samples, which are rarely available.4 As a consequence, previously published clinical series were largely based on morphologic interpretation supplemented by immunocytochemical determination of the cell-cycle fraction as suggested in the World Health Organization (WHO) classification.1,5–10 The classic morphologic features of BL are, however, variable, and affected by fixation, and as a consequence the diagnosis of BL has been subjective and poorly reproducible.11,12 In addition, 100% MKI67 expression is not specific for cells with cMYC rearrangement.
Previous studies have suggested that sporadic BL, defined using these criteria, is usually curable using rapidly cycling, high-intensity chemotherapy regimens with central nervous system (CNS) prophylaxis; CODOX-M/IVAC is one such regime described by Magrath5 and successfully used in the United Kingdom in modified form in a prospective trial (LY06).6 CODOX-M/IVAC and similar high-intensity regimens are considered essential for the treatment of BL, but are highly toxic and should be appropriately targeted to those with BL for whom standard therapy would be less effective.
This study was designed to develop reproducible diagnostic criteria for BL which could be applied to routine practice, and to study the clinical features and response to treatment of patients thus identified in comparison with other aggressive B-cell lymphomas. It was recognized that a morphologic diagnosis of BL was unreliable and in order to capture the maximum number of BL patients a more objective screening test was required. Based on the recommendations of the WHO classification1 a cell-cycle fraction of near 100% (> 95%), defined by immunocytochemical detection of MKI67, was adopted as a primary criteria for trial entry and treatment. The development of FISH techniques applicable to formalin-fixed, paraffin-embedded tissue13 enabled biopsies from patients in the trial to be characterized further in terms of key translocations with an extended panel of immunocytochemical features.
A principal cause of toxicity in LY066 was the use of high-dose methotrexate (6.7 g/m2) as a component of CODOX-M. The second aim of this study was to improve treatment tolerability while maintaining efficacy by dose-reducing methotrexate to 3 g/m2.
Methods
Study design
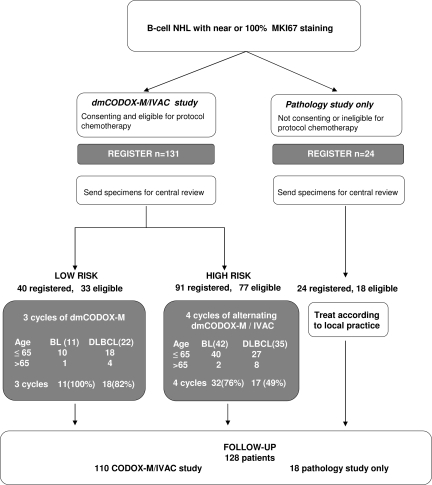
This prospective, international, nonrandomized phase 2 study was initiated and supported by the United Kingdom Medical Research Council Clinical Trials Unit (MRC CTU), London, and the National Cancer Research Institute Lymphoma Clinical Studies Group, and was funded by Cancer Research United Kingdom. It was conducted in collaboration with the Australasian Leukemia and Lymphoma Group (ALLG). The study schema is displayed in Figure 1. Written informed consent was obtained from all patients entered into any aspect of this study. Appropriate central ethical approval was obtained for this trial in the United Kingdom and Australia/New Zealand in accordance with the Declaration of Helsinki.
Figure 1.
Study profile. Study scheme, patient accrual, risk group, and reference diagnosis.
Eligibility
All patients meeting the following criteria were eligible for inclusion in the pathologic study. Solid tumors: B-cell lymphoma at any site expressing CD20 and/or CD79, and associated with 100% or near (> 95%) MKI67 expression defined using the antibody MIB1; leukemic presentation: evidence of a peripheral B-cell phenotype defined by flow cytometry with absence of CD34 and Tdt. At the time the study opened, MKI67 could not be demonstrated reliably by flow cytometry.
Additionally, to be eligible for the dose-modified (dm) CODOX-M/IVAC study, patients were required to be at least 16 years old, HIV negative, and sufficiently mentally and physically fit to tolerate the treatment regimens. Separate, reduced-dose protocols were recommended for patients older than 65 to increase treatment tolerability.
This protocol was written to allow a single course of COP- or CHOP-like chemotherapy to be given prior to dmCODOX-M induction in patients considered unfit (eg, because of lymphoma-related renal failure) or in whom an initial diagnosis of BL had not been established. It was recommended that protocol dmCODOX-M chemotherapy commence as soon as possible after this treatment, generally between days 14 and 21, or earlier if low-dose chemotherapy was given. Otherwise no previous chemotherapy, or irradiation, or previous malignant disease were allowed in the study.
Trial entry
Patients were registered by contacting the MRC CTU or ALLG (patients from Australia/New Zealand only). Data collection, management, and analyses were performed at the MRC CTU. Patients had to be registered prior to starting protocol therapy or exceptionally (eg, due to public holidays) up to 7 days after starting study treatment.
Pretreatment investigations
Following study entry, patients were evaluated urgently by physical examination with assessment of WHO performance status. Blood was obtained for complete blood cell count (cbc), biochemical profile, lactate dehydrogenase (LDH) urate levels, and HIV serology. A chest x-ray and computed tomography of the chest, abdomen, and pelvis were obtained. Bone scanning and magnetic resonance imaging of the head and axial skeleton was performed as indicated. All patients had a bone marrow trephine and aspirate with cytogenetics where relevant. Cerebrospinal fluid (CSF) was sent for cytology.
Pathology and cytogenetics
Pathology specimens for all patients registered were sent to the Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals NHS Trust for central review. Detailed immunophenotyping and interphase FISH studies on formalin-fixed, paraffin-embedded tissue sections were carried out. All cases were examined for expression of the following markers: CD20, CD79, C10, CD5, CD23, BCL-2, BCL-6, IRF4, MKI67, FOXP1, TP53, and P21. Biopsies were assessed as + (all or nearly all the tumor cells expressing the marker), − (none or very occasional positive cells) and +/− (a subpopulation of tumor cells expressing the marker). All specimens were studied for the presence of t(8;14)(q24;q32) and alternative cMYC rearrangements, t(14;18)(q32;q21) and 3q27 (BCL-6) rearrangements using interphase FISH on paraffin sections or isolated nuclei using previously published methods.13–15
Treatment
Treatment groups.
Patients were considered low risk if they had at least 3 of the following international prognostic index (IPI16) factors: normal LDH, WHO performance status 0-1, Ann Arbor stage I to II, and number of extranodal sites less than or equal to 1. These patients were treated with 3 cycles of dose-modified (dm) CODOX-M (the regimen described in our previous study LY066 with further adjustment to the methotrexate dose). All remaining cases were regarded as having high-risk disease and received alternating dmCODOX-M/IVAC twice (ie, dmCODOX-M/IVAC/dmCODOX-M/IVAC). Patients older than 65 years were treated with a separately designed protocol incorporating further dose reductions of dmCODOX-M and IVAC.
Protocol treatment schedule.
After risk group allocation all patients commenced treatment with dmCODOX-M. This regimen and IVAC are presented in Tables 1 and 2 together with the dose reductions for patients older than 65 years. Prior to chemotherapy all patients commenced oral allopurinol and/or received treatment with rasburicase. Before administration of high-dose methotrexate the measured creatinine clearance had to be greater than 50 mL per minute. Methotrexate was administered over 24 hours regardless of cbc; leucovorin rescue was commenced at 36 hours and continued until the methotrexate level was less than 5 × 10−8 M.
Table 1.
Dose-modified CODOX-M regimen, with further modification for age older than 65
| Day | Drug | Dose | Method | Time |
|---|---|---|---|---|
| 1 | Cyclophosphamide | 800 mg/m2 | IV | |
| Vincristine | 1.5 mg/m2 (max 2 mg) | IV | ||
| Doxorubicin | 40 mg/m2 | IV | ||
| Cytarabine | 70 mg | IT | ||
| 2 to 5 | Cyclophosphamide | 200 mg/m2 | IV | Daily |
| 3 | Cytarabine | 70 mg | IT | |
| 8 | Vincristine | 1.5 mg/m2 | IV | |
| 10 | Age 65 y or younger | |||
| Methotrexate | 300 mg/m2 | IV | 1 h | |
| Methotrexate | 2700 mg/m2 | IV | Given over next 23 h | |
| Age more than 65 y | ||||
| Methotrexate | 100 mg/m2 | IV | 1 h | |
| Methotrexate | 900 mg/m2 | IV | Given over next 23 h | |
| 11 | Leucovorin | 15 mg/m2 | IV | At h 36 from start of IV methotrexate |
| 15 mg/m2 | IV | Every 3 h between 36-48 h | ||
| 15 mg/m2 | IV | Then every 6 h until methotrexate level is < 5× 10−8 M | ||
| 13 | G-CSF | 5 μg/kg (1 ampoule) | SC | Daily until granulocyte count > 1×109/L then discontinue |
| 15 | Methotrexate | 12 mg | IT | |
| 16 | Leucovorin | 15 mg | PO | 24 h after IT methotrexate |
Next cycle on the day that the unsupported absolute granulocyte count is more than 1.0 × 109/L, with an unsupported platelet count of more than 75 × 109/L.
IV indicates intravenous; IT, intrathecal; SC, subcutaneous; and PO, oral.
Table 2.
IVAC regimen, with further modification for age older than 65
| Day | Drug | Dose | Method | Time |
|---|---|---|---|---|
| 1 to 5 | Etoposide | 60 mg/m2 (in 500 mL N saline or 5% dextrose) | IV | Daily over 1 h |
| Ifosfamide | IV | Daily over 1 h | ||
| Age 65 y or younger | 1.5 g/m2 | |||
| Age more than 65 y | 1 g/m2 | |||
| Mesna | IV | |||
| Age 65 y or younger | 300 mg/m2 (mixed with ifosfamide) | Over 1 h | ||
| Then 300 mg/m2 | Every 4 hours × 2 | |||
| Age more than 65 y | 200 mg/m2 (mixed with isosfamide) | Over 1 h | ||
| Then 200 mg/m2 | Every 4 hours × 2 | |||
| 1 to 2 | Cytarabine | IV | Over 3 h, 12 hourly; total of 4 doses | |
| Age 65 y or younger | 2 g/m2 | |||
| Age more than 65 y | 1 g/m2 | |||
| 5 | Methotrexate | 12 mg | IT | |
| 6 | Leucovorin | 15 mg | PO | 24 h after IT methotrexate |
| 7 | G-CSF | 5 μg/kg | SC | Daily until granulocyte count > 1.0 × 109/L |
IVAC starts on day 1 on the first day after CODOX-M that the unsupported absolute granulocyte count is more than 1.0 × 109/L, with an unsupported platelet count of more than 75 × 109/L. Next cycle (CODOX-M) commences on the day that the unsupported absolute granulocyte count is more than 1.0 × 109/L, with an unsupported platelet count of more than 75 × 109/L.
The second cycle was dmCODOX-M for low-risk patients and IVAC for high-risk patients. This, and subsequent cycles, commenced when the absolute granulocyte count without growth factor support was greater than 1.0 × 109/L with an unsupported platelet count of greater than 75 × 109/L. No dose modifications were recommended based on the degree or duration of myelosuppression in previous cycles.
All patients received additional CNS prophylaxis with intrathecal cytarabine and methotrexate (Tables 1 and 2). Patients with proven CNS disease received enhanced CNS-directed therapy either via lumbar puncture or an Ommaya reservoir. This comprised (in addition to intrathecal treatment shown in Tables 1 and 2) intrathecal cytarabine 70 mg on day 5 of dmCODOX-M, and days 7 and 9 of IVAC, and intrathecal methotrexate 12 mg (with leucovorin rescue) on day 17 of dmCODOX-M.
Evaluation.
Patients were assessed 3 to 4 weeks after final chemotherapy administration, with relevant repeat radiology and/or a bone marrow. As residual necrotic/fibrotic masses are not unusual in this malignancy, and PET scans were not available in most centers, the primary end point was not response, but progression-free survival, with clinical progression and death from any cause recorded as events.
Statistical considerations
Sample size.
In the dmCODOX-M/IVAC study, the primary outcome measure was progression-free survival (PFS). A minimum of 100 eligible patients were required; with an expected 1-year PFS rate of approximately 70% in the group undergoing protocol treatment, this would enable the PFS rate to be estimated with a standard error of less than 5%. The aim of the pathologic study was to register at least 120 patients with clinical and pathologic data. This would enable prognostic factors to be assessed, for example, differences of 25% in the 1-year PFS rate between groups of patients (eg, those with and without t(14;18)) to be detected with approximately 80% power at a 5% significance level.
Analysis methods.
Duration of PFS was calculated from the date of the start of chemotherapy to the date of the first appearance of progressive disease or death from any cause; patients known to be alive and without progressive disease at the time of analysis were censored at the time of their last follow-up. Overall survival (OS) was calculated from the date of the start of chemotherapy to the date of death from any cause; patients known to be alive at the time of analysis were censored at the time of their last follow-up. The Kaplan-Meier approach was used to display the PFS and OS estimates in different groups and the curves were compared using the log-rank test. The baseline characteristics between different groups were compared using the χ2 test for categorical data or χ2 test for trend for ordinal data when appropriate. All P values are 2-sided. An independent Data Monitoring Committee reviewed the trial data approximately annually.
Comparison with LY06.
We reanalyzed data from our previous trial (LY06) classifying patients according to the risk groups defined in LY10. We present data stratified by risk group, on patient characteristics, toxicity, and outcome, without formal comparison, in broadly comparable patients in the 2 trials aged younger than 60 years. While the major prognostic factors can be accounted for, it is acknowledged that there may be unknown factors that differ between the 2 study populations and further complicated the comparisons. The results of these comparisons should therefore be viewed cautiously.
Results
Accrual
Between April 2002 and May 2005, 155 patients were registered. These patients were recruited from the United Kingdom (123 patients), Poland (21 patients), Australia (10 patients), and New Zealand (1 patient).
After central pathology review (blind to treatment and outcome), 2 patients were regarded as ineligible. In a further 25 patients no or inadequate pathology material was received for review. Therefore, a total of 128 patients were fully eligible for study, comprising 110 patients treated according to protocol and 18 patients who refused trial entry or were unfit because of comorbid disease to receive protocol treatment.
Pathologic features of the study group
All tumor biopsies were first classified by the central review pathologist, using immunocytochemical criteria. The group was initially subdivided into germinal center (GC) and nongerminal center (nonGC) types using expression of BCL-6, CD10, and IRF4. These groups were further divided into BCL-2–positive and –negative cases and then by abnormal TP53 expression, defined as discordance between TP53 and P21 expression as previously reported.17–19
A cMYC rearrangement as the sole cytogenetic abnormality by interphase FISH occurred exclusively in tumors with a GC phenotype, absence of BCL-2 expression, and expression of abnormal TP53 (58 tumors, CD20+, CD79+, CD10+, BCL-6+, BCL-2−, P53+, P21−). However, 30% of tumors with this phenotype showed no evidence of cMYC rearrangement by FISH. t(14;18) was also found exclusively in the GC group, but BCL-2 was strongly expressed in all these cases. This group included 5 tumors with t(8;14) in combination with t(14;18). A 3q27 rearrangement was found in association with both GC and non-GC phenotypes. In a proportion of tumors with a GC phenotype there was aberrant coexpression of IRF 4 and FOX P1. Neither of these markers is expressed in normal GC B cells.20–22
Definition of BL
Based on these findings, BL was defined as a tumor with a germinal center phenotype, absence of BCL-2 expression, abnormal TP53 expression, a cMYC rearrangement, and the absence of t(14;18) or 3q27 rearrangements. This definition is used in the analysis of the study presented below. The remainder of the cases entered in the study were considered to be DLBCL, showing considerable heterogeneity in phenotype and cytogenetic characteristics.
Using these criteria, 58 patients were considered to have BL, 53 of whom were entered into the CODOX-M/IVAC study. The remaining 70 patients were diagnosed as having DLBCL, 57 of whom were entered into the CODOX-M/IVAC study. Five (4 CODOX-M/IVAC study, 1 pathology study) of these 70 patients with DLBCL proved to have dual t(8;14) and t(14;18) translocations.
Clinical features of the study group
The features of the patients with BL and DLBCL were clinically distinct and are described in Table 3. Comparison of these patient groups revealed a highly significant difference in median age (BL: 37 years, range 17-76 years; DLBCL: 56 years, range 19-83 years; P ≤ .001). In addition, the presence/absence of marrow involvement was significantly different (BL 44% vs DLBCL 24%; P = .016) as was the presence of B symptoms (BL present 63%, DLBCL 43%; P = .028). Risk group allocation also differed (BL low risk to high risk 24%:76%; DLBCL 39%:61%; P = .057).
Table 3.
Clinical features of BL and DLBCL in dmCODOX-M/IVAC and pathology studies
| BL |
DLBCL |
Total |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, y | |||||||
| CODOX-M/IVAC* | 60 or less | 48 | 91 | 38 | 67 | 86 | 78 |
| 61 to 65 | 2 | 4 | 7 | 12 | 9 | 8 | |
| More than 65 | 2 | 4 | 12 | 21 | 15 | 14 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Median (range) | 37 | (17-76) | 55 | (19-78) | 42 | (17-78) | |
| Both* | 60 or less | 52 | 90 | 41 | 59 | 93 | 73 |
| 61 to 65 | 2 | 3 | 10 | 14 | 12 | 9 | |
| More than 65 | 4 | 7 | 19 | 27 | 23 | 18 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| Median (range) | 37 | (17-76) | 56 | (19-83) | 43 | (17-83) | |
| Sex | |||||||
| CODOX-M/IVAC | Male | 42 | 79 | 42 | 74 | 84 | 76 |
| Female | 11 | 21 | 15 | 26 | 26 | 24 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both | Male | 46 | 79 | 46 | 66 | 92 | 72 |
| Female | 12 | 21 | 24 | 34 | 36 | 28 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| LDH level | |||||||
| CODOX-M/IVAC | Normal | 9 | 17 | 18 | 32 | 27 | 25 |
| Raised | 44 | 83 | 39 | 68 | 83 | 75 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both | Normal | 12 | 21 | 21 | 30 | 33 | 26 |
| Raised | 46 | 79 | 49 | 70 | 95 | 74 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| WHO PS | |||||||
| CODOX-M/IVAC | 0 | 18 | 34 | 15 | 26 | 33 | 30 |
| 1 | 15 | 28 | 16 | 28 | 31 | 28 | |
| 2 | 8 | 15 | 15 | 26 | 23 | 21 | |
| 3 | 10 | 19 | 10 | 18 | 20 | 18 | |
| 4 | 2 | 4 | 1 | 2 | 3 | 3 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both | 0 | 20 | 34 | 18 | 26 | 38 | 30 |
| 1 | 17 | 29 | 19 | 27 | 36 | 28 | |
| 2 | 9 | 16 | 18 | 26 | 27 | 21 | |
| 3 | 10 | 17 | 13 | 19 | 23 | 18 | |
| 4 | 2 | 3 | 2 | 3 | 4 | 3 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| Ann Arbor stage | |||||||
| CODOC-M/IVAC | I | 7 | 13 | 12 | 21 | 19 | 17 |
| II | 6 | 11 | 12 | 21 | 18 | 16 | |
| III | 4 | 8 | 5 | 9 | 9 | 8 | |
| IV | 36 | 68 | 28 | 49 | 64 | 58 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both | I | 10 | 17 | 14 | 20 | 24 | 19 |
| II | 6 | 10 | 16 | 23 | 22 | 17 | |
| III | 5 | 9 | 7 | 10 | 12 | 9 | |
| IV | 37 | 64 | 33 | 47 | 70 | 55 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| No. of extranodal sites of disease | |||||||
| CODOX-M/IVAC | 1 or less | 26 | 49 | 34 | 60 | 60 | 55 |
| More than 1 | 27 | 51 | 23 | 40 | 50 | 45 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both | 1 or less | 29 | 50 | 43 | 61 | 72 | 56 |
| More than 1 | 29 | 50 | 27 | 39 | 56 | 44 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| Modified IPI score | |||||||
| CODOX-M/IVAC* | 0 | 7 | 13 | 15 | 26 | 22 | 20 |
| 1 | 6 | 11 | 8 | 14 | 14 | 13 | |
| 2 | 22 | 42 | 12 | 21 | 34 | 31 | |
| 3 | 18 | 34 | 22 | 39 | 40 | 36 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both* | 0 | 10 | 17 | 17 | 24 | 27 | 21 |
| 1 | 6 | 10 | 11 | 16 | 17 | 13 | |
| 2 | 23 | 40 | 15 | 21 | 38 | 30 | |
| 3 | 19 | 33 | 27 | 39 | 46 | 36 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| Risk group | |||||||
| CODOX-M/IVAC | Low risk | 11 | 21 | 22 | 39 | 33 | 30 |
| High risk | 42 | 79 | 35 | 61 | 77 | 70 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both | Low risk | 14 | 24 | 27 | 39 | 41 | 32 |
| High risk | 44 | 76 | 43 | 61 | 87 | 68 | |
| Total | 58 | 100 | 70 | 100 | 128 | 100 | |
| CNS | |||||||
| CODOX-M/IVAC | Not involved | 47 | 89 | 48 | 87 | 95 | 88 |
| Involved | 6 | 11 | 7 | 13 | 13 | 12 | |
| Unknown | 0 | 2 | 2 | ||||
| Total | 53 | 100 | 57 | 110 | |||
| Both | Not involved | 50 | 88 | 60 | 90 | 110 | 89 |
| Involved | 7 | 12 | 7 | 10 | 14 | 11 | |
| Unknown | 1 | 3 | 4 | ||||
| Total | 58 | 70 | 128 | ||||
| Marrow | |||||||
| CODOX-M/IVAC | Not involved | 29 | 55 | 40 | 71 | 69 | 63 |
| Involved | 24 | 46 | 16 | 29 | 40 | 37 | |
| Unknown | 0 | 1 | 1 | ||||
| Total | 53 | 100 | 57 | 110 | |||
| Both | Not involved | 32 | 56 | 52 | 76 | 84 | 67 |
| Involved | 25 | 44 | 16 | 24 | 41 | 33 | |
| Unknown | 1 | 2 | 3 | ||||
| Total | 58 | 70 | 128 | ||||
| GI involved (ileocaecal, stomach) | |||||||
| CODOX-M/IVAC | Not Involved | 35 | 67 | 44 | 79 | 79 | 73 |
| Involved | 17 | 33 | 12 | 21 | 29 | 27 | |
| Unknown | 1 | 1 | 2 | ||||
| Total | 53 | 57 | 110 | ||||
| Both | Not involved | 39 | 70 | 54 | 79 | 93 | 75 |
| Involved | 17 | 30 | 14 | 21 | 31 | 25 | |
| Unknown | 2 | 2 | 4 | ||||
| Total | 58 | 70 | 128 | ||||
| B symptoms | |||||||
| CODOX-M/IVAC | No | 18 | 35 | 35 | 63 | 53 | 49 |
| Yes | 34 | 65 | 21 | 38 | 55 | 51 | |
| Unknown | 1 | 1 | 2 | ||||
| Total | 53 | 57 | 110 | ||||
| Both | No | 21 | 38 | 39 | 57 | 60 | 48 |
| Yes | 35 | 63 | 29 | 43 | 64 | 52 | |
| Unknown | 2 | 2 | 4 | ||||
| Total | 58 | 70 | 128 | ||||
| Preinduction chemo given | |||||||
| CODOX-M/IVAC* | No | 35 | 66 | 38 | 67 | 73 | 66 |
| CHOP | 7 | 13 | 10 | 18 | 17 | 15 | |
| COP | 0 | 0 | 1 | 2 | 1 | 1 | |
| Other | 11 | 21 | 8 | 14 | 19 | 17 | |
| Total | 53 | 100 | 57 | 100 | 110 | 100 | |
| Both* | No | 38 | 67 | 50 | 72 | 88 | 70 |
| CHOP | 7 | 12 | 10 | 14 | 17 | 13 | |
| COP | 0 | 0 | 1 | 1 | 1 | 1 | |
| Other | 12 | 21 | 8 | 12 | 20 | 16 | |
| Unknown | 1 | 1 | 2 | ||||
| Total | 58 | 70 | 128 | ||||
CODOX-M/IVAC indicates those patients entered into dmCODOX-M/IVAC study only; Both indicates the total patients included in dmCODOX-M/IVAC or pathology study.
Treatment and outcome
All patients entered into the dmCODOX-M/IVAC study, regardless of review pathology, were treated with dmCODOX-M or dmCODOX-M/IVAC according to risk group. Thirty-seven patients (4 low risk, 33 high risk) were initially treated with 1 course of CHOP (17 patients) or a CHOP-like regimen (20 patients) because of initial diagnostic uncertainty or poor physical condition.
Distribution of patients by diagnostic group, risk group, and age is shown in Figure 1. Fifty-three patients with BL (11 low risk, 42 high risk) and 57 patients with DLBCL (22 low risk, 35 high risk) were entered into the dmCODOX-M/IVAC study.
Low-risk protocol
Thirty-three patients (11 BL, 22 DLBCL) commenced this protocol, comprising 3 cycles of dmCODOX-M; 28 were aged 65 or younger and 5 were older than 65 years. Twenty-nine (88%) completed 3 cycles of chemotherapy with 19 of these receiving full-dose protocol treatment. All 11 patients with BL and 18 of 22 patients with DLBCL received 3 cycles of chemotherapy while 3 of 5 patients older than 65 years completed 3 cycles of chemotherapy. In total 4 patients stopped treatment early; one 78-year-old patient died from a CVA 15 days from start of cycle 1 (relationship to treatment not determined); and 3 other patients stopped early due to toxicity, change in diagnosis, and time to recover from surgery, respectively.
The median cycle 1-2 interval was 25 days (range 17-86 days), cycle 2-3 was 23 days (range 15-75 days). The comparable figures for our previous LY06 study5 were cycle 1-2, 22 days (range 18-32 days), cycle 2-3, 22 days (range 16-54 days).
High-risk protocol
Seventy-seven patients commenced this protocol comprising 42 patients with BL and 35 patients with DLBCL; 67 were aged 65 or younger and 10 were older than 65 years. These patients were treated with alternating dmCODOX-M/IVAC. Forty-nine patients (65%) completed 4 courses of treatment with 34 (44%) patients receiving full-dose protocol treatment. Treatment completion differed according to diagnostic group with 32 (76%) of 42 patients with BL and 17 (49%) of 35 patients with DLBCL receiving 4 cycles of chemotherapy. Only 3 of the 10 patients aged over 65 years received 4 cycles of chemotherapy.
In total, treatment was discontinued prematurely in 27 cases, reasons being lack of response or general poor condition (5 patients), progressive disease or disease-related death (12 patients), treatment toxicity (5 patients), treatment-related death (4 patients), and death from other cause (1 died from bronchopneumonia and peritonitis after 3 cycles of treatment). One patient had at least 1 cycle of treatment but further treatment details are missing.
The median cycle 1-2 interval was 27 days (range 17-67 days), cycle 2-3 was 21 days (range 11-37 days), and cycle 3-4, 29 days (range 20-55 days). The comparable figures in the LY06 study5 were, respectively, 24.5 days (range 16-40 days), 20 days (range 14-41 days), and 27 days (range 18-41 days).
Toxicity
The toxicity assessed using the NCIC Common Toxicity Criteria (CTC Version 2.0) during the chemotherapy is summarized in Table 4. There were 9 deaths (1 low risk, 8 high risk) reported to be treatment-related, of which 5, all high-risk patients, died within 12 weeks of starting treatment; 2 of the 9 patients were aged over 65 (66 and 67, respectively).
Table 4.
Worst toxicity experienced (CTC grade) during the treatment (for 109 patients who received at least 1 cycle of protocol treatment) in dmCODOX-M/IVAC study
| Low risk, N = 33 |
High risk, N = 76 |
Total, N = 109 |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| WBC | ||||||
| Grade 3 | 1 | 3 | 0 | 0 | 1 | 1 |
| Grade 4 | 32 | 97 | 75* | 99 | 107 | 98 |
| Neutropenic fever | ||||||
| Grade 3 | 20 | 61 | 67 | 88 | 87 | 80 |
| Neutrophil count | ||||||
| Grade 3 | 0 | 0 | 1 | 1 | 1 | 1 |
| Grade 4 | 32 | 97 | 75 | 99 | 107 | 98 |
| Platelets | ||||||
| Grade 3 | 5 | 15 | 1 | 1 | 6 | 6 |
| Grade 4 | 14 | 42 | 73 | 96 | 87 | 80 |
| Mucositis | ||||||
| Grade 3 | 10 | 31 | 29 | 38 | 39 | 36 |
| Grade 4 | 2 | 6 | 8 | 11 | 10 | 9 |
| Unknown | 1 | 0 | 1 | |||
| Neuropath, sensory/motor | ||||||
| Grade 3 | 3 | 10 | 3 | 4 | 6 | 6 |
| Grade 4 | 0 | 0 | 2 | 3 | 2 | 2 |
| Unknown | 3 | 0 | 3 | |||
One patient did not report grade 3/4 leukopenia but received only part of cycle 1 dmCODOX-M prior to disease progression.
Progression-free survival and overall survival dmCODOX-M/IVAC study
At the time of analysis, the median follow-up was 29 months (range 3-54 months) with only 2 surviving patients being followed less than 1 year. Forty patients died. The cause of death was disease-related in 29 and treatment-related in 9, with 1 other cause (bronchopneumonia, peritonitis) and 1 death in a 78-year-old patient from a CVA 15 days from start of cycle 1 (relationship to treatment not determined).
Sixty-five patients are alive without progression and 5 alive with progression; 13 patients died without reported progression and 27 patients died after disease progression. Of the 32 patients with disease progression, 20 of whom had further treatment, the median survival time from date of progression was 2 months.
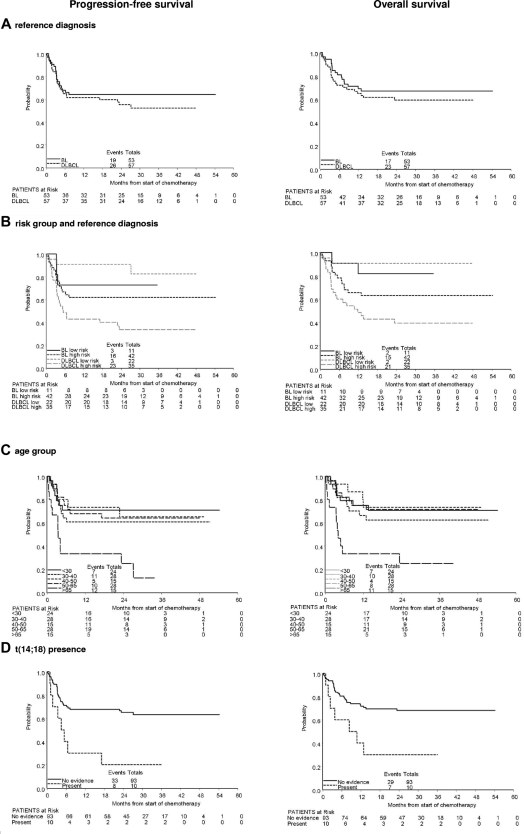
Progression-free survival (PFS) and overall survival (OS) according to risk group are summarized in Table 5. The 2-year PFS was 85% (95% CI 73%-97%) for low-risk patients and 49% (95% CI 38%-60%) for high-risk patients. The 2-year OS was 88% (95% CI 77%-99%) for low-risk patients and 52% (95% CI 41%-63%) for high-risk patients.
Table 5.
Summary of progression-free survival and overall survival in dmCODOX-M/IVAC study
| No. progression or death/total | 2-year PFS (95% CI) | Hazard ratio (95% CI), P | No. death/total | 2-year OS (95% CI) | Hazard ratio (95% CI), P | |
|---|---|---|---|---|---|---|
| All patients | 45/110 | 60% (51%-69%) | 40/110 | 63% (54%-72%) | ||
| Low risk versus high risk | 0.39 (0.21-0.72), .003 | 0.33 (0.17-0.63), <.001 | ||||
| Low risk | 6/33 | 85% (73%-97%) | 4/33 | 88% (77%-99%) | ||
| High risk | 39/77 | 49% (38%-60%) | 36/77 | 52% (41%-63%) | ||
| BL versus DLBCL | 0.77 (0.43-1.38), .38 | 0.76 (0.41-1.40), .38 | ||||
| BL | 19/53 | 64% (51%-77%) | 17/53 | 67% (54%-80%) | ||
| DLBCL | 26/57 | 55% (42%-68%) | 23/57 | 59% (46%-72%) | ||
| Age 65 y or younger versus age older than 65 y | 0.13 (0.05-0.36), <.001 | 0.11 (0.04-0.32), <.001 | ||||
| 65 y or less | 33/95 | 65% (55%-75%) | 29/95 | 69% (60%-78%) | ||
| Older than 65 y | 12/15 | 25% (2%-48%) | 11/15 | 25% (2%-48%) | ||
| Presence of t(14,18) | 0.18 (0.055-0.57), .004 | 0.19 (0.055-0.64), .008 | ||||
| No evidence | 33/93 | 65% (55%-75%) | 29/93 | 73% (64%-82%) | ||
| Present | 8/10 | 20% (0%-45%) | 7/10 | 40% (10%-70%) | ||
| Unknown | 7 | 11 | ||||
| BL versus DLBCL* | 0.33 (0.13-0.82), .017 | 0.38 (0.14-1.01), .052 | ||||
| BL | 19/53 | 64% (51%-77%) | 17/53 | 67% (54%-80%) | ||
| DLBCL* | 11/15 | 27% (5%-49%) | 9/15 | 40% (15%-65%) |
Indicates DLBCL with t(8;14)+, t(14;18)+, or 3q27 rearrangement.
Pathologic study
A total of 128 patients were in the pathologic study. At the time of analysis, the median follow-up was 27 months with 46 patients dead and 52 patients with disease progression or death. The PFS and OS by risk group and other prognostic factors were similar to those seen in the subset of 110 patients in the dmCODOX-M/IVAC study (data not shown) and hence subsequent analyses focused on the uniformly treated patients in the dmCODOX-M/IVAC study only.
Comparisons between BL and DLBCL
Overall survival of the BL and DLBCL groups was similar (Figure 2A, Table 5). However, these groups were clinically distinct as previously described, particularly with respect to risk group distribution. Figure 2B shows PFS and OS for patients divided according to risk group and pathology group. In the larger, high-risk group, patients with BL had significantly better PFS (HR = 0.79, P = .03) and OS (HR = 0.80, P = .05) than patients with DLBCL.
Figure 2.
Kaplan-Meier plots of progression-free survival and overall survival. By reference diagnosis (A), risk group and reference diagnosis (B), age group (C), and t(14;18) presence (D).
Age
Older patients (> 65 years old who were treated in a separate protocol) clearly have inferior PFS and OS when viewed across all histologies (Table 5). However, within the younger patients (≤ 65), there was no clear age-related trend. Figure 2C shows PFS and OS for patients divided according to age group.
t(14;18), t(8;14), and 3q27 rearrangement
Patients with t(14;18), all in the DLBCL group, have inferior PFS and OS to those with no evidence of t(14;18) (Table 5, Figure 2D). Patients with presence of t(8;14), t(14;18), or 3q27 rearrangement in the DLBCL group also have inferior PFS and OS (Table 5). Four patients were found to have a dual t(8;14) and t(14;18) translocation and this clearly resulted in a marked worsening of prognosis both with regard to PFS and OS. All 4 patients were dead less than 5 months from the start of treatment.
Prognostic factors
Further exploratory analyses on reference diagnosis, the clinical factors listed in Table 3, and the presence of t(14;18) were performed in the consistently treated high-risk patients (n = 77). There was some evidence of poorer prognosis associated with reference diagnosis of DLBCL (HR = 0.79, P = .03), increasing age (continuous variable, HR = 1.03, P = .02), CNS involvement (HR = 2.0, P = .07), and t(14;18) presence (HR = 2.7, P = .02) on univariate analysis.
LY10 versus LY06
The entry criteria for our previous BL study, LY06,6 were based on morphologic assessment and MKI67 immunocytochemistry. All patients were younger than 60 years old. FISH was not available at the time of this study, and the pathologic material was not available for further review. BL was therefore diagnosed using the cytologic appearances of the tumor and standard immunophenotyping available at that time. The 2 trial BL populations are therefore not directly comparable, and to minimize bias in the comparison with LY10 with respect to toxicity and outcome, all patients diagnosed with BL or DLBCL and with adequate data on the LY10 risk factors were included (in LY06, 51 were reported to have BL, and 12 were considered to have DLBCL).
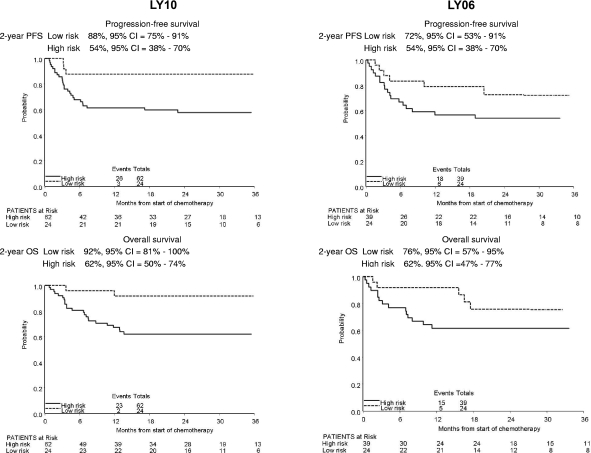
Risk group definitions differed between LY10 and LY06, therefore the 63 LY06 patients were reanalyzed according to the LY10 risk group definitions, resulting in 9 LY06 high-risk patients moving to the LY10 low-risk group. The clinical features of patients younger than 60 years in the 2 trials are summarized by risk group in Table 6 and were comparable. PFS and OS curves were remarkably similar (Figure 3). In LY06 the 2-year PFS in the low-risk group was 72% (95% CI 53%-91%) and in the high-risk group 54% (95% CI 38%-70%). The comparable figures in LY10 were 88% (95% CI 75%-91%) and 54% (95% CI 38%-70%). In LY06 the 2-year OS in the low-risk group was 76% (95% CI 57%-95%) and in the high-risk group 62% (95% CI 47%-77%). In LY10 the 2-year OS in the low-risk group was 92% (95% CI 81%-100%), and in the high-risk group 62% (95% CI 50%-74%).
Table 6.
Clinical features for patients in LY10 and LY06 (risk group defined as in LY10)
| LY10 CODOX-M/IVAC study patients, risk group defined as in LY10 |
LY06 patients, risk group defined as in LY10 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Low risk, N = 24 |
High risk, N = 62 |
Low risk, N = 24 |
High risk, N = 39 |
|||||
| n | % | n | % | n | % | n | % | |
| Risk group defined as in LY06 | ||||||||
| Low risk | NA | NA | 15 | 63 | 0 | 0 | ||
| High risk | NA | NA | 9 | 38 | 39 | 100 | ||
| Median age, y (range) | 38 | (20-56) | 39 | (17-60) | 32 | (15-59) | 43 | (17-60) |
| Sex | ||||||||
| Male | 20 | 83 | 47 | 76 | 17 | 71 | 22 | 56 |
| Female | 4 | 17 | 15 | 24 | 7 | 29 | 17 | 44 |
| Ann Arbor stage | ||||||||
| I | 16 | 67 | 0 | 0 | 13 | 54 | 0 | 0 |
| II | 8 | 33 | 4 | 6 | 9 | 38 | 3 | 8 |
| III | 0 | 0 | 7 | 11 | 2 | 8 | 6 | 15 |
| IV | 0 | 0 | 51 | 82 | 0 | 0 | 30 | 77 |
| WHO PS | ||||||||
| 0 | 14 | 58 | 12 | 19 | 21 | 88 | 6 | 15 |
| 1 | 10 | 42 | 17 | 27 | 3 | 13 | 11 | 28 |
| 2 | 0 | 0 | 17 | 27 | 0 | 0 | 14 | 36 |
| 3 | 0 | 0 | 14 | 23 | 0 | 0 | 6 | 15 |
| 4 | 0 | 0 | 2 | 3 | 0 | 0 | 2 | 5 |
| LDH level | ||||||||
| Normal | 17 | 71 | 1 | 2 | 22 | 92 | 2 | 5 |
| Raised | 7 | 29 | 61 | 98 | 2 | 8 | 37 | 95 |
| IPI | ||||||||
| 0 | 17 | 71 | 0 | 0 | 20 | 83 | 0 | 0 |
| 1 | 7 | 28 | 2 | 3 | 4 | 17 | 0 | 0 |
| 2 | 0 | 0 | 30 | 48 | 0 | 0 | 22 | 56 |
| 3 | 0 | 0 | 30 | 48 | 0 | 0 | 17 | 44 |
Figure 3.
Kaplan-Meier plots of progression-free survival and overall survival in LY10 and LY06 patients with risk group defined as in LY10.
Nadir WBC and neutrophil counts were similar between the 2 trials; however, mucositis was slightly reduced in LY10; the incidence of grade 3/4 mucositis in low-risk patients in LY10 and LY06 were, respectively, 39%/0% and 26%/22% with corresponding figures for high-risk patients of 35%/8% in LY10 and 34%/18% in LY06.
Discussion
BL is a rare form of non-Hodgkin lymphoma comprising no more than 1% of cases. There are no randomized trials in this condition. Previous published series have comprised modest-sized single institution or multigroup evaluations of complex cycling chemotherapy using diverse entry criteria.5–10 Most commonly in older studies this has comprised typical tumor morphology on hematoxylin and eosin (H&E) histology; more recently 100% MKI67 staining has been used as a surrogate for the diagnosis of BL, occasionally supported by cytogenetics demonstrating a cMYC rearrangment.4 This study is the first to treat all patients consistently with a highly active BL regime using robust diagnostic criteria based on immunophenotyping and interphase FISH.
The use of high MKI67 expression as the primary entry criterion in this study was designed to increase the consistency of recruitment of patients and reduce dependence on unreliable and subjective morphologic criteria. However, the patient population recruited using these criteria proved to be highly heterogeneous with respect to immunophenotypic and cytogenetic features. Based on these findings high MKI67 alone should not be used as a screening test for BL.
All of the tumors in this study with a cMYC rearrangement had a GC phenotype with expression of CD10 and BCL-6, suggesting that somatic hypermutation and class switch recombination may be important in the generation of the translocation. In most cases this appeared to be a highly aberrant germinal center phenotype with coexpression of BCL-6, IRF-4, and FOXP1 not seen in normal germinal center B cells. In experimental systems, inactivation of P53 is essential for cMYC-mediated oncogenesis23–25 and in this study the cMYC rearranged tumors all showed evidence of mutated P53 with strong nuclear staining for the P53 protein in the absence of P21. In tumors with this set of features and absence of BCL-2 expression there was no evidence of a t(14;18), 3q27 rearrangement or significant aneuploidy by FISH. For the purpose of this study this was adopted as the definition of BL. This group showed distinctive clinical and demographic differences from the other patients in the trial. There were, however, no significant differences in multiple other parameters including disease sites at presentation, incidence of CNS disease, stage, IPI score, or other clinical features. Although highly uniform in many respects only a minority of these cases showed classic Burkitt-type morphology. At a practical level, the immunophenotype described above identifies BL with a sensitivity of 100% and specificity of around 70%. When these features are present FISH studies should always be carried out. It is possible that the specificity may be further improved by the addition of further markers such as TCL1.26
This approach to the diagnosis of Burkitt lymphoma has been supported by the publication of 2 gene expression studies27,28 that identified a unique BL gene expression signature distinct from other types of aggressive B-cell lymphoma. Hummel et al27 emphasized the distinctive characteristics of the molecular BL group in which a cMYC rearrangement was the sole abnormality. Gene expression analysis is, however, not a routine diagnostic technique, and the rapid deterioration of many BL samples due to apoptosis is a significant obstacle. However, it is likely that the group defined in our studies using immunocytochemistry and FISH is the same as the molecular BL categories defined in these studies.
The definition of BL needs to be justified by reference to clinical data and in particular the utility of this approach in targeting high-intensity chemotherapy. In this study dmCODOX-M/IVAC proved to be a highly effective treatment in the BL group with an overall survival of 67%, and an excellent outcome in the low-risk group defined by IPI. The effectiveness of the IPI in predicting outcome was much less for the BL group than for DLBCL and in high-risk patients there was a greatly reduced risk of disease progression after chemotherapy in the BL group compared with the DLBCL group. Of the 110 dmCODOX-M/IVAC study patients, the deaths of 9 (8%) were attributed to treatment toxicity, emphasizing the need to target this therapy to those with disease not adequately treated by CHOP-R. Although historic comparison needs to be treated with caution, particularly in view of the diagnostic problems in previous trials, it does not appear that the reduction in methotrexate dose was associated with a less favorable outcome. This should improve the tolerability of the regimen for a broader group of patients.
The majority of patients with a high MKI67 did not meet the definition of BL used in this study and so can act as a comparison group in which to assess the efficacy of this treatment in patients with DLBCL. The overall survival in this group was not significantly different from the BL group as a whole. However, the outcome of this group was also broadly similar to that achieved in most recent trials of CHOP-R–containing regimens, which are much less toxic.29–32 In contrast to BL, there is a continuing rate of relapse in this group, as would be expected in any series of DLBCL. An important question is the efficacy of CODOX-M/IVAC in patients with DLBCL who have poor prognostic factors. In this study the difference in outcome between IPI groups persisted, those with high IPI having a significantly worse outcome. In the absence of randomized data it is not possible to comment on whether the high IPI patients had a better survival than would be expected with CHOP-type therapy or whether the addition of rituximab to CODOX-M/IVAC would improve the outcome of this poor prognosis group.
One group that is known to have a very poor outcome are patients who have tumors that have multiple chromosomal abnormalities4,33,34 with combinations of cMYC, BCL-6, and BCL-2 rearrangements. In this study, high-intensity chemotherapy does not appear to have been effective in overcoming the adverse effect of the translocation. A poorer outcome was also found in patients without cMYC rearrangement but with t(14:18) or 3q27 abnormalities. These data suggest that increasing the intensity of treatment of patients with DLBCL using dmCODOX-M/IVAC does not negate significantly the effects of the adverse prognostic factors that apply to CHOP-treated patients.
This trial incorporated dose reduction of methotrexate, given as part of CODOX-M from 6.7 g/m2 to 3 g/m2. There was some evidence that this approach was less toxic. It was also anticipated that this dose reduction may enable chemotherapy to be more rapidly cycled because of lesser degrees of mucositis. However, this did not prove to be the case when the cycling time was compared with our previous study. There is little or no prospect that randomized trials will be performed in this condition now or in the future and it can be reasonably concluded from our and other data7 that methotrexate given at a dose of 3g/m2 is of similar efficacy and can be regarded as an acceptable standard.
The trial was designed to include older patients who are poorly represented in previously published studies. A dose-modified protocol was recommended, but relatively few patients were accrued. As previously described, results from this patient group were inferior and the treatment poorly tolerated with few patients treated effectively.
Rituximab is now in widespread use in B-cell lymphoma treatment and has been shown to increase cure rates in DLBCL. This drug was not incorporated into the treatment given in this trial as it was not a standard treatment and not licensed when the study was designed. Rituximab has been described as having possible beneficial effects, particularly in older patients.10 It seems highly unlikely that randomized trials testing the efficacy of this drug will be performed in the future. Clinicians may decide they wish to add this agent to treatments in this young patient population.
In conclusion we have proposed a set of robust diagnostic criteria for the identification of patients with nonendemic BL that can be used to effectively target high-intensity chemotherapy. These patients have a good outcome when treated with dose-modified CODOX-M/IVAC, and this should now be considered as a standard therapy for BL. In contrast, the trial does not support the routine use of high-intensity therapy in other highly proliferative B-cell lymphomas. There was no clear evidence that this treatment would be efficacious for non–BL patients who would currently conventionally be treated with CHOP-R with more acceptable toxicity.
Supplementary Material
Acknowledgments
Dr G. M. Mead (Southampton University Hospitals Trust, Southampton, United Kingdom) was the chief investigator. Dr A. Jack and Dr S. Barrans (Haematological Malignancy Diagnostic Service, Leeds General Infirmary, Leeds, United Kingdom) were the reference pathologist and the clinical scientist, respectively. Statisticians were W. Qian and S. Stenning, MRC Clinical Trials Unit, London, United Kingdom. Central trial and data management was performed by S. Clawson, C. Goldstein, and C. Yule, MRC Clinical Trials Unit, London, United Kingdom. Local coordinator for the ALLG was J. Stone at the Peter MacCallum Cancer Institute, Melbourne, Australia. The Independent Data Monitoring Committee was made up of R. Glynne-Jones, R. Prescott, and H. Scarffe (chair). We would like to thank all clinicians and patients who participated in the study. A list of participating clinicians is available on the Blood website; see the Supplemental Materials link at the top of the online article.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.M.M., A.J., and S.P.S. designed the study; S.B. and A.J. performed the central pathology review and laboratory analyses and derived the diagnostic criteria for BL; G.M.M., J.W., J.A.R., and M.W. (representing the ALLG) entered more than 10 patients; S.M.C. and C.L.Y. were responsible for the data management; W.Q. and S.P.S. performed statistical analysis; G.M.M., W.Q., S.P.S., and A.J. drafted the manuscript; all authors contributed to and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Wendi Qian, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, United Kingdom; e-mail: wendi.qian@ctu.mrc.ac.uk.
References
- 1.Diebold J, Jaffe ES, Raphael M, Warnke RA. Burkitt lymphoma. In: Jaffe E, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics: Tumours of Haematopoietic and Lymphoid Tumours. Lyon, France: AIARC Press; 2001. pp. 181–184. [Google Scholar]
- 2.Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104:3009–3020. doi: 10.1182/blood-2004-02-0405. [DOI] [PubMed] [Google Scholar]
- 3.Hecht JL, Aster JC. Molecular biology of Burkitt's lymphoma. J Clin Oncol. 2000;18:3707–3721. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson N, Lesack D, Klasa R, et al. Small noncleaved, non-Burkitt's (Burkitt-like) lymphoma: cytogenetics predict outcome and reflect clinical presentation. J Clin Oncol. 1999;17:1558–1567. doi: 10.1200/JCO.1999.17.5.1558. [DOI] [PubMed] [Google Scholar]
- 5.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 6.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 7.Lacasce A, Howard O, Li S, et al. Modified Magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymph. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 8.Rizzieri DA, Johnson JL, Niedzwiecki D, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukaemia and lymphoma: final results of Cancer and Leukaemia Group B study 9251. Cancer. 2004;100:1438–1448. doi: 10.1002/cncr.20143. [DOI] [PubMed] [Google Scholar]
- 9.Divine M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–1935. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukaemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 11.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 12.Lones MA, Raphael M, Perkins SL, et al. Mature B-cell lymphoma in children and adolescents: international group pathologist consensus correlates with histology technical quality. J Pediatr Hematol Oncol. 2006;28:568–574. doi: 10.1097/01.mph.0000212980.67114.a5. [DOI] [PubMed] [Google Scholar]
- 13.Barrans SL, Evans PA, O'Connor SJ, Owen RG, Morgan GJ, Jack AS. The detection of t(14;18) in archival lymph nodes: development of a fluorescence in situ hybridization (FISH)-based method and evaluation by comparison with polymerase chain reaction. J Mol Diagn. 2003:168–175. doi: 10.1016/S1525-1578(10)60469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrans SL, Evans PA, O'Connor SJ, et al. The t(14;18) is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome. Clin Cancer Res. 2003;9:2133–2139. [PubMed] [Google Scholar]
- 15.Barrans SL, O'Connor SJ, Evans PA, et al. Re-arrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br J Haematol. 2002;117:322–332. doi: 10.1046/j.1365-2141.2002.03435.x. [DOI] [PubMed] [Google Scholar]
- 16.The International Non-Hodgkin's Lymphoma Prognostic Factors Project: a predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 17.Barrans SL, Carter I, Owen RG, et al. Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood. 2002;99:1136–1143. doi: 10.1182/blood.v99.4.1136. [DOI] [PubMed] [Google Scholar]
- 18.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 19.Bai M, Tsanou E, Skyrlas A, Sainis I, Agnantis N, Kanavaros P. Alterations of the p53, Rb and p27 tumor suppressor pathways in diffuse large B-cell lymphomas. Anticancer Res. 2007;27(4B):2345–2352. [PubMed] [Google Scholar]
- 20.Barrans SL, Fenton JA, Banham A, Owen RG, Jack AS. Strong expression of FOXP1 identifies a distinct subset of diffuse large B-cell lymphoma (DLBCL) patients with poor outcome. Blood. 2004;104:2933–2935. doi: 10.1182/blood-2004-03-1209. [DOI] [PubMed] [Google Scholar]
- 21.Banham AH, Connors JM, Brown PJ, et al. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11:1065–1072. [PubMed] [Google Scholar]
- 22.Klein U, Casola S, Cattoretti G, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 23.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118:79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattoretti G, Shaknovich R, Smith PM, Jäck HM, Murty VV, Alobeid B. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol. 2006;177:6930–6939. doi: 10.4049/jimmunol.177.10.6930. [DOI] [PubMed] [Google Scholar]
- 26.Rodig SJ, Vergilio JA, Shahsafaei A, Dorfman DM. Characteristic expression patterns of TCL1, CD38, and CD44 identify aggressive lymphomas harboring a MYC translocation. Am J Surg Pathol. 2008;32:113–122. doi: 10.1097/PAS.0b013e3180959e09. [DOI] [PubMed] [Google Scholar]
- 27.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 28.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 29.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 30.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 31.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 32.Pfreundschuh M, Trümper L, Osterborg A, et al. MabThera International Trial Group. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good prognosis diffuse large B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 33.Au WY, Gascoyne RD, Viswanatha DS, et al. Concurrent chromosomal alterations at 3q27, 8q24 and 18q21 in B-cell lymphomas. Br J Haematol. 1999;105:437–440. [PubMed] [Google Scholar]
- 34.Kanungo A, Medeiros LJ, Abruzzo LV, Lin P. Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol. 2006;19:25–33. doi: 10.1038/modpathol.3800500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.