Abstract
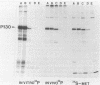
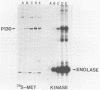
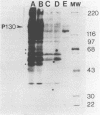
Phosphorylation of the major autophosphorylation site (Tyr-1073) within Fujinami sarcoma virus P130gag-fps activates both the intrinsic protein-tyrosine kinase activity and transforming potential of the protein. In this report, a second site of autophosphorylation Tyr-836 was identified. This tyrosine residue is found within a noncatalytic domain (SH2) of P130gag-fps that is required for full protein-kinase activity in both rat and chicken cells. Autophosphorylation of this tyrosine residue implies that the SH2 region lies near the active site in the catalytic domain in the native protein and thus possibly regulates its enzymatic activity. Four mutations have occurred within the SH2 domain between the c-fps and v-fps proteins. Tyr-836 is one of these changes, being a Cys in c-fps. Site-directed mutagenesis was used to investigate the function of this autophosphorylation site. Substitution of Tyr-836 with a Phe had no apparent effect on the transforming ability or protein-tyrosine kinase activity of P130gag-fps in rat-2 cells. Mutagenesis of both autophosphorylation sites (Tyr-1073 and Tyr-836) did not reveal any cooperation between these two phosphorylation sites. The implications of the changes within the SH2 region for v-fps function and activation of the c-fps oncogenic potential are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariizumi K., Shibuya M. Construction and biological analysis of deletion mutants of Fujinami sarcoma virus: 5'-fps sequence has a role in the transforming activity. J Virol. 1985 Sep;55(3):660–669. doi: 10.1128/jvi.55.3.660-669.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Gabrilove J. L., Tam J. P., Moore M. A., Hanafusa H. Specific expression of the human cellular fps/fes-encoded protein NCP92 in normal and leukemic myeloid cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2379–2383. doi: 10.1073/pnas.82.8.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Vass W. C., Tambourin P. E. Human cellular fps/fes cDNA rescued via retroviral shuttle vector encodes myeloid cell NCP92 and has transforming potential. Oncogene Res. 1987 Sep-Oct;1(4):441–458. [PubMed] [Google Scholar]
- Foster D. A., Hanafusa H. A fps gene without gag gene sequences transforms cells in culture and induces tumors in chickens. J Virol. 1983 Dec;48(3):744–751. doi: 10.1128/jvi.48.3.744-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. A., Shibuya M., Hanafusa H. Activation of the transformation potential of the cellular fps gene. Cell. 1985 Aug;42(1):105–115. doi: 10.1016/s0092-8674(85)80106-9. [DOI] [PubMed] [Google Scholar]
- Greer P. A., Meckling-Hansen K., Pawson T. The human c-fps/fes gene product expressed ectopically in rat fibroblasts is nontransforming and has restrained protein-tyrosine kinase activity. Mol Cell Biol. 1988 Feb;8(2):578–587. doi: 10.1128/mcb.8.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Herrera R., Rosen O. M. Autophosphorylation of the insulin receptor in vitro. Designation of phosphorylation sites and correlation with receptor kinase activation. J Biol Chem. 1986 Sep 15;261(26):11980–11985. [PubMed] [Google Scholar]
- Huang C. C., Hammond C., Bishop J. M. Nucleotide sequence and topography of chicken c-fps. Genesis of a retroviral oncogene encoding a tyrosine-specific protein kinase. J Mol Biol. 1985 Jan 20;181(2):175–186. doi: 10.1016/0022-2836(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Kornbluth S., Hanafusa H. Enzymatically inactive p60c-src mutant with altered ATP-binding site is fully phosphorylated in its carboxy-terminal regulatory region. Cell. 1987 Sep 11;50(6):937–943. doi: 10.1016/0092-8674(87)90520-4. [DOI] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- MacDonald I., Levy J., Pawson T. Expression of the mammalian c-fes protein in hematopoietic cells and identification of a distinct fes-related protein. Mol Cell Biol. 1985 Oct;5(10):2543–2551. doi: 10.1128/mcb.5.10.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Hanafusa H., Kawai S. A cellular protein is immunologically crossreactive with and functionally homologous to the Fujinami sarcoma virus transforming protein. Cell. 1982 Apr;28(4):897–906. doi: 10.1016/0092-8674(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Meckling-Hansen K., Nelson R., Branton P., Pawson T. Enzymatic activation of Fujinami sarcoma virus gag-fps transforming proteins by autophosphorylation at tyrosine. EMBO J. 1987 Mar;6(3):659–666. doi: 10.1002/j.1460-2075.1987.tb04805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Wang J. Y. Protein tyrosine phosphorylation in the cell cycle of BALB/c 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8191–8195. doi: 10.1073/pnas.83.21.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Verbeek J. S., Van den Ouweland A. M., Onnekink C., Bloemers H. P., Van de Ven W. J. The structure of the human c-fes/fps proto-oncogene. EMBO J. 1985 Nov;4(11):2897–2903. doi: 10.1002/j.1460-2075.1985.tb04020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Pawson T. Catalytic and non-catalytic domains of the Fujinami sarcoma virus P130gag-fps protein-tyrosine kinase distinguished by the expression of v-fps polypeptides in Escherichia coli. Oncogene. 1987 May;1(2):181–191. [PubMed] [Google Scholar]
- Sadowski I., Stone J. C., Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986 Dec;6(12):4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarut J., Mathey-Prevot B., Hanafusa H. Preferential expression of the c-fps protein in chicken macrophages and granulocytic cells. Mol Cell Biol. 1985 May;5(5):1067–1072. doi: 10.1128/mcb.5.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Stadtmauer L., Rosen O. M. Phosphorylation of synthetic insulin receptor peptides by the insulin receptor kinase and evidence that the preferred sequence containing Tyr-1150 is phosphorylated in vivo. J Biol Chem. 1986 Jul 25;261(21):10000–10005. [PubMed] [Google Scholar]
- Stone J. C., Atkinson T., Smith M., Pawson T. Identification of functional regions in the transforming protein of Fujinami sarcoma virus by in-phase insertion mutagenesis. Cell. 1984 Jun;37(2):549–558. doi: 10.1016/0092-8674(84)90385-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Hinze E., Pawson T. Mapping of multiple phosphorylation sites within the structural and catalytic domains of the Fujinami avian sarcoma virus transforming protein. J Virol. 1983 Apr;46(1):29–41. doi: 10.1128/jvi.46.1.29-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Pawson T. Protein kinase activity of FSV (Fujinami sarcoma virus) P130gag-fps shows a strict specificity for tyrosine residues. J Biol Chem. 1986 Jan 5;261(1):328–333. [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Pawson T. A lysine in the ATP-binding site of P130gag-fps is essential for protein-tyrosine kinase activity. EMBO J. 1986 Jan;5(1):69–76. doi: 10.1002/j.1460-2075.1986.tb04179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]