Abstract
Xanthorhodopsin is a light-driven proton pump like bacteriorhodopsin, but made more effective for collecting light by its second chromophore, salinixanthin, a carotenoid. Action spectra for transport and fluorescence of the retinal upon excitation of the carotenoid indicate that the carotenoid functions as an antenna to the retinal. The calculated center-to-center distance and angle of the transition moments of the two chromophores are 11 Å and 56°, respectively. As expected from their proximity, the carotenoid and the retinal closely interact: tight binding of the carotenoid, as indicated by its sharpened vibration bands and intense induced circular dichroism in the visible, is removed by hydrolysis of the retinal Schiff base, and restored upon reconstitution with retinal. This antenna system, simpler than photosynthetic complexes, is well-suited to study features of excited-state energy migration.
Keywords: xanthorhodopsin, retinal protein, antenna carotenoid, energy transfer, fluorescence anisotropy
1. Introduction
Sunlight is the major source of energy in biology, and evolution has produced elaborate systems to efficiently collect it. In the photosynthetic complexes of plants and bacteria, a large number of chlorophyll and carotenoid molecules absorb the incident light as antennae, and funnel the resulting excited-state energy to the reaction centers where the photochemistry takes place. Nearly forty years ago, a second, retinal-based, light-energy conversion system was discovered in the haloarchaea [1]. As a light-driven proton pump, bacteriorhodopsin [2–5] produces transmembrane proton-motive force to be used for ATP synthesis, as photosynthetic centers, but it is a far simpler system, a small heptahelical membrane protein with a single retinal as chromophore. Such retinal-based proton pumps are now known to be wide-spread among eubacteria, fungi, and even eukaryotes [6]. Is there a need, and even room, for an antenna to assist the retinal in such simple proteins?
Action spectra have hinted at the possibility of an antenna to retinal in photoreceptors. Sensitivity of fly rhodopsin to UV light is enhanced by retinol [7, 8], and a porphyrin seems to enhance the photoresponse of a deep-sea fish rhodopsin [9–11]. Energy transfer from carotenoids or flavins to a retinal-based photoreceptor was suggested to account for the action spectrum for phototaxis in the green alga H. pluvialis [12]. The properties of the recently discovered xanthorhodopsin [13, 14] demonstrate that carotenoid to retinal excited-state energy transfer is indeed possible. It is a well-defined retinal/carotenoid protein with 1:1 chromophore stoichiometry, and a light-driven proton pump with substantial sequence homology to bacteriorhodopsin and proteorhodopsin. Xanthorhodopsin is found in the cell membrane of Salinibacter ruber, a halophilic eubacterium [15, 16], which is one of the few non-archaeal retinal-proteins available for study in its native host organism. This brief review will summarize the evidence for excited-state energy transfer in this protein, the nature of the antenna binding site, the interaction between the carotenoid and the retinal, and structural considerations in accomodating the second chromophore inside the protein. The latter is aided by a high-resolution crystallographic model that we very recently been able to refine from x-ray diffraction.
2. Homology with other bacterial rhodopsins
The S. ruber genome contains open reading frames that appear to code for three categories of type II rhodopsins [17, 18]: a proton pump [13], a chloride pump [19], and the two sensory rhodopsins. Transport measurements with cell membrane vesicles show evidence for a proton transporter and only traces, if any, chloride transporter, and purified membranes contain a protein identified by mass spectrometry as the product of the gene similar to those of bacterio-opsin and proteo-opsin [13]. The protein was named xanthorhodopsin to signify that it contains both carotenoid and retinal.
All residues demonstrated as essential for binding retinal and for translocating protons are present in the xanthorhodopsin gene sequence, consistent with its function as proton pump. The sequence has somewhat more homology to bacterio-opsin (23%) than to proteo-opsin (19%), but the constellation of the functional residues is more like in proteo-opsin. Thus, there is no homologue for Glu-204 of bacteriorhodopsin, suggesting that proton release on the extracellular side during or immediately after deprotonation of the Schiff base in the photocycle is not a feature of the photocycle, as in proteorhodopsin. Experiments with pyranine as pH indicator of transient changes during the photocycle show this to be so. The homologue of the proton donor Asp-96 to the Schiff base in bacteriorhodopsin is a glutamic acid, also as in proteorhodopsin. There are few clues in the primary structure concerning the location of the large carotenoid molecule.
3. Absorption spectra, action spectra
The absorption spectrum of S. ruber membranes is dominated by the bands of salinixanthin [20] at 458, 486 and 521 nm. After purification that removes nearly all other proteins and most of non-bond carotenoid, the salinixanthin bands are seen to be sharp peaks and the retinal chromophore appears as a distinct shoulder with an estimated maximum at ca. 560 nm (Fig. 1A). The separation of vibronic states in the highly structured carotenoid band of the complex contrasts with the spectrum of the same carotenoid in organic solvents and when not bound to salinixanthin, where motion of the keto ring relative to the polyene chain averages its various states. Although the extinctions of neither carotenoid nor retinal in salinixanthin are known exactly, the former must be near 130,000 M−1 cm−1 [20] and the latter 60,000 M−1.cm−1 (as in bacteriorhodopsin). Thus, the stoichiometry of the two chromophores is 1:1. Because this protein is homologous to bacteriorhodopsin (see below), this means that it contains one retinal and one salinixanthin [13, 21].
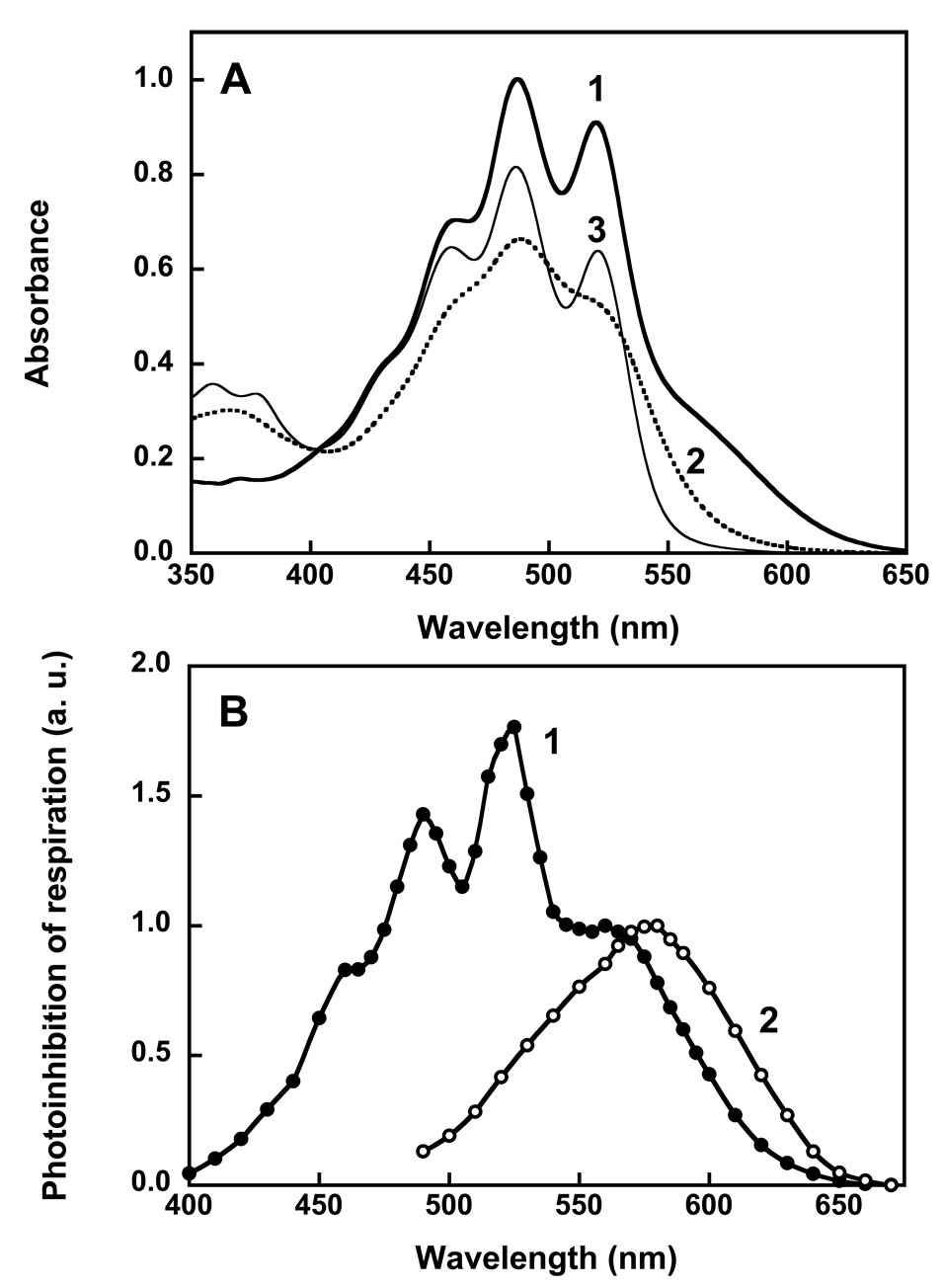
Fig. 1.
Absorption spectra (A) and action spectra (B) of xanthorhodopsin. In A: spectrum 1, xanthorhodopsin; spectrum 2, after hydrolysis of the retinal Schiff base with hydroxylamine; spectrum 3, after borohydride reduction of the retinal Schiff base. Hydroxylamine releases the retinal and that decreases the resolution of the vibronic bands of the carotenoid, while borohydride leaves the reduced retinal in its binding site and does not significantly alter the carotenoid spectrum. In B: spectra 1 and 2, action spectra for photoinhibition of respiration by S. ruber and Archaebacterium sp. (Halorubrum sp.) cells, respectively. After [13, 22, 25].
The protonation state of the counter-ion to the retinal Schiff base of bacterial rhodopsins affects their spectra. In bacteriorhodopsin and proteorhodopsin the maximum shifts from 565 to 605 nm, and 520 to 543 nm at acid pH, with pKa values of 2.5 and ca. 7, respectively. In xanthorhodopsin this shift is only a few nm, and could be identified as the consequence of counter-ion protonation only indirectly, using the fact that the Schiff base will not deprotonate in the photocycle (producing the M intermediate) when its proton acceptor is unavailable because it is already protonated [21]. Its pKa is ca. 6.
The dependence of proton transport on the wavelength of actinic light, when the light-intensity is low enough to limit, will reveal the spectra of the chromophores involved in the photoreaction, and thus their identity. To this end, transport was assayed by a) light-induced pH change in cell membrane vesicles [13], and b) light-induced inhibition of respiration in whole S. ruber cells [13, 22], which originates from light-induced proton gradient back-pressure. The latter assay had been used for bacteriorhodopsin-containing H. salinarum cells [23, 24], and demonstrated at the time, as did action spectra by other methods, that retinal and not the carotenoid bacterioruberin was the chromophore that drives proton transport. Both pH change and inhibition of respiration were abolished by uncoupler, indicating that they originate from extrusion of protons from the vesicle or cell interior by the S. ruber pump. The action spectra from these measurements were complex and revealed the participation of the retinal, with a broad maximum at ca. 560 nm as expected, but more interestingly, the participation of a second chromophore, with narrow bands at 458, 486, and 521 nm (Fig. 1B). The latter corresponds to the spectrum of salinixanthin, the carotenoid whose red color is responsible for the name, “ruber.” In Archeabacteria, the action spectrum reveals no such participation of its carotenoid, bacterioruberin, in transport (Fig. 1B).
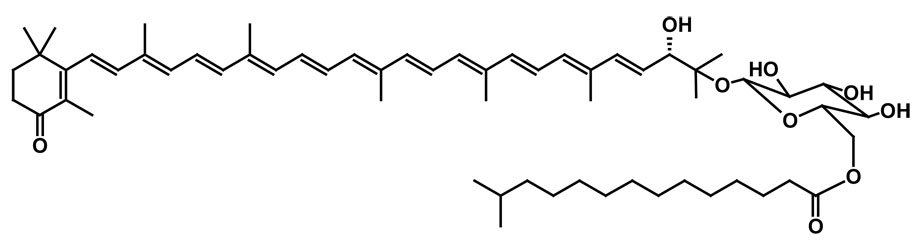
Salinixanthin is a complex carotenoid (Fig. 2), in which the C40 conjugated chain with 13 double bonds and a fatty acid chain are linked to a β-D-glycoside [20]. It bears more similarity to the carotenoids of photosynthetic complexes, e.g., rhodopin glucoside, than the simpler and symmetrical carotenoid, bacterioruberin, which confers onto the halophilic archaea their familiar red color.
Fig. 2.
Chemical structure of salinixanthin, from [20].
The sequence homology with other type II rhodopsins, and particularly those with proton pump function, as well as the photochemical cycle (see below), make it unavoidable to conclude that the configurational changes of the retinal after its photoisomerization drive proton translocation in xanthorhodopsin in a similar way as in bacteriorhodopsin. Thus, the action spectra raised the strong possibility that the carotenoid functions as antenna to the retinal. If so, comparison of the contribution of the carotenoid to the action spectra and the absorption spectra indicate that excitation energy must be transferred with high efficiency (calculated from such data as ca. 40%) between the chromophores [13]. However, the observations did not rule out the possibility that the role of salinixanthin in the transport is less direct. An unspecified light-induced change in the carotenoid might poise the protein, which transports protons otherwise much like bacteriorhodopsin or proteorhodopsin, to be more effective (by a factor of about 2, to produce the observed action spectrum) in the ion translocation. This possibility is ruled out, as there is direct evidence for the antenna function of the carotenoid, as shown below.
4. Excited-state energy transfer
Excited-state energy transfer can be probed by steady-state and ultra-rapid spectroscopy. The simplest evidence for excited-state energy migration is fluorescence of the acceptor upon excitation of the donor. Because the two xanthorhodopsin chromophores have very different absorption and fluorescence spectra, this could be tested by steady-state methods [25].
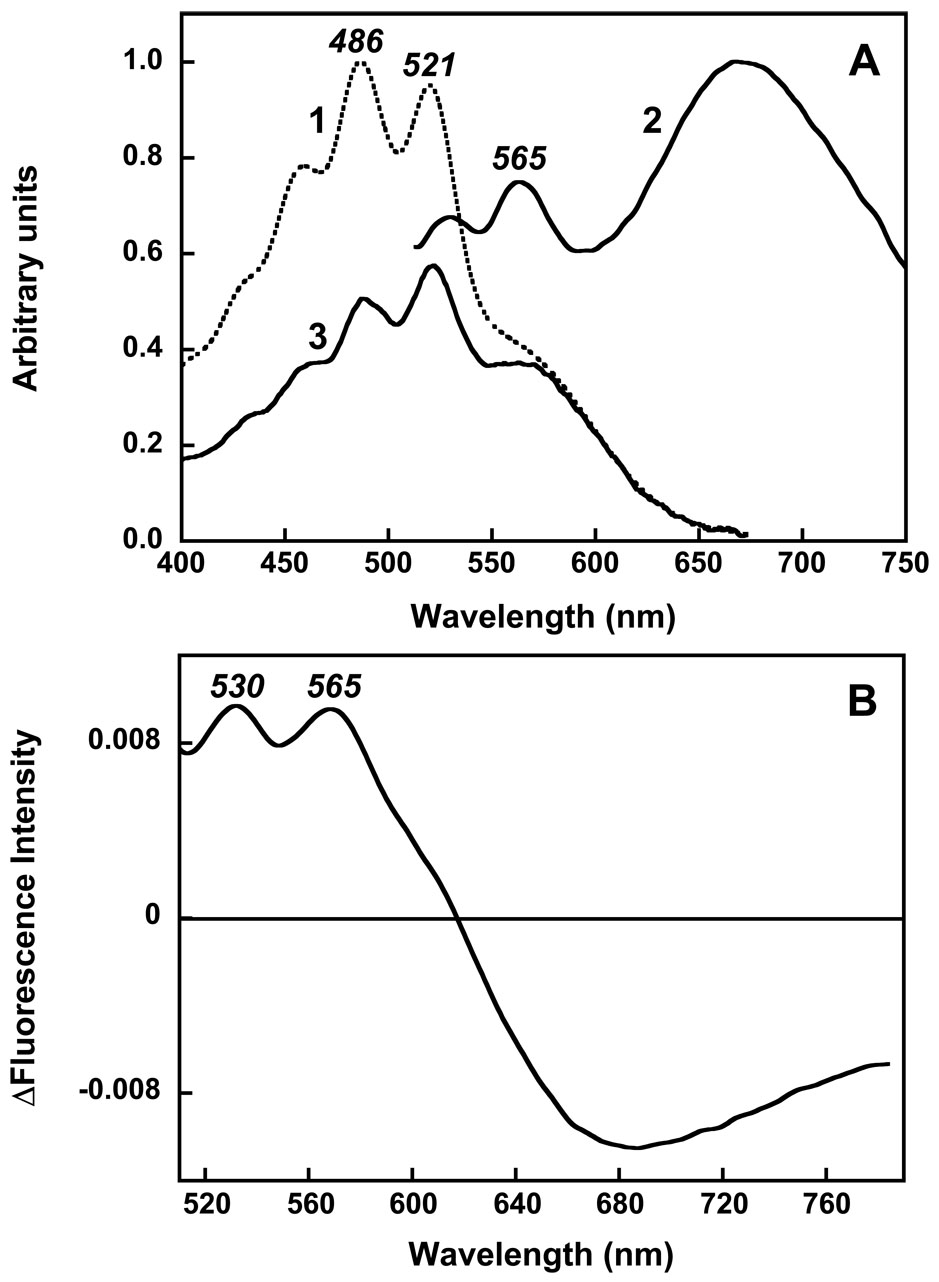
The fluorescence emission of retinal in bacteriorhodopsin, and in xanthorhodopsin also, is a broad band at ca. 680 nm, with low quantum yield (Fig. 3A). This is emission from the S1 excited state. As expected, it is fully removed by removal of the retinal upon hydrolysis of its Schiff base with hydroxylamine. The fluorescence emission of salinixanthin (bands at 529, 565 and ca. 600 nm), corresponds to the approximate mirror image of its structured absorption spectrum, with bands at 458, 486, and 521 nm. This is emission from the S2 state, and its level overlaps, ideally for energy transfer, with the absorption of the retinal chromophore. Importantly, the excitation spectrum for retinal emission shows contributions not only from retinal but also from salinixanthin absorption, demonstrating that excitation of the carotenoid results in retinal fluorescence [25]. When this excitation spectrum is measured at “magic-angle” polarization so as to remove the effect of anisotropy, the contribution of the carotenoid is ca. 45% of its contribution to the absorption spectrum. Thus, from this measurement the efficiency of energy transfer is ca. 45%, indicating that decay of the carotenoid S2 state via energy transfer to the retinal has about the same rate as its alternative decay to the lower-lying S1 carotenoid state. The implication that the S2 state is the donor of the excitation energy is supported by increase in the intensity of the fluorescence of the carotenoid when the absorption band of the retinal is shifted to 360–380 nm through reduction of the Schiff base with borohydrate (Fig. 3B), so that though it remains the binding site and keeps the carotenoid in its native conformation but cannot accept energy from the antenna. The observed 1.8–2 fold increase in intensity of the carotenoid fluorescence bands at 530 and 565 nm [25] is consistent with increase in the lifetime of S2 due to the absence of quenching through the energy transfer channel.
Fig. 3.
Fluorescence of the chromophores of xanthorhodopsin A: 1, absorption spectrum of xanthorhodopsin; 2, fluorescence emission spectrum when excited at 470 nm, on an arbitrary scale, pH 5.5; 3, excitation spectrum for emission at 700 nm. Emission from salinixanthin is a set of structured bands at 530–600 nm, emission from the retinal is a broad band at 680 nm. Energy transfer from salinixanthin to the retinal is indicated by the carotenoid bands in the excitation spectrum. B: difference in the fluorescence emission spectrum upon reduction of the retinal with borohydride, pH 8.5. The broad retinal emission band is removed, but the carotenoid emission bands have increased in amplitude because the excited state decay channel to the energy acceptor is no longer present. After [25].
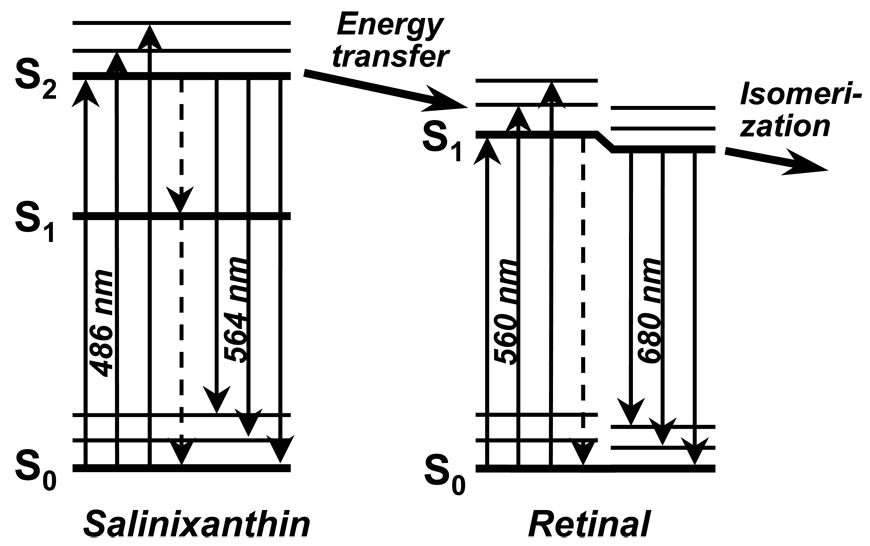
Thus, we could conclude that energy transfer occurs from the S2 level of salinixanthin to the S1 level of the retinal (Fig. 4). The low quantum yield of the salinixanthin fluorescence in xanthorhodopsin (ca 4 10−5) indicates that, as in other carotenoids of this kind, the lifetime of the S2 level is no greater than 100 fsec. The high efficiency of energy migration in spite of this short lifetime places a severe restriction on the geometry of the two chromophores.
Fig. 4.
Simplified scheme of excited-state energy transfer from carotenoid to retinal. Absorption of a photon by the carotenoid produces the shortlived S2 state, as S1 is forbidden. Energy migration to the S1 state of the retinal occurs only from S2, because the carotenoid S1 level is lower than the retinal S1. It competes with internal conversion to S1 of carotenoid (dashed vertical arrow). Decay of the all trans retinal S1 state produces 13-cis,15-anti configuration.
5. The carotenoid binding site
After the retinal of xanthorhodopsin is released from its binding site upon hydroxylamine treatment, as indicated by the appearance the negative band at ca. 560 nm of the Schiff base-linked retinal and the positive band at ca. 370 nm of the oxime produced, addition of retinal (or retinal analogues) will reconstitute the chromophore. The removal of the retinal is accompanied by specific changes in the carotenoid absorption bands. In the bleached protein the sharp vibronic bands are broadened, suggesting greater motional freedom of the carotenoid at its keto ring, but the maxima are only slightly shifted. The absorption spectrum of the carotenoid now resembles the spectrum in an organic solvent. The changes are fully reversed upon reconstitution with retinal. It appears, therefore, that the binding of the two chromophores are strongly coupled [13].
More evidence for the strong chromophore coupling is provided by circular dichroism spectra. Salinixanthin and retinal in organic solvents are only weakly optically active, but when they are bound in xanthorhodopsin there are strong bands in the visible region from both [26]. The acquired optical activity suggests that the polyenes are either forced into asymmetric configurations by the constraints of the binding sites, or they are influenced by asymmetry of the protein in the binding sites. Hydroxylamine treatment removes not only the contribution of the retinal to the CD spectrum (because it no longer absorbs in the visible), but also the contribution of the carotenoid. It appears, therefore, that when the retinal is released from its binding site, the carotenoid (or at least its keto terminus) is also released.
The fluorescence studies (see above) yielded information also on the geometric relationship of the retinal and salinixanthin [25]. The approximate center-to-center distance of the two chromophore transition moments could be calculated from the overlap integral of the absorption spectrum of the retinal chromophore and the salinixanthin contribution to the fluorescence excitation spectrum, and the fluorescence quantum yield. Assuming a simple Förster mechanism, which is not quite justified when the two polyene chains are long and close to one another, the obtained value is ca. 11 Å. If the overall structure of xanthorhodopsin is like that of bacteriorhodopsin [27], this would place the carotenoid in close proximity of the seven-helical bundle. The angle between the two transition dipole moments could be calculated from the anisotropy of fluorescence excitation spectrum. At > 560 nm, where only the retinal absorbs, the anisotropy is 0.39, which is near the theoretical value of 0.4 for emission that fully preserves the excitation polarization. This is as expected for a very short excited state lifetime where no significant motion can occur. Between 400 and 560 nm, where salinixanthin contributes to the excitation spectrum, as determined by its absorption spectrum and the efficiency of energy transfer, the total anisotropy is lower and wavelength dependent. The observed anisotropy spectrum could be well matched with a calculated spectrum from the measured parameters, with the relative angle between the donor and acceptor chromophores as the only free variable. The best fit gave an angle of 56 ± 3° between the retinal and the carotenoid. The transition dipoles should be roughly parallel with the conjugated chains, although with a deviation as much as 9° from the axis of the carbon skeleton of the carotenoid [28]. The retinal transition moment in bacteriorhodopsin is 21° inclined from the membrane plane [29], and this is likely to be so for xanthorhodopsin also. One of the two orientations allowed by the 56° angle places the carotenoid transition moment about parallel with the membrane normal (at 13°), and the other at a considerably inclined angle (at 55°). Other than the possible off-axis orientation of the transition moment, these angles should correspond roughly to the physical orientation of the polyene chain.
Bacteriorhodopsin does not bind bacterioruberin, the main carotenoid in H. salinarum, but another retinal-protein, archaerhodopsin, does [30]. In this system also, CD spectra indicate induced optical activity for the carotenoid and therefore specific binding [26, 30]. However, hydroxylamine bleaching of the retinal in this case does not perturb the absorption spectrum of the carotenoid, and nor does it remove its CD bands in the visible. Lack of strong coupling between the two chromophores is consistent with the observed lack of antenna function in archaerhodopsin [22]. In the recent crystal structure of this protein [31], the carotenoid is found at the protein periphery, at a 17 Å distance from the retinal. Its location at the contact points in the archaerhodopsin trimer suggests a structure role [31].
6. Photocycle of the retinal and the carotenoid
Transient absorption changes in the visible region upon illumination reveal a photocycle comparable to those of bacteriorhodopsin and proteorhodopsin [13]. Intermediate states with absorption shifts resembling K, L, M, and N or O, are observed to rise and decay in sequence, suggesting a similar overall mechanism for proton transport in this protein, although differences in the mechanism of proton release when compared to bacteriorhodopsin (see above) are already apparent.
There is no compelling reason to suspect that salinixanthin plays a direct role in proton translocation, but it is located near enough to the retinal to expect that it would sense the retinal transformations during the photochemical cycle. Indeed, static light minus dark difference spectra at a cryogenic temperature and time-resolved absorption measurements after flash excitation at ambient temperature both show changes not only in the retinal but also in the carotenoid. Dissection of the contribution of salinixanthin to the complex series of difference spectra that rise and decay between 100 ns and 100 ms is difficult and still underway, but preliminary conclusions can be made. Roughly coincident with the K state, the carotenoid shows a small blue-shift in its structured spectrum, suggesting an electrochromic effect on its π–system. This change relaxes later in the cycle, and is replaced in M and the later states by a broadening of the spectrum similar to that which occurs when the retinal is removed by hydroxylamine treatment. The broadening is likely to report on a conformational change in the protein that lessens the interaction of the carotenoid with the retinal.
7. Prospects for a structural description
If salinixanthin is within 11 Å of the retinal, as the fluorescence experiments indicate, the high-resolution crystallographic structure of the homologous protein, bacteriorhodopsin, by itself, provides no clues where the carotenoid might be located. Seemingly, there is no room in this small, tightly packed heptahelical membrane protein for a large molecule like salinixanthin.
However, at the time of this writing we have been able to produce well-diffracting (to 1.9 Å) xanthorhodopsin crystals by vapor-phase crystallization. The crystals are square rods, and dichroic: rotation around the long axis changes the color from deep red to yellow. The structural model from a full data-set is now in the final stages of refinement, and confirms, by and large, the predictions about the location of the carotenoid from the fluorescence experiments.
In conclusion, xanthorhodopsin appears to be a simple system that utilizes an antenna, preceding perhaps in evolution the much more complex multi-chromophore photosynthetic complexes. The gains in optical cross-section are modest [13], but must confer advantages over the other retinal-proteins that do not make use of a carotenoid. Present and future studies of the events during the short-lived excited state of carotenoid and retinal in this protein promise a simple model for energy migration between polyene chromophores.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nature (London), New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 2.Mathies RA, Lin SW, Ames JB, Pollard WT. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu.Rev. Biophys.Biophys.Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- 3.Lanyi JK. Bacteriorhodopsin. Annu.Rev.Physiol. 2004;66:665–688. doi: 10.1146/annurev.physiol.66.032102.150049. [DOI] [PubMed] [Google Scholar]
- 4.Lanyi JK, Varo G. The photocycles of bacteriorhodopsin. Isr.J.Chem. 1995;35:365–385. [Google Scholar]
- 5.Haupts U, Tittor J, Oesterhelt D. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu.Rev.Biophys.Biomol.Struct. 1999;28:367–399. doi: 10.1146/annurev.biophys.28.1.367. [DOI] [PubMed] [Google Scholar]
- 6.Spudich JL. The multitalented microbial sensory rhodopsins. Trends Microbiol. 2006;14:480–487. doi: 10.1016/j.tim.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Kirschfeld K, Franceschini N. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- 8.Stark WS, Tan KE. Ultraviolet light: photosensitivity and other effects on the visual system. Photochem.Photobiol. 1982;36:371–380. doi: 10.1111/j.1751-1097.1982.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 9.Douglas RH, Partridge JC, Dulai KS, Hunt DM, Mullineaux CW, Hynninen PH. Enhanced retinal longwave sensitivity using a chlorophyll-derived photosensitiser in Malacosteus niger, a deep-sea dragon fish with far red bioluminescence. Vision Res. 1999;39:2817–2832. doi: 10.1016/s0042-6989(98)00332-0. [DOI] [PubMed] [Google Scholar]
- 10.Isayama T, Alexeev D, Makino CL, Washington I, Nakanishi K, Turro NJ. An accessory chromophore in red vision. Nature. 2006;443:649. doi: 10.1038/443649a. [DOI] [PubMed] [Google Scholar]
- 11.Douglas RH, Partridge JC, Dulai KS, Hunt DM, Mullineaux CW, Tauber AY, Hynninen PH. Dragon fish see using chlorophyll. Nature. 1998;393:423–424. [Google Scholar]
- 12.Litvin FF, Sineshchekov OA, Sineshchekov VA. Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature. 1978;271:476–478. doi: 10.1038/271476a0. [DOI] [PubMed] [Google Scholar]
- 13.Balashov SP, Imasheva ES, Boichenko VA, Anton J, Wang JM, Lanyi JK. Xanthorhodopsin: a proton pump with a light-harvesting carotenoid antenna. Science. 2005;309:2061–2064. doi: 10.1126/science.1118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balashov SP, Lanyi JK. Xanthorhodopsin: Proton pump with a carotenoid antenna. Cell Mol.Life Sci. 2007;64:2323–2328. doi: 10.1007/s00018-007-7167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anton J, Rossello-Mora R, Rodriguez-Valera F, Amann R. Extremely halophilic bacteria in crystallizer ponds from solar salterns. Appl.Environ. Microbiol. 2000;66:3052–3057. doi: 10.1128/aem.66.7.3052-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anton J, Oren A, Benlloch S, Rodriguez-Valera F, Amann R, Rossello-Mora R. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int.J.Syst.Evol. Microbiol. 2002;52:485–491. doi: 10.1099/00207713-52-2-485. [DOI] [PubMed] [Google Scholar]
- 17.Papke RT, Douady CJ, Doolittle WF, Rodriguez-Valera F. Diversity of bacteriorhodopsins in different hypersaline waters from a single Spanish saltern. Environmental Microbiology. 2003;5:1039–1045. doi: 10.1046/j.1462-2920.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 18.Mongodin EF, Nelson KE, Daugherty S, DeBoy RT, Wister J, Khouri H, Weidman J, Walsh DA, Papke RT, Sanchez PG, Sharma AK, Nesbo CL, MacLeod D, Bapteste E, Doolittle WF, Charlebois RL, Legault B, Rodriguez-Valera F. The genome of Salinibacter ruber: convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc.Natl.Acad.Sci.U.S.A. 2005;102:18147–18152. doi: 10.1073/pnas.0509073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pena A, Valens M, Santos F, Buczolits S, Anton J, Kampfer P, Busse HJ, Amann R, Rossello-Mora R. Intraspecific comparative analysis of the species Salinibacter ruber. Extremophiles. 2005;9:151–161. doi: 10.1007/s00792-005-0430-y. [DOI] [PubMed] [Google Scholar]
- 20.Lutnaes BF, Oren A, Liaaen-Jensen S. New C(40)-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J.Nat.Prod. 2002;65:1340–1343. doi: 10.1021/np020125c. [DOI] [PubMed] [Google Scholar]
- 21.Imasheva ES, Balashov SP, Wang JM, Lanyi JK. pH Dependent Transitions in Xanthorhodopsin. Photochem.Photobiol. 2006;82:1406–1413. doi: 10.1562/2006-01-15-RA-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boichenko VA, Wang JM, Anton J, Lanyi JK, Balashov SP. Functions of carotenoids in xanthorhodopsin and archaerhodopsin, from action spectra of photoinhibition of cell respiration. Biochim.Biophys.Acta. 2006;1757:1649–1656. doi: 10.1016/j.bbabio.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litvin FF, Balashov SP, Sineshchekov VA. Investigation of Primary Photochemical Conversions of Bacteriorhodopsin in Purple Membranes and Cells of Halobacterium-Halobium by Low-Temperature Spectrophotometry Method. Bioorg.Khim. 1975;1:1767–1777. [Google Scholar]
- 24.Danon A, Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc.Nat.Acad.Sci. U.S.A. 1974;71:1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balashov SP, Imasheva ES, Wang JM, Lanyi JK. Carotenoid to retinal excited-state energy transfer in xanthorhodopsin. Biophys.J. 2007 doi: 10.1016/j.bpj.2009.01.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balashov SP, Imasheva ES, Lanyi JK. Induced chirality of the light-harvesting carotenoid salinixanthin and its interaction with the retinal of xanthorhodopsin. Biochemistry. 2006;45:10998–11004. doi: 10.1021/bi061098i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luecke H, Schobert B, Richter HT, Cartailler JP, Lanyi JK. Structure of bacteriorhodopsin at 1.55 Å resolution. J.Mol.Biol. 1999;291:899–911. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]
- 28.Georgakopoulou S, Cogdell RJ, van Grondelle R, van Amerongen H. Linear-dichroism measurements on the LH2 antenna complex of Rhodopseudomonas acidophila strain 10050 show that the transition dipole moment of the carotenoid rhodopsin glucoside is not collinear with the long molecular axis. J.Phys.Chem.B. 2003:655–658. [Google Scholar]
- 29.Heyn MP, Borucki B, Otto H. Chromophore reorientation during the photocycle of bacteriorhodopsin: experimental methods and functional significance. Biochim.Biophys.Acta. 2000;1460:60–74. doi: 10.1016/s0005-2728(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 30.Mukohata Y, Ihara K, Uegaki K, Miyashita Y, Sugiyama Y. Australian halobacteria and their retinal-protein ion pumps. Photochem.Photobiol. 1991;54:1039–1045. doi: 10.1111/j.1751-1097.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura K, Kouyama T. Structural role of bacterioruberin in the trimeric structure of archaerhodopsin-2. J.Mol.Biol. 2008;375:1267–1281. doi: 10.1016/j.jmb.2007.11.039. [DOI] [PubMed] [Google Scholar]