Abstract
Valganciclovir is commonly used for cytomegalovirus (CMV) prophylaxis in renal transplant patients. A fixed dose of 900 mg daily is typically recommended, however, there has never been a formal pharmacokinetic study comparing various doses in renal transplant patients. We therefore compared the pharmacokinetic characteristics of intravenous ganciclovir (IV GCV) and oral ganciclovir (GCV) with two different doses of valganciclovir (VGCV) in an open-label crossover study. Ten adult kidney recipients participated in a four-phase crossover treatment schedule of IV GCV (2.5 mg/kg every 12 h), VGCV (900 mg daily), VGCV (450 mg daily) and oral GCV (1000 mg Q8 H). IV GCV and oral VGCV 900 mg daily achieved similar values for AUC0–24 (median 60.63 vs. 62.86 μg/h/mL). Oral VGCV 450 mg achieved comparable AUC0–24 values as oral GCV 1000 mg Q8 H (median AUC0–24 35.9 vs. 29.04 μg/h/mL). Oral VGCV 900 mg daily provided systemic GCV exposure similar to IV GCV and confirms PV 16 000 study results. Further, VGCV 450 mg daily provided comparable systemic exposure versus oral GCV. Due to its favorable pharmacokinetic profile, data herein suggest that VGCV can be used in the early post-kidney transplant period, and that 450 mg daily provides ample drug exposure for effective CMV prophylaxis in kidney transplant patients.
Keywords: Kidney transplantation, pharmacokinetics, prophylaxis, valganciclovir
Introduction
Human cytomegalovirus (CMV) is one of the most common viral infections occurring following solid organ transplant and has been associated with significant morbidity and mortality (1). CMV infection and disease generally occurs from 1 to 6 months following transplantation (1). Risk of CMV infection is dependent on donor (D) and recipient (R) serostatus with a CMV seropositve donor to a CMV seronegative recipient (D+/R−) representing the highest risk of CMV acquisition for the recipient. Use of lymphocyte depleting therapies such as antithymocyte globulin in R+ patients is also associated with a significant risk of CMV disease secondary to CMV reactivation in these individuals (2). In transplant recipients, CMV infection is associated with organ rejection, decreased graft survival and decreased immune function resulting in comorbid infections (3). As such, prevention of CMV infection in transplant patients continues to be a top priority.
Intravenous ganciclovir (IV GCV) 5 mg/kg/day and oral ganciclovir (GCV) 1000 mg three times daily have been shown to prevent CMV in high risk individuals (D+/R−) and in R+ organ transplant recipients receiving lymphocyte depleting agents (4). Valganciclovir (VGCV), a prodrug of GCV with improved bioavailability and convenient once daily dosing, was approved in 2001 for the treatment of CMV retinitis in patients infected with the human immunodeficiency virus (HIV) and in 2003 for the prevention of CMV in kidney, heart and pancreas transplant recipients based on the results of the PV 16 000 Study, which showed VGCV 900 mg daily is as effective as oral GCV (5,6). Several studies have reported similar efficacy between low dose VGCV (450 mg/day) and oral GCV 1000 mg three times daily (7–9).
The purpose of this crossover pharmacokinetic study was to characterize GCV disposition in kidney transplant recipients receiving different dose formulations of the drug. Despite the seeming effectiveness of VGCV 450 mg daily for CMV prevention, recent guidelines recommend giving the drug at 900 mg daily for prevention of CMV in solid organ transplant recipients (10,11). Unfortunately, pharmacokinetic studies comparing different GCV regimens have not been conducted in kidney transplant recipients. Comparative pharmacokinetic data are necessary to assess the appropriateness of the various VGCV and GCV regimens currently in use for CMV treatment and prophylaxis in solid organ transplant recipients.
Methods
Patients
Eleven patients undergoing a kidney transplant and requiring CMV prophylaxis were enrolled after informed consent for study participation from August 2002 through January 2005. The trial was approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Institutional Review Board. Patients were excluded if they were: less than 18 years old, neutropenic, thrombocytopenic, severely anemic (hgb < 8 mg/dL) or experiencing persistent diarrhea. All patients were CMV antigenemia negative at the time of enrollment and were screened for CMV at 1, 6 and 12 months posttransplant as part of the kidney transplant protocol. Patients were eligible to begin the treatment protocol when their estimated glomerular filtration rate was 60 mL/min or greater as calculated by the urine 24-h creatinine clearance (CrCl) or the Modification of Diet in Renal Disease 4 variable equation (MDRD 4) (12). with individual body surface area.
Protocol therapy
The study was an open label four-phase crossover, pharmacokinetic study. Patients not able to participate in all four phases, could be enrolled for a two-phase comparison (IV GCV vs. VGCV 900 mg or oral GCV vs. VGCV 450 mg). Patients received depletional induction therapy with rabbit antithymocyte globulin at a mean total dose of 10.5 mg/kg ± 0.72 mg/kg or alemtuzumab at a total dose of 60 mg (with daily i.v. methylprednisolone for 2 to 6 days), and were at least 7 days post-renal transplant with a calculated GFR ≥ 60 mL/min. The four study phases consisted of IV GCV 2.5 mg/kg every 12 h (Phase I), VGCV 900 mg daily (Phase II), oral VGCV 450 mg daily (Phase III), oral GCV 1000 mg (PO GCV) every 8 h (Phase IV). Individuals with a CMV status of D+/R− also received cytomegalovirus immune globulin. Seven evaluable patients were used for each comparison. Pharmacokinetic samples were collected under steady-state conditions (after 2–4 days), after the morning dose of medication, in patients with stable renal function (at least two consecutive serum creatinines no more than 2 days apart within 0.3 mg/dL) and an estimated GFR of 60 mL/min or greater for all treatments. If GFR was less than 60 mL/min, pharmacokinetic sampling was delayed until GFR increased beyond this point. Patients had a 24-h urine collection for creatinine clearance determination on day 7 per transplant protocol. PO GCV and VGCV doses were administered with a standard low fat breakfast (30% fat). IV GCV at 2.5 mg/kg every 12 h adjusted for renal function was administered for one dose preoperatively and continued postoperatively. Patients receiving full dose IV GCV (2.5 mg/kg every 12 h) for at least 2 days and at least 7 days post-surgery with an estimated glomerular filtration rate (GFR) of 60 mL/min or greater (using MDRD 4 variable equation) were eligible to undergo pharmacokinetic sampling. After insertion of a cannula into a peripheral vein, 3 mL blood samples were drawn immediately before the intravenous dose and then at 0.25, 0.5, 1, 1.5, 2, 4, 6, 8 and 12 h after the start of the 1-h ganciclovir infusion. Patients received their last dose of IV GCV after the 12-h blood sample. The following day, patients began VGCV 900 mg daily with food for 7 to 21 days. After at least 4 doses of 900 mg of oral VGCV (between day 4 and day 21 after starting valganciclovir) patients underwent pharmacokinetic sampling. VGCV 900 mg was given immediately following a standardized breakfast and serial blood samples were collected at 0, 0.5, 1, 1.5, 2, 4, 6, 8, 12 and 24 h after the dose. After receiving 7 to 21days of VGCV 900 mg daily, the dose was reduced to VGCV 450 mg daily with food. After at least 4 doses of VGCV 450 mg serial blood samples were collected as described for the 900 mg dose. The following day patients were instructed to stop VGCV and begin taking PO GCV 1000 mg every 8 h with food. After patients had received oral GCV for at least 4 days, they underwent pharmacokinetic sampling. PO GCV was given immediately following a standardized breakfast and serial blood samples (3 mL) were collected at 0, 0.5, 1, 1.5, 2, 4, 6, 8 h after the dose. The patient took the remaining two doses of PO GCV as scheduled after the 8-h blood sample was drawn; the following day patients resumed taking VGCV 450 mg daily.
Pharmacokinetic parameters
Ganciclovir concentrations were determined using a newly developed high performance liquid chromatography method. The bioanalytical assay was performed using a precipitation extraction method with 25.0 M perchloric acid. The HPLC system consisted of a Waters 2795 Alliance HT separations module, and a 2996 photodiode array detector set at λ = 254 nm (Waters Corp., Milford, MA). The HPLC system and the assay parameters were controlled using the Empower (Version 5.0) chromatography manager software (Waters Corp., Milford, MA). Ganciclovir and 7-methyl xanthine internal standard were isolated from human plasma using precipitation extraction with 25.0M perchloric acid. Briefly, a plasma sample of 300 μL was placed in a 1.5-mL eppendorf microcentrifuge tube followed by the addition of 30 μL 7-methyl xanthine internal standard solution (20.0 μg/mL). The sample was vortexed for 5–10 s, and immediately thereafter 10 μL of 25.0M perchloric acid was added to the sample. The samples were subsequently vortexed for 30–35 s and centrifuged at 7200 rpm for 10 min. The supernate was transferred to a clean 13 × 100 mm test tube and evaporated to dryness using a Zymark TurboVap for 45 min at 40°C. The samples were reconstituted with 110 μL of mobile phase and transferred into HPLC vials. Subsequently, 80.0 μL was injected onto an Xterra™ MSC18, 5 μm, 4.6 × 150 mm reverse-phase analytical column (Waters Corp.) and eluted isocratically at 1.2 mL/min. (T = 34°) for 15 min using a mobile phase consisting of (48:52, v/v) acetonitrile and 25 mM K2HPO4 buffer.
Calibration curves were linear from 0.050 μg/mL to 15.0 μg/mL (R2 > 0.998). Percentage of errors, as a measure of accuracy, were < 15%, and the inter-and intra-assay coefficients of variation were 2.10–7.91% and 3.43–9.03%, respectively, at four different drug concentrations. The limit of quantitation was 0.050 μg/mL and the limit of detection was 0.030 μg/mL. During the validation, short-term stability of the drug in plasma and repeated freezing and thawing of plasma were evaluated. The overall recovery of ganciclovir and 7-methyl xanthine (IS) using the precipitation extraction method was > 95%.
Ganciclovir pharmacokinetic parameters were determined using noncompartmental methods with the WinNonlin Professional(tm) computer program (version 5.0, Pharsight Corporation, Mountain View, CA). Maximal serum concentrations (Cmax) and time to reach Cmax (Tmax) were determined by visual inspection of the concentration-time profiles. The elimination rate constant (λZ) was estimated as the absolute value of the slope of a linear regression of natural logarithm of concentration versus time. Half life was calculated as ln2/λZ. Values for area under the concentration versus time curve from 0 h to the last quantifiable concentration (AUC0–last) were determined by the linear trapezoidal rule. To allow for AUC0–24 comparisons between the study groups, IV GCV AUC0–12 values and oral GCV AUC0–8 values were multiplied by 2 and 3 respectively to yield predicted values for AUC0–24. Apparent oral clearance (CL/F) was estimated as dose/AUC. Absolute bioavailability was calculated using AUC0–∞ and the actual IV GCV, VGCV 900 mg and PO GCV 1000 mg doses (AUCpo *IV dose/AUCiv *PO dose) for patients undergoing IV GCV pharmacokinetic testing.
Statistics
Sample size calculations were based on a previously published cross-over study that compared GCV pharmacokinetics in liver transplant patients who received GCV and VGCV in separate phases (13). The study was designed as a one-sided equivalence study to test noninferiority of VGCV 900 mg relative to IV GCV, and VGCV 450 mg relative to oral GCV. Intra- and interpatient coefficients of variation (CVs) of approximately 8.8% and 21% respectively, for AUC0–24 were seen for ganciclovir in HIV-infected patients (13). Interpatient CVs for oral GCV and 900 mg of VGCV have been reported to be 22% and 24% respectively in liver transplant patients (13). Thus, an intrapatient CV of 15% and interpatient CV of 24% were used to calculate the sample size. An acceptable bioequivalence range for AUC0–24 is between 80% and 125%, a typical range for this type of study. Assuming a one-sided test at the 0.05 level and requiring 90% power, the required sample size, using nQuery Advisor was estimated to be seven patients. To account for any nonevaluable subjects, 11 patients were included in the study. Patients not able to complete all four phases of the study could be included in the analysis if they completed one of the two-phase comparisons (either phase I and II or phase III and IV).
AUC0–24 and Cmin values were compared between study phases using an exact version of the nonparametric Wilcoxon signed-rank test for paired data. Cmin values were also dichotomized into success and failure categories (with a cut off value of 0.6 μg/mL) and compared with the exact McNemar test for such paired data. The cut off of 0.6 μg/mL was chosen because this is the trough GCV concentration that is believed necessary to provide adequate prophylaxis against CMV infection (14). Summary statistics are reported as nonparametric medians and ranges. All p-values are two sided, unless explicitly noted otherwise, with p < 0.05 considered statistically significant.
Results
Eleven patients were enrolled into the study. One patient withdrew after IV GCV testing due to a postoperative complication and one patient completed phases I through III but was unable to complete phase IV due to a rise in serum creatinine. Patient demographics are listed in Table 1. Maintenance immunosuppression did not include oral corticosteroids.
Table 1.
Patient demographics
| IV GCV vs. VGCV 900 | PO GCV vs. VGCV 450 mg | |
|---|---|---|
| Age | 39 (29–54) | 39 (28–64) |
| Gender (male/female) | ||
| Male | 4 | 4 |
| Female | 3 | 3 |
| Estimated GFR (mL/min) | Phase I: 66 (61–107) | Phase III: 64 (61–73) |
| Phase II: 62 (60–68) | Phase IV: 66 (56–102) | |
| Race | ||
| White | 3 | 3 |
| Black | 4 | 4 |
| CMV status | ||
| D−/R− | 2 | 2 |
| D+/R− | 2 | 2 |
| D+/R+ | 1 | 1 |
| D−/R+ | 2 | 2 |
| Induction therapy | ||
| Rabbit ATG | 5 | 5 |
| Alemtuzumab | 2 | 2 |
| Immunosuppresion | ||
| Sirolimus/tacrolimus | 5 | 5 |
| Tacrolimus | 2 | 2 |
Median with ranges in parentheses.
GFR = glomerular filtration rate.
Four patients completed all four study phases, although one patient’s data from Phase II was excluded from the final analysis when it was discovered that the patient had not taken their VGCV 900 mg dose on the day of pharmacokinetic analysis. Six patients completed at least two study phases (phases I and II or phases III and IV). Median GCV pharmacokinetic parameters with ranges in parentheses are shown in Table 2. Ganciclovir systemic exposure (AUC0–24) for each dosing regimen and for each patient is shown in Figure 1.
Table 2.
Median pharmacokinetic parameters of GCV following dosing with IV Ganciclovir (GCV), Valganciclovir (VGCV) and oral Ganciclovir (GCV)
| Parameter | IV GCV * n = 6 | VGCV 900 mg * n = 6 | VGCV 450 mg n = 7 | Oral GCV N = 7 |
|---|---|---|---|---|
| AUC0–24 (μg/h/mL) | 60.631 (47.24–85.35) | 62.861 (47.273–77.413) | 35.896 (27.986–43.386) | 29.041 (15.18–51.30) |
| Cmax (μg/mL) | 6.824 (6.465–7.768) | 7.972 (3.814–13.798) | 4.146 (3.063–4.678) | 1.435 (0.788–2.614) |
| Cmin (μg/mL) | 0.864 (0.641–2.024) | 0.545 (0.322–1.003) | 0.255 (0.171–0.804) | 0.759 (0.385–1.528) |
| Tmax (h) | 1 (1–1.5) | 1.75 (1–2) | 2 (1–6) | 2 (1.5–4) |
| F (%) | 34 | 6 |
Medians with range in parentheses.
One patient was excluded from the analysis when it was discovered that they did not take their VGCV dose on the morning of pharmacokinetic sampling. The missing patient’s measured values for AUC0–24, Cmax, Cmin, Tmax, were 128.519 μg/h/ml, 11.623 μg/mL, 2.902 μg/mL, 1 h respectively for IV GCV and 12.635 μg/h/mL, 1.701 μg/mL, 0.147 μg/mL, 0 h, 8.1 h, respectively, for VGCV 900 mg.
Figure 1. Ganciclovir systemic exposure (AUC 0–24) after administration of IV ganciclovir, valganciclovir 900 mg daily, valganciclovir 450 mg daily and PO ganciclovir 1000 mg every 8 h.
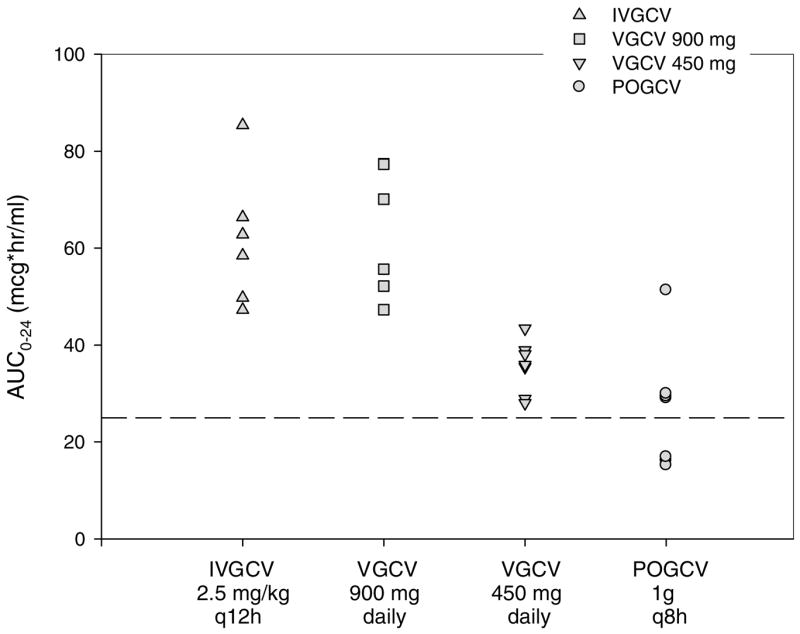
Broken line represents the mean ganciclovir systemic exposure achieved with PO ganciclovir in previous studies.
GCV maximum concentrations and AUC0–24 following oral VGCV 900 mg were nearly twice the values following a 450 mg dose. The rate of absorption following oral GCV was similar to VGCV, however, the median maximum concentration attained was only 1.435 μg/mL. The GCV AUC0–24 was not statistically different between oral GCV and VGCV 450 mg (p = 0.11) and values were slightly less in the oral GCV in six of seven patients. One patient experienced greater exposure with oral GCV as a result of a temporary decline in estimated GFR to 56 mL/min. Minimum concentrations of GCV following VGCV 450 mg were only 50% of the minimum concentrations obtained with oral GCV and tended to be below 0.6 μg/mL. AUC0–24 values were similar between our IV GCV dose and VGCV 900 mg daily (p =0.84). However, minimum concentrations (Cmin) were lower in the VGCV group (p = 0.032) compared to IV GCV. This can be attributed to the IV GCV dosing of 2.5 mg/kg every 12 h used in this study as opposed to 5 mg/kg once daily.
All treatments were generally well tolerated. Those side effects that were reported were expected and consistent with ganciclovir administration. The most common side effects were anemia, leukopenia, diarrhea, fatigue and insomnia. Leukopenia and anemia occurred most often in the IV GCV and oral VGCV 900 mg daily phases. Three patients became neutropenic during the study (ANC < 1000 cells mm3), during which time each was receiving VGCV 900 mg daily.
Discussion
Our study demonstrates that oral VGCV 900 mg daily provides similar systemic ganciclovir exposure to IV GCV 2.5 mg/kg every 12 h and that VGCV 450 mg daily provides comparable exposure to PO GCV 1000 mg Q8 H in kidney transplant recipients. GCV exposure for IV GCV and PO GCV found in this study is higher compared to 22 μg/h/mL and 15.40 μg/h/mL respectively in HIV positive/CMV positive adults as previously reported (15). Our results for VGCV 900 mg confirm previous results in solid organ transplant recipients (5).
The FDA approval of VGCV 900 mg for CMV prophylaxis in solid organ transplant recipients was based on findings reported by Paya and colleagues where 364 patients received VGCV 900 mg daily or PO GCV 1000 mg TID for 100 days in a randomized, prospective, double-blind, double dummy study (PV 16 000 study) (5,16). The range of GCV AUC0–24 results at steady state following VGCV 900 mg in our patients was comparable to patients in the PV 16 000 study (mean AUC0–24 48.2 μg/h/mL) (5) and as reported by Pescovitz et al. in a cohort of liver transplant recipients (41.7 μg/h/mL) who received a single dose of the drug, thus confirming their results (13). The increase in GCV exposure demonstrated in only some of our patients may be due to the type of organ transplant, age and days posttransplant. Although patients in our study had a creatinine clearance of 60 mL/min or greater, it is still possible that their renal function was inferior compared to the PV 16 000 study and liver transplant patients. The fact that we measured steady state GCV concentrations and avoided a washout period may also have contributed to the increased exposure observed in some patients compared to a single-dose investigation in liver transplant patients (13). Of note, the degree of GCV exposure we observed with VGCV 900 mg was within the realm demonstrated to prevent CMV viremia: an AUC0–24 of 40–60 μg/h/mL reported by Wiltshire and colleagues (17).
Previous studies measured GCV levels following VGCV 900 mg at 1 month or greater posttransplant or following a single dose (5,13). We were interested in the extent of VGCV absorption early posttransplant since medication absorption may be erratic following surgery. Our patients were sampled at 11 to 28 days posttransplant and achieved adequate GCV exposure; thereby confirming that the drug is well absorbed in this early transplant period. Bioavailablity of VGCV measured lower than what was previously reported in healthy volunteers and HIV-positive/CMV-positive adults (34% vs. 60%) and may reflect our patient population and the administration with a low fat meal (18). Most likely a reduction in the clearance of ganciclovir accounts for the AUC0–24 results in our study despite the lower bioavailability.
GCV prophylaxis is often given to CMV positive recipients receiving depletional induction therapy, and to patients at high risk for CMV infection (CMV D+/R−) for several months after transplant (19,20). We were interested in the results of a single dose study in liver transplant recipients in which similar GCV exposure between VGCV 450 mg and oral GCV 1000 mg three times daily was reported, with a mean AUC0–24 of 21 and 20.7 μg/h/mL, respectively (13). We chose to study this lower dose of VGCV (450 mg daily) in kidney transplant recipients to demonstrate noninferiority in GCV exposure compared with GCV 1000 mg Q8 H, which was standard of care during the study period. Others have shown PO GCV 1000 mg Q8 H to be efficacious in preventing CMV infection and disease and this dose has yielded a mean GCV exposure of 25 to 28 μg/h/mL (4,5, 21). After repeated dosing, we found GCV exposure to be similar between VGCV 450 mg and oral GCV (AUC0–24 35.896 μg/h/mL vs. 29.041 μg/h/mL respectively) and within the level of exposure previously reported for PO GCV in the PV 16 000 Study. Our results showed a higher exposure in six of seven patients with VGCV 450 mg, compared to PO GCV 1000 mg Q8 H. However this study was not powered to show superiority; hence it cannot be assumed that VGCV 450 mg will routinely produce higher GCV exposures compared to the exposures achieved with PO GCV. Although we found ganciclovir exposure to be similar and above 25 μg/h/mL, Wiltshire et al. has reported that an AUC0–24 of 40–50 μg/h/mL suppressed CMV viremia in CMV D+/R− recipients during prophylaxis with PO GCV or VGCV 900 mg daily (17). However, this level of exposure may not be necessary in all organ transplant recipients since others have demonstrated efficacy with VGCV 450 mg daily (8,9,22). In addition, the incidence of CMV viremia and disease was similar in both PO GCV and VGCV 900 mg at 6 and 12 months posttransplant despite the higher exposure with the VGCV 900 mg group (17).
A minimum GCV trough concentration in plasma necessary to prevent CMV infection has not been identified in organ transplant recipients. However, it is unclear whether or not GCV trough concentrations are associated with the drug’s prophylactic efficacy since the intracellular triphosphate form of the drug (not the parent compound in plasma) is pharmacologically active. Trough levels of ganciclovir in the range of 0.25 μg/mL to 2.75 μg/mL have been reported to inhibit most CMV isolates in vitro (23). In a small study of AIDS patients receiving oral GCV for CMV retinintis, trough plasma concentrations of ganciclovir below 0.6 μg/mL demonstrated a trend toward a higher risk for progression of CMV (14). To our knowledge, our study is the first to analyze trough GCV levels following oral VGCV in adult kidney transplant recipients. In our study, the median trough concentration for VGCV 450 mg was below 0.6 μg/mL (median: 0.255 μg/mL, SD 0.231 μg/mL), which was statistically different compared with oral GCV (0.759 μg/mL, SD 0.390 μg/mL, p = 0.016); however, most trough concentrations remained above 0.25 μg/mL. If trough concentrations greater than 0.6 μg/mL are necessary to achieve adequate CMV prophylaxis, it may be necessary during periods of high immunosuppression such as during transplant induction or rejection treatment, to use IV GCV or VGCV 900 mg (adjusted for renal function) to maintain higher trough levels especially in high-risk patients.
Several limitations are present in this study. The study was not randomized, the sample size was small and a washout period was not included. Interruption of CMV prophylaxis for a washout period could increase the risk of infection and since ganciclovir has a short half-life, a majority of the dose would be cleared and have minimal impact on our results. In addition, this study was not designed to address issues such as efficacy, CMV resistance and long-term safety. Future longitudinal prospective studies are necessary to address these issues with VGC 450 mg daily dosing since most efficacy studies have been retrospective (7,8,24).
Our study was conducted entirely in kidney transplant recipients, thus it is unclear to what degree, if any, and our data can be generalized to other transplant recipients. Indeed GCV drug exposure and minimum concentration needed to limit GCV resistance may differ depending on the transplanted organ and its associated CMV risk. Kidney transplant recipients historically have a lower CrCl than other transplanted organs and because of the significant renal clearance of GCV dosage forms, they will therefore have higher GCV concentrations. Accordingly, clinicians must be vigilant with regard to making GCV dose adjustments based on changes in renal function but are limited to available dosage forms.
In summary, we found that oral VGCV 900 mg daily provided similar systemic exposure to IV GCV and exceeded exposure achieved with PO GCV, confirming results of the PV 16 000 Study in kidney transplant recipients (5). We report systemic availability under steady state conditions for VGCV, which displayed excellent absorption during the early post transplant period and appears to be a suitable alternative for continued maintenance dose treatment in the outpatient setting. Moreover, we found similar GCV exposures between VGCV 450 mg daily and oral GCV 1000 mg Q8 h under steady state conditions confirming findings from a single dose in liver transplant recipients (13). This is the first report of trough values, which were lower with VGCV 450 mg compared to oral GCV, and perhaps the higher dose of VGCV (equivalent of 900 mg daily) should be used during severe immunosuppression such as with depletional induction therapy and rejection therapy to minimize CMV replication and resistance in this more vulnerable population. Kidney transplant recipients achieve higher GCV concentrations compared to other organ recipients, thus necessitating constant GCV dosage adjustments based on renal function. Further study on renal dose adjustments for the VGCV 450 mg dose is needed to limit bone marrow toxicity especially with extended prophylaxis treatment.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, Clinical Center Pharmacy Department and NIDDK, Transplantation Branch. There was no commercial sponsorship.
The authors gratefully acknowledge the nurses and transplant coordinators of the Organ and Tissue Transplant Research Center of the Warren G. Magnuson Clinical Center for their excellent patient care skills, Eric Elster, MD, John Swanson, MD, and Y. Hon for their assistance with this research.
References
- 1.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RB, Paya C, Pescovitz MD, et al. Risk factors for cytomegalovirus viremia and disease developing after prophylaxis in high-risk solid-organ transplant recipients. Transplantation. 2004;78:1765–1773. doi: 10.1097/01.tp.0000142619.01510.a5. [DOI] [PubMed] [Google Scholar]
- 3.Rubin RH. Importance of CMV in the transplant population. Transpl Infect Dis. 1999;1(1 Suppl):S3–S7. [PubMed] [Google Scholar]
- 4.Gane E, Saliba F, Valdecasas GJ, et al. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The oral ganciclovir international transplantation study group [corrected] Lancet. 1997;350:1729–1733. doi: 10.1016/s0140-6736(97)05535-9. [DOI] [PubMed] [Google Scholar]
- 5.Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346:1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 7.Akalin E, Sehgal V, Ames S, et al. Cytomegalovirus disease in high-risk transplant recipients despite ganciclovir or valganciclovir prophylaxis. Am J Transplant. 2003;3:731–735. doi: 10.1034/j.1600-6143.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 8.Gabardi S, Magee CC, Baroletti SA, Powelson JA, Cina JL, Chandraker AK. Efficacy and safety of low-dose valganciclovir for prevention of cytomegalovirus disease in renal transplant recipients: A single-center, retrospective analysis. Pharmacotherapy. 2004;24:1323–1330. doi: 10.1592/phco.24.14.1323.43152. [DOI] [PubMed] [Google Scholar]
- 9.Weng FL, Patel AM, Wanchoo R, et al. Oral ganciclovir versus low-dose valganciclovir for prevention of cytomegalovirus disease in recipients of kidney and pancreas transplants. Transplantation. 2007;83:290–296. doi: 10.1097/01.tp.0000251371.34968.ca. [DOI] [PubMed] [Google Scholar]
- 10.Preiksaitis JK, Brennan DC, Fishman J, Allen U. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218–227. doi: 10.1111/j.1600-6143.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 11.Cytomegalovirus. Am J Transplant. 2004;4(10 Suppl):S51–S58. [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Pescovitz MD, Rabkin J, Merion RM, et al. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000;44:2811–2815. doi: 10.1128/aac.44.10.2811-2815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piketty C, Bardin C, Gilquin J, Gairard A, Kazatchkine MD, Chast F. Monitoring plasma levels of ganciclovir in AIDS patients receiving oral ganciclovir as maintenance therapy for CMV retinitis. Clin Microbiol Infect. 2000;6:117–120. doi: 10.1046/j.1469-0691.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RD, Griffy KG, Jung D, Dorr A, Hulse JD, Smith RB. Ganciclovir absolute bioavailability and steady-state pharmacokinetics after oral administration of two 3000-mg/d dosing regimens in human immunodeficiency virus- and cytomegalovirus-seropositive patients. Clin Ther. 1995;17:425–432. doi: 10.1016/0149-2918(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 16.Wiltshire H, Hirankarn S, Farrell C, et al. Pharmacokinetic profile of ganciclovir after its oral administration and from its prodrug, valganciclovir, in solid organ transplant recipients. Clin Pharmacokinet. 2005;44:495–507. doi: 10.2165/00003088-200544050-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wiltshire H, Paya CV, Pescovitz MD, et al. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation. 2005;79:1477–1483. doi: 10.1097/01.tp.0000164512.99703.ad. [DOI] [PubMed] [Google Scholar]
- 18.Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39:800–804. doi: 10.1177/00912709922008452. [DOI] [PubMed] [Google Scholar]
- 19.Said T, Nampoory MR, Johny KV, et al. Cytomegalovirus prophylaxis with ganciclovir in kidney transplant recipients receiving induction antilymphocyte antibodies. Transplant Proc. 2004;36:1847–1849. doi: 10.1016/j.transproceed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Taber DJ, Ashcraft E, Baillie GM, et al. Valganciclovir prophylaxis in patients at high risk for the development of cytomegalovirus disease. Transpl Infect Dis. 2004;6:101–109. doi: 10.1111/j.1399-3062.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 21.Pescovitz MD, Pruett TL, Gonwa T, et al. Oral ganciclovir dosing in transplant recipients and dialysis patients based on renal function. Transplantation. 1998;66:1104–1107. doi: 10.1097/00007890-199810270-00023. [DOI] [PubMed] [Google Scholar]
- 22.Akalin E, Bromberg JS, Sehgal V, Ames S, Murphy B. Decreased incidence of cytomegalovirus infection in thymoglobulin-treated transplant patients with 6 months of valganciclovir prophylaxis. Am J Transplant. 2004;4:148–149. doi: 10.1046/j.1600-6143.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 23.Plotkin SA, Drew WL, Felsenstein D, Hirsch MS. Sensitivity of clinical isolates of human cytomegalovirus to 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Infect Dis. 1985;152:833–834. doi: 10.1093/infdis/152.4.833. [DOI] [PubMed] [Google Scholar]
- 24.Gruber SA, Garnick J, Morawski K, et al. Cytomegalovirus prophylaxis with valganciclovir in African-American renal allograft recipients based on donor/recipient serostatus. Clin Transplant. 2005;19:273–278. doi: 10.1111/j.1399-0012.2005.00337.x. [DOI] [PubMed] [Google Scholar]


