Abstract
Background
A positive family history (FH) of alcohol use disorders (AUD) has been linked to increased risk for the development of AUD, and neurocognitive factors have been postulated as important underlying mechanisms of familial alcoholism transmission.
Methods
We used functional magnetic resonance imaging (fMRI) during a spatial working memory (SWM) and vigilance paradigm to investigate potential neurodevelopmental differences linked to familial density of AUD in 72 adolescents aged 12 to 14 years.
Results
Youth with denser family histories of AUD showed less activation during a simple vigilance condition relative to SWM in cingulate and medial frontal gyri (β = 0.28, p = 0.03), and a trend for more relative activity during rest (β = −0.25, p = 0.07) in this cluster.
Conclusions
Youth with greater familial densities of AUD may be less successful at modulating activity of the default network, potentially indicating a greater propensity for task-independent thought or reduced inhibition of task-irrelevant processing. Failure to moderate activation of the default network may have implications for cognitive efficiency and goal directed behavior in youth with dense FH. Further, aberrant activation in cingulate regions may be linked to genetic variation in GABA receptor units, suggesting a useful endophenotype for risk associated with alcohol dependence.
Keywords: Adolescence, fMRI, Spatial Working Memory, Family History of Alcoholism, Default Network, Cingulate
Youth with a positive family history (FHP) of alcohol use disorders (AUD) are 2 to 5 more times likely to develop drinking problems than those negative for this trait (FHN) (Cloninger et al., 1986; Schuckit, 1985). Underlying neurobiological mechanisms have been proposed as moderating this increased risk, as FHP youth often perform differently than FHN controls on tests of neurocognitive function. For example, nonabusing FHP adolescent males performed worse than FHN boys on tests of language functioning and academic achievement (Giancola et al., 1993; Hegedus et al., 1984; Najam et al., 1997; Poon et al., 2000; Sher et al., 2000; Tarter et al., 1984; Viken et al., 1999), organization of new information (Peterson et al., 1992), executive cognitive functioning (Giancola et al., 1996; Harden and Pihl, 1995), perseveration (Giancola et al., 1993), working memory (Corral et al., 1999; Harden and Pihl, 1995; Ozkaragoz et al., 1997), nonverbal memory (Sher et al., 1991), visuospatial skills (Berman and Noble, 1995; Corral et al., 1999; Garland et al., 1993; Ozkaragoz and Noble, 1995; Ozkaragoz et al., 1997; Sher et al., 1991) and attention (Tarter et al., 1989). Multigen-erational transmission (Conrod et al., 1995; LeMarquand et al., 1999; Peterson et al., 1996; Pihl and Bruce, 1995), high familial density (Hill et al., 2000a), early AUD onset in father (Tarter et al., 1989), active paternal alcoholism (Ozkaragoz et al., 1997), and genotypic features (Berman and Noble, 1995) increase the link between FHP and cognitive functioning. However, other studies have not observed relationships between FHP status and neurocognition (Finn and Justus, 1999; Schuckit et al., 1987), and the neural mechanisms predicting FHP individuals’ risk for AUD remains unclear.
In an attempt to link neurobehavioral differences in FHP individuals to their increased risk for AUD, it has been proposed that a positive FH may involve familial transmission of a subtle neurodevelopmental delay. FHP youth have demonstrated smaller volumes of the right amygdala (Hill et al., 2001), which tends to increase in volume over childhood and adolescence. Slowed rate of change in P300 amplitude (Almasy et al., 1999; Begleiter et al., 1984; Polich et al., 1994; Porjesz et al., 1998) and delayed maturation of postural sway (Hill et al., 2000a,b) have also been found among FHP youth. Children of alcoholics demonstrate smaller intracranial volume, which may indicate reduced brain growth (Gilman et al., 2007). Differences in P300 amplitude and postural stability disappear over time, suggesting that these abnormalities may correspond to an inherited developmental lag (Bauer and Hesselbrock, 1999; Hill et al., 1999; Polich et al., 1994). During this period of prolonged neurodevelopment, FHP youth who initiate drinking could be at increased risk for problem behaviors (Hill et al., 2000a; Hingson et al., 2006; King and Chassin, 2007). Therefore, understanding the neural characteristics of FHP youth may help determine potential premorbid abnormalities in the brain functioning of individuals who develop AUD and how these factors contribute to increased risk.
The neural substrate of spatial working memory (SWM) may be especially relevant in identifying mechanisms of increased risk in FHP individuals. The neural mechanisms of spatial working memory (SWM) processing involve a front-oparietal network including the dorsolateral prefrontal cortex, posterior parietal cortices, cingulate regions, and medial frontal cortex (Casey et al., 1997; Courtney et al., 1998; Goldman-Rakic, 1987; McCarthy et al., 1996). While frontal regions are linked to executive control and parietal cortices to accurate and effortful performance (Nelson et al., 2000), the cingulate regions and medial frontal gyrus contribute to tasks effecting conflict and discrimination between responses (Casey et al., 1997), and are part of a “default network” activating in response to simple or rest conditions (Greicius et al., 2003), particularly after cognitively challenging tasks, as processing resources reallocate from areas engaged in task requirements (McKiernan et al., 2003). Studies of nondrinking FHP individuals have identified disorganization of P300 source density maps in the frontoparietal network (Hada et al., 2001), potentially indicating aberrant frontoparietal circuitry (Rangaswamy et al., 2004a) involved in SWM processing, and raising the possibility that reorganization of alcoholics’ frontoparietal pathways used to complete SWM tasks (Pfefferbaum et al., 2001; Tapert et al., 2001, 2004) may be moderated by premorbid FH effects. Therefore, differences in the neural substrate of SWM may be linked to FH status, and aid identification of factors promoting alcohol drinking or related behaviors.
Because SWM substrates reorganize during adolescence (Nelson et al., 2000; Schweinsburg et al., 2005a; Thomas et al., 1999), its examination during this period may capture a subtle neurodevelopmental lag in FHP youth. For example, compared to adults, children show more widespread (Thomas et al., 1999) and bilateral (Courtney et al., 1997; Jonides et al., 1993; Smith et al., 1996) dorsolateral prefrontal cortex activation, while adults show more parietal activity (Thomas et al., 1999). Across adolescence (i.e. ages 12 to 18), increasing pre-frontal and inferior parietal activation and decreasing superior parietal response as well as medial deactivation (i.e., activating more during baseline) is seen in response to SWM (Schweinsburg et al., 2005a). Thus, the underlying neural mechanisms of SWM develop over adolescence, shifting more inferior and posterior, and lateralizing to the right hemisphere, possibly reflecting shifts in strategic and cortical organization (Scherf et al., 2006; Schweinsburg et al., 2005a; Thomas et al., 1999). Considering these and other adolescent neurodevelopments (Giedd et al., 1999; Gogtay et al., 2004; Sowell et al., 2001), lags in neuromaturation may be detected, highlighting potential endophenotypes for the development of AUD.
Subtle delays in neurodevelopment might place FHP adolescents at increased risk for subsequent AUD due to lagged acquisition of cognitive and behavioral correlates. Typical neurodevelopments such as cortical thinning (Sowell et al., 2001), prefrontal myelination (Giedd et al., 1996; Gogtay et al., 2004), regional specialization (Durston et al., 2006) accompany improved performances on cognitive abilities throughout adolescence. Progressed spatial working memory, verbal working memory, and executive functioning often parallel specific neurodevelopmental processes (Conklin et al., 2007; Durston et al., 2006; Eigsti et al., 2006; Shaw et al., 2006). Changes in reward neurocircuitry are also theorized to develop across adolescence (Galvan et al., 2007). In sum, delayed neurodevelopment during adolescence may lead to increased vulnerability for problem behaviors. The aim of this study is to contrast blood oxygen level dependent (BOLD) response patterns to SWM and simple vigilance in youth with varied familial densities of AUD, utilizing a neurocognitive substrate that (1) changes in a predictable manner over adolescent development and (2) engages brain regions that appear sensitive to FH-related abnormalities. We hypothesize that youths with dense familial AUD will demonstrate BOLD activation patterns indicative of protracted neurodevelopment. Specifically, we predict that with increasing FH density, there will be greater BOLD activation to SWM in more superior and bilateral regions than among FH negative counterparts.
METHODS
Participants
Youth were selected from an ongoing longitudinal study that follows youth with and without risk factors for AUD (i.e., conduct disorder and positive family histories) from ages 12 to 14 through young-adulthood to identify neurocognitive risk factors for substance problems (Anderson et al., 2005; Nagel et al., 2005, 2006; Schweinsburg et al., 2005b). The focus of this analysis was to identify neural features of FHP youth vis-à-vis fMRI response to a SWM task. Recruitment fliers were mailed to households of San Diego area middle school students. Youth eligibility was preliminarily ascertained through a brief telephone interview. After a description of the study, written informed consent and assent, approved by the University of California San Diego Human Research Protections Program, were obtained from parents and adolescents, respectively. Eligible youth were administered a 90-minute detailed screening interview covering personal substance use histories using the Customary Drinking and Drug Use Record (Brown et al., 1998), psychiatric diagnoses using the Computerized Diagnostic Interview Schedule for Children (C-DISC-4.0; Shaffer et al., 2000), and FH using the Family History Assessment Module (FHAM; Rice et al., 1995). The FHAM and C-DISC were also administered to parents by different interviewers to corroborate adolescent reports. Diagnoses were considered present if either respondents’ report or the combined report indicated the disorder. In cases of discrepancies, additional data were obtained, or data were coded to represent the lower level of functioning. Exclusionary criteria were: premature birth; reports of maternal drinking or drug use during pregnancy; parental history of psychosis, bipolar I, or antisocial personality disorder; history of neurological problems, head trauma, learning disabilities, Axis I psychiatric disorder other than conduct disorder or oppositional defiant disorder, or psychiatric medication use; MRI contraindications (e.g., braces); noncorrectable sensory impairments; nonfluency in English; and left-handedness.
After excluding for excessive movement (n = 5), abnormal brain anatomy (n = 4), signal drop-out (n = 4), neuropsychological scores below the normal range (n = 1), or ≤50% accuracy on the fMRI task (n = 2), remaining participants (n = 72, 43% female, 78% Caucasian) were ages 12 to 14, typically from upper middle to upper class families, and average to high average intellectually. History of alcohol and other drug use was minimal (see Table 1). Adolescents who met for conduct disorder (n = 7 “mild” and n = 5 “moderate” in severity) were included, as this was evenly distributed across FH density values.
Table 1.
Participant Characteristics and Correlations With Family History Density (n = 72)
| M (SD) | Pearson r | p-value | |
|---|---|---|---|
| Age in years (range 12–14) | 13.25 (0.81) | −0.11 | 0.34 |
| Pubertal development | |||
| Girls | 3.65 (0.71) | −0.21 | 0.26 |
| Boys | 2.89 (0.70) | −0.03 | 0.88 |
| Father education | 16.35 (2.20) | −0.20 | 0.11 |
| Mother education* | 15.49 (2.32) | −0.25 | 0.03 |
| Father annual salary ($K) | 87.88 (68.04) | 0.34 | 0.77 |
| Mother annual salary ($K) | 35.59 (42.49) | −0.12 | 0.31 |
| CBCL Internalizing T-score | 46.55 (6.41) | 0.15 | 0.22 |
| CBCL Externalizing T-score | 45.85 (6.41) | 0.08 | 0.55 |
| Beck Depression Inventory total | 2.60 (3.12) | 0.05 | 0.71 |
| Spielberger State Anxiety T-score | 28.68 (7.03) | 0.16 | 0.19 |
| Lifetime uses of alcohol | 0.85 (2.68) | −0.10 | 0.40 |
| Alcoholic drinks per occasion | 0.17 (0.53) | −0.08 | 0.50 |
| Lifetime uses of cigarettes | 0.10 (0.42) | −0.14 | 0.25 |
| Lifetime uses of marijuana | 0.23 (0.74) | −0.07 | 0.54 |
| ANOVAs comparing FH density across categories: | |||
|
|
%
|
F-value
|
|
| Sex (% female) | 43 | 1.65 | 0.15 |
| Ethnicity (% Caucasian) | 78 | 1.12 | 0.36 |
| Conduct disorder diagnosis | 16 | 1.92 | 0.09 |
| Parental marital status | 1.34 | 0.25 | |
| Single | 4 | ||
| Married to each other | 81 | ||
| Divorced | 14 | ||
| Other | 1 | ||
| Living arrangement* | 2.67 | 0.02 | |
| Both biological parents | 81 | ||
| Single parent | 10 | ||
| Parent and step-parent | 8 | ||
p < 0.05.
Measures
Family History of Substance Use Disorders
Biological parents’ and grandparents’ lifetime history of AUD was obtained from both parents and participant using the FHAM. Most participants had 2 biological parents as informants, and all participants had at least one. For each participant, an index of FH density was computed: each parent with a history of AUD contributed 0.5 and each grandparent with AUD history added 0.25 to the score (range 0 to 2) (Zucker et al., 1994). Stoltenberg et al. (1998) found this density measure provided a valid characterization of familial AUD and may provide greater sensitivity than categorical approaches.
Family Background
Socioeconomic status scores for each participant were derived using the Hollingshead scale, which combines educational attainment and occupation of each parent (Hollingshead, 1965).
Psychiatric Functioning
Diagnoses were derived for participants using youth and parent reports from the C-DISC-4.0 (Shaffer et al., 1996). The Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2001) provided levels of psychopathological syndromes based on parent report.
State Measures
Mood was assessed at the time of scanning with the Beck Depression Inventory (Beck, 1978) and state scale of the Spielberger State Trait Anxiety Inventory (Spielberger et al., 1970).
Self-Rating Scale for Pubertal Development
Physical development was assessed with a sex-specific 5-item self-report measure (Petersen et al., 1988). Youth selected 1 of 5 sex-specific statements ranging from “has not begun yet” to “seems complete” indicating current level of pubertal development. Multiple domains of secondary sex characteristic development (e.g., menstruation, facial hair growth, etc.) were sampled to most closely ascertain Tanner staging, characterized as pre-, early, mid-, late, or postpubertal. Pubertal staging is relevant here, as any FH-related differences could account for developmental discrepancies in brain activation.
Youth Substance Use
The Customary Drinking and Drug Use Record (Brown et al., 1998) provides a detailed assessment of past 3-month and lifetime use of alcohol, nicotine, and other drugs.
Neuropsychological Testing
The Vocabulary, Similarities, Block Design, and Matrices subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) were used to ensure participants’ scores fell within the normal range (Wechsler, 1999). Right-handedness was confirmed with the Edinburgh Handedness Inventory (Oldfield, 1971).
Procedures
Participants completed the protocol across 2 appointments, which included a 1-hour scan session. Images were acquired on a 1.5 Tesla General Electric Signa LX scanner. A high-resolution structural image was collected in the sagittal plane using an inversion recovery prepared T1-weighted 3-dimensional spiral fast-spin echo sequence (repetition time = 2,000 ms; echo time = 16 ms; field of view = 240 mm; resolution = 0.9375 mm × 0.9375 mm × 1.328 mm) (Wong et al., 2000). Functional imaging was collected in the axial plane using T2-weighted spiral gradient recall echo imaging (repetition time = 3,000 ms; echo time = 40 ms; flip angle = 90°; field of view = 240 mm; 20 continuous slices; slice thickness = 7 mm; in-plane resolution = 1.875 mm × 1.875 nm; 156 repetitions).
Task stimuli were presented from a laptop computer through a data projector to a screen in the MRI room near the foot of the scanner bed. The participant viewed stimuli through a mirror mounted on the head coil. The fMRI task (Kindermann et al., 2004; Tapert et al., 2001), adapted from McCarthy and colleagues (McCarthy et al., 1994), was chosen to explore the neural substrates of SWM functioning and probe the integrity of these brain regions in adolescents at risk for AUD. The task consists of 18 20-second blocks alternating between experimental (SWM) and baseline (vigilance) condition, with blocks of rest (fixation cross in the center of the screen) in the beginning, middle, and end. In the SWM condition, abstract line drawings appear 1 at a time in 1 of 8 locations. Participants are to press a button when a design appears in a location already occupied in that block. On average, 3 of the 10 trials in each block are repeat locations, and repeats are 2-back. In the vigilance condition, the same stimuli are presented in the same locations, but a dot appears above figures on 30% of trials, and participants are to press a button when a dot appears. The purpose of the vigilance condition is to control for simple motor and attention processes involved in the SWM condition. In both conditions, stimuli are presented for 1,000 ms, and each interstimulus interval is 1,000 ms (total time: 7 minutes, 48 seconds). Upon completion, participants and parents are financially compensated for their time.
Data Analysis
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI; afni.nimh.nih.gov; Cox, 1996). Motion in the time series data was corrected by registering each acquisition to a minimally deviant repetition with an iterated least squares algorithm (Cox and Jesmanowicz, 1999); 3 rotational and 3 displacement parameters were output for each repetition of each participant. Trained raters examined the corrected time series data and removed repetitions containing residual visually discernible head motions. If >22% of repetitions were removed, the participant was not included (n = 5 not described in this paper). On average, 6% of repetitions were removed due to movement. Time series data were deconvolved with a reference vector coding the alternating task conditions while covarying for linear trends and the motion correction applied to control for spin history effects (Bandettini et al., 1993), and modeling typical delays in the hemodynamic response (Bandettini et al., 1993; Boynton et al., 1996). Functional data were transformed into standardized space (Lancaster et al., 2000; Talairach and Tournoux, 1988) and resampled into 3.5 mm3 voxels, and a spatial smoothing Gaussian filter (full-width half maximum = 3.5 mm) was applied. These steps resulted in a fit coefficient for each voxel representing BOLD response to SWM relative to the vigilance task condition; separate coefficients modeled SWM relative to fixation, and vigilance relative to rest.
To determine whether bulk motion during the task differed as a function of FH, each participant’s absolute mean for each of the 6 motion parameters across the time series data was evaluated with Spearman rank-order correlations. For estimating task-correlated motion, the 6 parameters were correlated with the task reference vector for each participant. These values were also correlated with FH density scores with Spearman rank-order correlations.
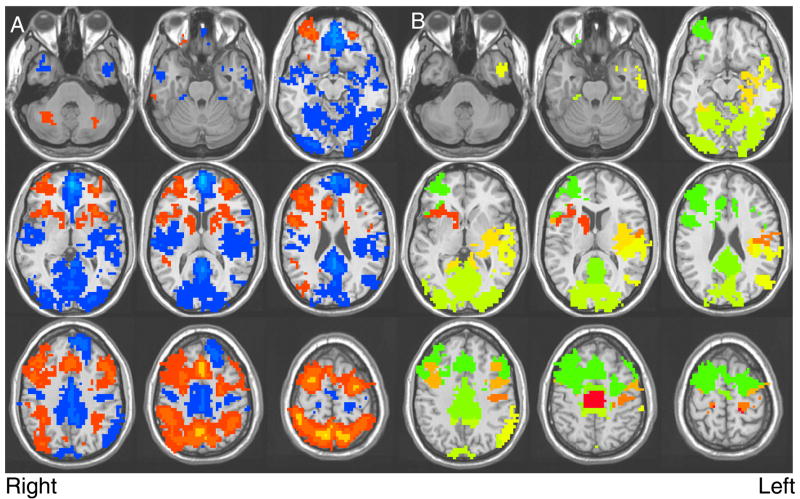
A single sample t-test was conducted to indicate voxels responding to SWM relative to vigilance across all participants (n = 72). To control for Type I error, a combination of t-statistic magnitude and cluster volume thresholding was applied (Forman et al., 1995; Ward, 1997) by only interpreting clusters comprised of at least 22 contiguously activated voxels at a < 0.025 (≥943 μl in volume). Eleven clusters surpassed this threshold. The largest cluster (342,486 μl) was reduced into 9 smaller regions of interest defined using regional proximity, established correlates of brain structure to function (Heilman and Valenstein, 1972, 2003), and the direction of BOLD activation (i.e., areas activating preferentially to SWM were grouped separately from those activating to vigilance; see Fig. 1). For each of the resulting 19 clusters (see Table 3), average activation for each participant was converted into z-scores.
Fig. 1.
(A) Results of the single sample t-test, showing regions with significant activation among adolescent participants (n = 72) during the SWM task; red indicates increased BOLD response contrast to the SWM blocks, and blue indicates increased BOLD response contrast to the vigilance blocks (p < 0.025, clusters > 943 μl). (B) Delineation of regions resulting from reduction of the largest cluster that significantly activated to the SWM task.
Table 3.
Regions Activated to SWM Relative to Vigilance Conditions (Clusters > 943 μl, p < 0.025, n = 72)
| RL | AP | IS | BA | +/− SWM | Brain regions |
|---|---|---|---|---|---|
| −13 | −18 | 37 | 6, 8, 47 | + | Medial middle, superior & inferior frontal gyri; anterior to posterior cingulatea |
| −7 | 56 | 51 | 19, 7, 40, 39, 5 | + | Precuneui, superior & inferior parietal lobules |
| 1 | 74 | 5 | − | Occipital, lingual & fusiform gyria | |
| 6 | −46 | 11 | 9,10 | − | Medial & middle frontal gyri |
| 0 | 34 | 29 | 18, 30 | − | Cingulate regions and middle frontal gyrus, posterior cingulate, medial frontal gyria |
| −42 | 10 | 6 | 13, 4, 41, 42, 22, 21, 40 | − | Insula, striatum; inferior frontal gyrus; transverse, superior & middle temporal gyri; para/hippocampal gyri, amygdala; inferior parietal lobule |
| 51 | 32 | 8 | 42, 21, 34 | − | Temporal gyri, inferior parietal lobule, supramarginal, middle & superior temporal gyria |
| 25 | 21 | 3 | 35, 28 | − | Parahippocampal gyri, hippocampus, insula, thalamusa |
| 26 | −12 | 8 | 44, 47 | + | Caudate, insula, cingulate, middle frontal gyrus; thalamus, putamen; inferior frontal gyrus |
| −23 | −11 | 10 | + | Insula, caudate, striatuma | |
| 31 | −47 | 9 | 10 | + | Middle & superior frontal gyri |
| 2 | 25 | 47 | − | Paracentral lobulesa | |
| −32 | −7 | −34 | 38, 36, 21 | − | Superior & middle temporal gyri, uncus |
| 54 | −21 | 10 | 45 | − | Inferior frontal gyrus, precentral gyrus |
| −56 | 29 | −15 | 20 | + | Middle temporal gyrus into inferior temporal gyrus |
, clusters activated preferentially to the SWM condition;
, clusters activated preferentially to the vigilance condition.
Divided from the largest cluster.
Z-scores representing SWM response relative to vigilance for each of the 19 clusters were screened for outliers using 3 regression-based diagnostic approaches: Studentized deleted residuals > ±3, leverage values >0.17, and Mahalanobis distance > critical value > 16.27 (p = 0.001, dfbetas > ±1 or dffits > ±1). One case was determined as unduly influential in the right cerebellar tonsil.
To determine whether FH density predicted BOLD response in regions that reliably activated to SWM relative to vigilance, the average activations for 15 of these 19 clusters were entered as dependent variables for hierarchical regressions performed in SPSS 14.0 (Chicago, IL). The right cerebellar tonsil cluster was excluded as a statistical outlier (see above). Three other clusters (left cerebellar tonsil, and left and right precentral motor cortices) were not entered into the equation because FH effects were not expected in these regions. Bulk motion in the roll and pitch directions were included as covariates, and FH density was entered on the second step. The presence of conduct disorder was not related to independent or dependent variables, and mothers’ education was related to FH but not activation, and therefore not included. Bonferroni correction was applied, and regressions were considered significant at α ≤ 0.003.
RESULTS
Participant Characteristics
FH density was not significantly related to gender, conduct disorder diagnosis, CBCL scores, or other indicators of mood or demography. FH density negatively correlated with maternal years of education (r = −0.25, p = 0.03, see Table 1). Performance on neuropsychological tests and the SWM task did not differ as a function of FH density (see Table 2). Increased age was related to faster reaction time in the vigilance condition (r = −0.31, p = 0.01) and to better accuracy in SWM (r = 0.28, p = 0.02) and vigilance conditions (r = 0.28, p = 0.02). Pubertal development scores indicated that, on average, males were nearly at mid-puberty, while females were between mid- to late puberty. Pubertal development was not related to SWM task performance or neuropsychological test scores (ps > 0.05).
Table 2.
Neurocognitive Scores and Correlations With Family History Density (n = 72)
| Mean | SD | Pearson r | p-value | |
|---|---|---|---|---|
| Spatial working memory accuracy % | 91.00 | 6.24 | 0.16 | 0.20 |
| Spatial working memory reaction time (ms) | 604.38 | 79.96 | −0.18 | 0.16 |
| Vigilance accuracy % | 95.67 | 2.60 | −0.09 | 0.47 |
| Vigilance reaction time (ms) | 654.32 | 58.26 | 0.02 | 0.89 |
| WASI Vocabulary T-score | 56.56 | 8.05 | −0.10 | 0.41 |
| WASI Similarities T-score | 56.40 | 9.14 | 0.07 | 0.55 |
| WASI Block Design T-score | 56.26 | 7.72 | 0.14 | 0.24 |
| WASI Matrix Reasoning T-score | 54.50 | 6.07 | −0.06 | 0.59 |
Motion
The maximum values of rotation and displacement were modest (roll = 0.18°, pitch = 1.28°, yaw = 0.35°, superior = 0.80 mm, left = 0.24 mm, posterior = 0.39 mm). Spearman rank-order correlations demonstrated that FH density was not related to bulk motion in any direction (ps > 0.20), but FH density was related to task-correlated motion in the roll (i.e., ear-to-shoulder) and yaw (i.e., nodding) rotations (β = 0.27, p = 0.03; β = 0.28, p = 0.02, respectively).
SWM Activation and Family History
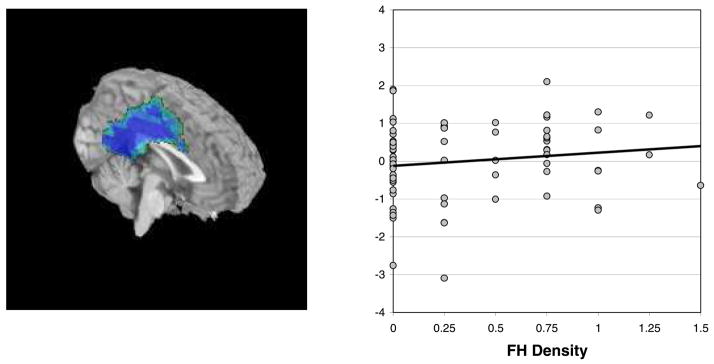
Across participants, the pattern of SWM relative to vigilance was consistent with existing literature (Barch et al., 1997; Courtney et al., 1998; D’Esposito et al., 1998; Jonides et al., 1993; Smith and Jonides, 1998), involving bilateral dorsolateral prefrontal and parietal cortices activating to SWM blocks, and medial frontal and cingulate regions activating to vigilance blocks (see Table 3). A hierarchical regression determined whether FH density would predict SWM relative to vigilance response in the 15 areas of interest that reliably activated to the task. Task-correlated motion (roll and yaw) parameters were entered on step 1, and centered FH density was entered on step 2. Considering a Bonferroni correction for the number of regressions evaluated (α = 0.05/15 = 0.003), greater FH density was related to decreased SWM response relative to vigilance in a region encompassing bilateral cingulate, posterior cingulate, and extending into the medial frontal gyri at a trend level, above and beyond effects of motion [F (3,66) = 4.26, p = 0.008; R2Δ = 7%, β = 0.28, p = 0.03] (see Fig. 2).
Fig. 2.
Denser FH was linked to less vigilance response relative to SWM response (β = 0.28, p = 0.03) in the cingulate gyrus, posterior cingulate, and medial frontal gyrus (highlighted area). Most participants activated more to the vigilance condition than to the SWM condition in this region, but this was less true for those with greater FH density.
Follow-Up Analyses
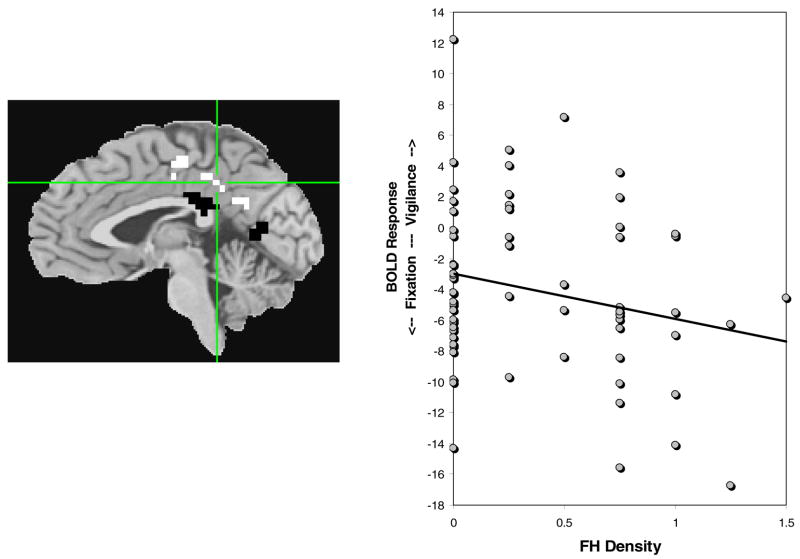
To clarify this pattern of activation, 2 single-sample t-tests in AFNI compared SWM relative to fixation, and vigilance relative to fixation activations (a < 0.025, ≥943 μl, n = 70). In the SWM relative to fixation contrast, many regions activated preferentially to SWM, and the cingulate/medial frontal gyral region activated more during fixation blocks than during SWM blocks. In the vigilance relative to fixation contrast, the cingulate/medial frontal gyrus cluster, superior cingulate, medial frontal gyrus, and paracentral lobule showed significantly greater BOLD response during fixation blocks, while more inferior areas of the cingulate activated preferentially to the vigilance condition (see Fig. 3). Two separate regressions examined the relationship between BOLD response and FH density for each subregion of activation within the larger cluster, for the vigilance relative to fixation contrast. The superior portion of the cluster, which activated preferentially to the fixation condition, showed a trend-level negative relationship between BOLD response to vigilance relative to fixation and increasing FH density [F(3,62) = 2.49, p = 0.07, β = −0.25, p = 0.07]. The inferior area, which activated preferentially to the demands of vigilance, did not relate to FH density. Taken together, these results suggest less BOLD response during vigilance and more activity during fixation (or rest-related response) with increasing FH density.
Fig. 3.
Activation during vigilance relative to fixation was examined in the region in which SWM relative to vigilance related to FH density. The area in white (superior cingulate; denoted with crosshairs) preferentially activated to fixation, and was related to FH density at a trend level (see graph; β = −0.25, p = 0.07). The regions in black (posterior cingulate) preferentially activated to vigilance condition, but were not related to FH density scores.
DISCUSSION
The goal of this study was to explore the relationship between FH of AUD and brain response to SWM and simple vigilance in adolescents ages 12 to 14 with very limited histories of alcohol or drug use. We predicted there would be greater BOLD activation to SWM in more superior and bilateral brain regions with increasing FH density, suggesting a neurodevelopmental lag in brain regions sensitive to FH-related abnormalities. Contrary to our hypothesis, FH status did not predict activation to the SWM task. Therefore, findings do not necessarily support a neurodevelopmental delay as a mechanism through which youth with familial AUD are at risk for AUD. However, adolescents with greater familial densities of AUD demonstrated less response than FHN youth during a simple vigilance baseline condition in medial brain regions including the posterior cingulate, cingulate gyrus, and medial frontal gyrus. In addition, participants with greater FH densities demonstrated more BOLD response than FHN youth during rest periods in the more superior aspects of this medial region. While unrelated to the a priori hypothesis, these findings may provide interesting insights to the cognitive style of youth with familial AUD.
Midline structures such as the posterior cingulate cortex, medial frontal gyri, and anterior cingulate have been consistently found to “deactivate,” or respond preferentially to the baseline condition as compared to the more demanding condition of many fMRI paradigms (Binder et al., 1999; Greicius et al., 2003; Hampson et al., 2006; McKiernan et al., 2003). This increased midline activity during a passive state (e.g., fixation) or conditions requiring little cognitive processing (e.g., vigilance), relative to regions needed for cognitively demanding conditions, has been linked to a propensity for stimulus independent thought, or mind wandering (Gusnard et al., 2001; Mason et al., 2007; McGuire et al., 1996; McKiernan et al., 2006). This could in turn relate to compromised appraisal of emergent risk situations and reduced tendencies to carefully consider outcomes of behavioral selections. We observed that increased FH density was linked to more fixation-related BOLD response and less vigilance response in the cingulate. Potentially, this may indicate that adolescents with denser FH engage in less stimulus independent thought during easy task conditions (i.e., in response to a vigilance task), but more such thought when task demands cease (i.e., during fixation). A task with varied rest durations and task demands could help ascertain if this activation pattern is beneficial to performance by maintaining cognitive arousal (Mason et al., 2007), or counter-productive, by allocating resources away from the target task.
Previous work investigating the utility of the default network suggests that activation in midline structures decreases in response to greater task difficulty (Greicius and Menon, 2004; Greicius et al., 2003; McKiernan et al., 2003). McKiernan et al. (2003) contrasted BOLD activation during a parametrically-manipulated short-term memory condition to response obtained during a target discrimination condition. As task demands increased, processing reserves were reallocated from default areas to regions necessary for the active condition. The authors concluded that the magnitude by which midline activation changed represented the degree to which resting state activity was inhibited to maintain successful task-relevant processing. Functional connectivity studies also support the notion that opposing activation patterns between default and higher-order cortical regions may serve to enhance performance (Greicius et al., 2003). Hampson et al. (2006) reported that increasing working memory performance positively correlated with the strength of connectivity in the default network, and proposed that lateral prefrontal regions may actively suppress activation of midline structures to augment performance. Similarly, a greater inverse correlation between activity in the default network and task-related activation was positively associated with intra-individual consistency in behavioral performance (Clare Kelly et al., 2008). In other words, the strength of the correlation may represent an index of neural regulation between default and task-active “modes” (Clare Kelly et al., 2008). Together, current findings suggest that adolescents with dense FH may be less successful in moderating the default network in response to cognitive load. This difference could have potential consequences for downstream cognitive functioning, or serve as an additional endophenotype to help identify youth at greater risk of developing CNS disinhibitory related behaviors.
In FH dense adolescents, compromised neural regulation of midline regions may have direct behavioral sequelae. The functional consequences of these findings may be evident in the poorer neurocognitive and academic performance of some individuals (Corral et al., 1999; Giancola et al., 1996; Hegedus et al., 1984; Najam et al., 1997; Poon et al., 2000), or mark behavioral disinhibition. However, in this sample, youth with greater familial AUD did not score differently from FHN youth on neuropsychological tests, and measures of behavioral inhibition were not linked to this pattern of activation. Thus, an alternative explanation for smaller magnitudes of vigilance-related activation with increasing FH density is that FH-dense adolescents were less challenged by the SWM condition than FHN youth, thus requiring fewer cognitive resources to complete the task. Future studies that parametrically manipulate task difficulty will help characterize how the default network develops across adolescence, and elucidate whether this finding supports an FH-related developmental lag. Additionally, studies that include participants with more behavioral problems might uncover a relationship.
Our findings have potentially interesting implications for the interpretation of anomalous electrophysiological characteristics theorized to be biological vulnerabilities for AUD and behavioral disinhibition, such as reduced P300 amplitude and increased resting beta frequency (Bauer and Hesselbrock, 1993; Hesselbrock et al., 2001; Polich et al., 1994; Porjesz et al., 2005; Rangaswamy et al., 2004a). The relationship between genetic features that determine characteristics of brain oscillations could participate in the mechanism for such predispositions. Specifically, EEG beta frequency has been linked to receptor genes for gamma-aminobutyric acid (GABA) that are involved in inhibitory neural networks (Rangaswamy et al., 2004b). The balance of inhibitory inter-neurons and excitatory pyramidal cells depends on the action of GABA subunits (Whittington et al., 2000). Because alcoholics and their children demonstrate increased beta activity, this may indicate an imbalance between excitation and inhibition (Porjesz and Rangaswamy, 2007). Variations in GABA receptor genes affect neural inhibition and thus the level of neural excitability, thereby influencing the predisposition to develop alcohol dependence and related disinhibitory disorders (Begleiter and Porjesz, 1999). Interestingly, GABA appears to specifically influence activity in the default network regions (Northoff et al., 2007) that here demonstrated decreased vigilance and increased fixation response in FHP youth. Northoff et al. (2007) reported that GABA concentration predicted the magnitude of negative BOLD response (relative to a resting state) in the anterior cingulate. Additionally, the anterior and posterior cingulate gyri and medial frontal gyri have been indicated as potential sources of the P300. For instance, Ardekani et al. (2002) found a correlation between event-related potentials and BOLD activation to a visual odd-ball task. However, studies mapping anatomical origins of electrophysiological data have yet to reach consensus. Nonetheless, an intriguing body of emerging evidence suggests that suboptimal modulation of default networks in response to cognitive demands may relate to atypical electrophysiological activity and behavioral disinhibition in nondrinking FHP individuals.
Additional data support the link between substance use risk and activation of structures involved in the default network. Paulus et al. (2005) reported that decreased BOLD activation in response to a decision making task in the posterior cingulate and medial frontal gyrus predicted relapse in stimulant dependent adults. They proposed that relapsing individuals may be impaired in gathering adequate controlled processing resources during decision making, resulting in poor assessment abilities (Paulus et al., 2005). Taken together with our results, difficulty engaging this region even for simple tasks may be connected to CNS disinhibition and risk for impulsive behaviors. While the neural underpinnings of relapse and risk for AUD are likely distinct, we suggest that subtle neural response differences may influence the risk FHP adolescents have for developing patterns of problem drinking.
This study has several limitations. First, participant motion, although relatively modest, was related to FH density, and thus needed to be controlled statistically. Second, characterizing the relationship between brain regions supporting higher-order cognitive demands and the default network will require functional connectivity analyses, which are beyond the scope of this report. Third, most participants had highly educated, affluent parents, and all were free from medical and psychiatric problems; while this minimizes a variety of potential confounds, results may not generalize to the broad population of youths at risk for AUD. Fourth, activation in the default network has not been characterized across adolescence, so we cannot yet determine if the response pattern observed here represents a developmental lag. Finally, FH results were relatively modest and did not survive strict Bonferroni correction.
The current investigation suggests that functional neuroimaging may be useful for identifying neurocognitive risk factors associated with familial density of AUD. Although we did not find evidence that BOLD response specifically during spatial working memory demands was related to FH, we observed that greater neural activity during rest and reduced activity during an active baseline condition were linked to denser FH. We theorize that this may indicate an imbalance between excitatory and inhibitory action during neural transmission determined by genetic variation in GABAergic mechanisms. Behaviorally, CNS disinhibition might translate into reduced ability to inhibit task irrelevant stimuli or an increased risk for impulsive behavior. Further understanding of the default network in relation to CNS disinhibition and behavioral inhibition may help inform prevention programming and identify youth at risk for substance problems. Longitudinal studies may establish a causal link between neurocognitive characteristics and the risk for developing AUD.
Acknowledgments
Research supported by NIAAA grants R01 AA13419, T32 AA013525, and F31 AA016727. The authors thank Valerie Barlett, Lisa Caldwell, and Sonja Eberson for assistance with subject recruitment and data management, and Sandra A. Brown, Ph.D. and Edward P. Riley, Ph.D. for their continued support.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O’Connor SJ, Kuperman S, Rohrbaugh J, Bauer LO, Reich T, Polich J, Begleiter H. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88:383–390. [PubMed] [Google Scholar]
- Anderson KG, Schweinsburg AD, Paulus MP, Brown SA, Tapert SF. Examining personality and alcohol expectancies using fMRI with adolescents. J Stud Alcohol. 2005;66:323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Choi SJ, Hossein-Zadeh GA, Porjesz B, Tanabe JL, Lim KO, Bilder R, Helpern JA, Begleiter H. Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Res Cogn Brain Res. 2002;14:347–356. doi: 10.1016/s0926-6410(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol. 1993;54:577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) Psychological Corp; San Antonio, TX: 1978. [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Berman SM, Noble EP. Reduced visuospatial performance in children with the D2 dopamine receptor A1 allele. Behav Genet. 1995;25:45–58. doi: 10.1007/BF02197241. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Clare Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Reich T, Bohman M. Inheritance of risk to develop alcoholism. NIDA Res Monogr. 1986;66:86–96. [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol. 2007;31:103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Ditto B. Autonomic reactivity and alcohol-induced dampening in men at risk for alcoholism and men at risk for hypertension. Alcohol Clin Exp Res. 1995;19:482–489. doi: 10.1111/j.1530-0277.1995.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Corral MM, Holguin SR, Cadaveira F. Neuropsychological characteristics in children of alcoholics: familial density. J Stud Alcohol. 1999;60:509–513. doi: 10.15288/jsa.1999.60.509. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, Davidson MC, Lawrence Aber J, Casey BJ. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol Sci. 2006;17:478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Finn PR, Justus A. Reduced EEG alpha power in the male and female offspring of alcoholics. Alcohol Clin Exp Res. 1999;23:256–262. [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Garland MA, Parsons OA, Nixon SJ. Visual spatial learning in nonalcoholic young adults with and those without a family history of alcoholism. J Stud Alcohol. 1993;54:219–224. doi: 10.15288/jsa.1993.54.219. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Martin CS, Tarter RE, Pelham WE, Moss HB. Executive cognitive functioning and aggressive behavior in preadolescent boys at high risk for substance abuse/dependence. J Stud Alcohol. 1996;57:352–359. doi: 10.15288/jsa.1996.57.352. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Peterson JB, Pihl RO. Risk for alcoholism, antisocial behavior, and response perseveration. J Clin Psychol. 1993;49:423–428. doi: 10.1002/1097-4679(199305)49:3<423::aid-jclp2270490317>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Bjork JM, Hommer DW. Parental alcohol use and brain volumes in early- and late-onset alcoholics. Biol Psychiatry. 2007;15:15. doi: 10.1016/j.biopsych.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. Handbook of physiology, the nervous system, higher functions of the brain. 1987;5:373–417. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Chorlian DB, Begleiter H, Polich J. Auditory P3a deficits in male subjects at high risk for alcoholism. Biol Psychiatry. 2001;49:726–738. doi: 10.1016/s0006-3223(00)01049-0. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. J Abnorm Psychol. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Hegedus AM, Alterman AI, Tarter RE. Learning achievement in sons of alcoholics. Alcohol Clin Exp Res. 1984;8:330–333. doi: 10.1111/j.1530-0277.1984.tb05522.x. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Frontal lobe neglect in man. Neurology. 1972;22:660–664. doi: 10.1212/wnl.22.6.660. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Clinical Neuropsychology. 4. Oxford University Press; New York: 2003. [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O’Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism–evidence from the collaborative study on the genetics of alcoholism. J Biomed Sci. 2001;8:77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000b;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill EM, Stoltenberg SF, Burmeister M, Closser M, Zucker RA. Potential associations among genetic markers in the serotonergic system and the antisocial alcoholism subtype. Exp Clin Psychopharmacol. 1999;7:103–121. doi: 10.1037//1064-1297.7.2.103. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Lowers L, Steinhauer S, Konicky C. Developmental changes in postural sway in children at high and low risk for developing alcohol-related disorders. Biol Psychiatry. 2000a;47:501–511. doi: 10.1016/s0006-3223(99)00175-4. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophr Res. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J Stud Alcohol Drugs. 2007;68:256–265. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. Am J Psychiatry. 1999;156:1771–1779. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman-Rakic P, Shulman RG. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci U S A. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. Neuroreport. 1996;7:2095–2099. [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Barlett VC, Schweinsburg AD, Tapert SF. Neuropsychological predictors of BOLD response during a spatial working memory task in adolescents: what can performance tell us about fMRI response patterns? J Clin Exp Neuropsychol. 2005;11:631–644. doi: 10.1080/13803390490919038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najam N, Tarter RE, Kirisci L. Language deficits in children at high risk for drug abuse. J Child Adolesc Subst Abuse. 1997;6:69–80. [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozkaragoz TZ, Noble EP. Neuropsychological differences between sons of active alcoholic and non-alcoholic fathers. Alcohol Alcohol. 1995;30:115–123. [PubMed] [Google Scholar]
- Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14:31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. A self report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. J Stud Alcohol. 1992;53:154–160. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcohol Clin Exp Res. 1996;20:1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pihl R, Bruce K. Cognitive impairment in children of alcoholics. Alcohol Health Res World. 1995;19:142–147. [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: differences related to subtypes of familial alcoholism. Alcohol Clin Exp Res. 2000;24:1020–1027. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcohol Clin Exp Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Scientific World Journal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004a;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Reich T, Begleiter H. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004b;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetics and the risk for alcoholism. Jama. 1985;254:2614–2617. [PubMed] [Google Scholar]
- Schuckit MA, Butters N, Lyn L, Irwin M. Neuropsychologic deficits and the risk for alcoholism. Neuropsychopharmacology. 1987;1:45–53. doi: 10.1016/0893-133x(87)90009-1. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005a;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescent development. J Int Neuropsychol Soc. 2005b;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Fisher P, Trautman P, Moreau D, Kleinman M, Flory M. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53:339–348. doi: 10.1001/archpsyc.1996.01830040075012. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–829. [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; New York: 1988. Coplanar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tarter RE, Hegedus AM, Winsten NE, Alterman AI. Neuropsychological, personality, and familial characteristics of physically abused delinquents. J Am Acad Child Psychiatry. 1984;23:668–674. doi: 10.1016/s0002-7138(09)60534-3. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Jacob T, Bremer DL. Specific cognitive impairment in sons of early onset alcoholics. Alcohol Clin Exp Res. 1989;13:786–789. doi: 10.1111/j.1530-0277.1989.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behav Genet. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank LR. Single slab high resolution 3D whole brain imaging using spiral FSE. Proc Int Soc Magn Reson Med. 2000;8:683. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. In: Babor TF, Hesselbrock V, Meyer RE, Shoemaker W, editors. Biopyschosocial variation among pathways into symptomatic difficulty, in Types of Alcoholics: Evidence from Clinical, Experimental and Genetic Research. The New York Academy of Sciences; New York: 1994. pp. 134–146. [DOI] [PubMed] [Google Scholar]