Abstract
BACKGROUND
To reduce the risk of medication errors in paediatric patients, the Canadian Council on Health Services Accreditation endorsed the standardization and limiting of drug concentrations available within an organization.
METHODS
Standard concentrations (SCs) were implemented in the emergency department, operating room and paediatric intensive care unit at the Children’s Hospital of Eastern Ontario in Ottawa, Ontario. The change in practice involved addressing concerns raised during stakeholder consultations, developing a computer program, and educating and testing staff in the new method. The software for SC selection and infusion rate calculation featured redundant inputs, a ‘deviation’ column comparing the prescribed and infused doses, and a printout of patient information that also facilitated dose verification back-calculation.
RESULTS
The major barrier to acceptance of SCs was possible fluid overload in lower weight patients. Thus, infusions received by 48 successive infants in the paediatric intensive care unit were compared with theoretical SC infusions. Volumes were not significantly increased, and there was no trend toward proportionally larger volumes in lower weight patients. Medication error reporting was very low before implementation, and SC errors remained low; new online reporting led to higher reporting of other errors after implementation. A survey indicated excellent staff acceptance and beliefs that patient safety and continuity of care were improved.
INTERPRETATION
SCs were successfully instituted with computer support, in lieu of ‘smart pumps,’ across multiple critical care units in a paediatric institution. The initial program is being expanded to 40 continuous infusion drugs, plus paediatric advanced life support bolus medications.
Keywords: Critical care, High-alert medications, Intravenous, Medication safety, Paediatrics, Standard concentrations
Abstract
HISTORIQUE
Pour réduire le risque d’erreurs de médicaments chez les patients d’âge pédiatrique, le Conseil canadien d’agrément des services de santé a approuvé la normalisation et les restrictions des concentrations de médicaments disponibles dans une organisation.
MÉTHODOLOGIE
Les concentrations normalisées (CN) ont été adoptées au département d’urgence, au bloc opératoire et à l’unité de soins intensifs pédiatriques du Centre hospitalier pour enfants de l’est de l’Ontario d’Ottawa, en Ontario. Le changement de pratique visait à réagir aux préoccupations soulevées pendant les consultations avec les intervenants, à créer un programme informatique et à apprendre la nouvelle méthode au personnel puis à leur faire passer des tests. Le logiciel de sélection des CN et du calcul du taux de perfusion comportait des entrées redondantes, une colonne d’écarts comparant les doses prescrites et perfusées et un imprimé de l’information propre aux patients, ce qui facilitait le rétrocalcul de vérification des doses.
RÉSULTATS
Le principal obstacle à l’acceptation des CN provenait de la possibilité de surcharge liquidienne des patients de petit poids. Ainsi, les perfusions qu’avaient reçues 48 nourrissons consécutifs à l’unité de soins intensifs ont été comparées à des perfusions aux CN théoriques. Les volumes n’augmentaient pas de manière significative, et on ne remarquait pas de tendance vers une augmentation des volumes proportionnellement plus élevée chez les patients de plus petit poids. Les déclarations d’erreurs de médicaments étaient très faibles avant l’adoption du protocole, et les erreurs de CN le sont demeurées. Les nouvelles déclarations électroniques ont entraîné une plus forte déclaration d’autres erreurs après l’adoption du protocole. Selon un sondage, le personnel démontrait une excellente acceptation du protocole et pensait qu’il favorisait une amélioration de la sécurité des patients et de la continuité des soins.
INTERPRÉTATION
Les CN ont été implantées avec succès avec un soutien informatique en remplacement des pompes intelligentes dans de multiples unités de soins intensifs d’un établissement pédiatrique. Le programme initial est étendu à 40 médicaments à perfusion continue et aux bolus de médicaments pour les soins spécialisés en réanimation pédiatrique.
Paediatric patients are all sizes and weights, and thus dosing of intravenous medication infusions for children must be patient-specific. Calculations using methods such as the ‘rule of six’ and preparation of individualized medication solutions may be serious sources of preventable medication errors, particularly for ‘high-alert’ drugs in paediatric patients (1,2). This is especially important in critical care when time is of the essence and high-alert drugs are more frequently used (3,4). The Institute for Safe Medication Practices, the Joint Commission for Accreditation of Healthcare Organizations (JCAHO) in the United States and the Canadian Council on Health Services Accreditation recommend against the use of unique or variable concentration solutions (1,5,6). As of 2008, the JCAHO will require that hospitals stock and prepare a limited number of standard concentrations (SCs) of intravenous medications (1,5).
Traditionally, doses for infusions have been translated into a medication concentration such that the numerical value of the infusion flow rate matches the dose. A common method is the ‘rule of six’ – the dose (μg/kg/min) equals the infusion rate (mL/h) when the concentration of drug (mg/100 mL) is six times the patient weight (kg) (7). However, when using such a system, solution concentrations may exceed the recommended maximum for heavier patients. Also, to meet unit-specific preferences, nurses and physicians, using a variety of possible formulas, may re-do calculations and change medication solutions on transfer. This practice not only wastes time and materials, it poses risks due to the interruption of medication (particularly for cardiac patients on medications such as adrenaline or dopamine) and repeats the risks inherent in providing medication.
The traditional variable concentration approach is subject to errors in the calculation of the solution drug concentration, preparation of the individual infusion or the setting of the solution infusion rate (8–10). Using a small number of centrally prepared SCs reduces the potential for error in solution preparation and the risk of contamination from multiple-use medication vials. ‘Smart pumps’ may be used to implement SCs, reducing calculation and rate-setting errors, but this technology is expensive and may not be in place.
It was hypothesized that hospital departments caring for critically ill children could make the transition from unit-specific variable concentration drug preparation to SCs without substantial additional infrastructure or funding, in a manner acceptable to frontline users. The present report describes the design and implementation of SCs of selected high-alert medications, with computer support, at a Canadian children’s hospital. The intent was to improve and standardize provision of medications across three main hospital areas – the emergency department (ED), the paediatric intensive care unit (PICU) and the operating room (OR).
METHODS
Setting
The Children’s Hospital of Eastern Ontario (CHEO) in Ottawa, Ontario, is a quaternary care paediatric teaching hospital associated with the University of Ottawa. In 2006, the year that SCs were introduced across these areas of critical care, there were 54,000 visits to the ED, 7239 surgeries and 594 patients admitted to the PICU. Patients previously received variable drug concentrations, with differences among units and clinicians regarding formulation of solutions.
Initial consultations
On the initiative of the pharmacy department, key stakeholders were invited to participate in a multidisciplinary team to explore the feasibility and to plan the implementation of a SC system. Broad initial consultations were held with medical, nursing and administrative staff in the ED, the OR and the PICU. Inclusive meetings were also held with key individuals in the departments of cardiology, cardiovascular surgery and diagnostic imaging, as well as with the resuscitation team.
It was agreed that SCs should be implemented for the most frequently prescribed high-alert drugs. The consultations resulted in a comprehensive list of specific concerns, and a staged action plan to address them. It was decided to examine the effect of SCs on the volume of fluid patients received; to provide an accurate infusion rate calculation tool to minimize human error; and to plan for a seamless transition to the new system.
Assessment of potential impact on infusion volume
A retrospective chart review was performed to study the potential for fluid overload in infants receiving multiple medications. The 24 h fluid volume from variable concentration drug infusions for PICU patients weighing less than 20 kg and receiving one or more continuous intravenous medication infusion(s) was calculated. The volumes actually administered were compared with the volumes that would have been received had SCs been used, using a paired t test. A potential trend between fluid volume differences and weight was tested using Pearson’s correlation. It was decided a priori that a 10% increase in solution volumes was clinically significant.
Data were compiled and analyzed using SPSS version 14.0 (SPSS Inc, USA). The present study was approved by the CHEO Ethics Committee.
Computer program to choose drug concentration and to determine infusion rate
A program based on Microsoft Excel 2000 (Microsoft Corporation, USA) was developed for use in the PICU, the ED and the OR. It was modified from a program that was originally developed for the neonatal intensive care unit (NICU) at The Ottawa Hospital, and was introduced into the CHEO NICU in 2005.
Educational program
Educational packages and in-service programs were provided to all nurses and physicians in the PICU and the ED, anesthesiologists and anesthesia assistants in the OR, and pharmacists. Information-only sessions were given to nurses in the OR, recovery room and diagnostic imaging. Nurses in the ED and the PICU completed a test which was graded pass or fail, with follow-up and retesting for failed tests. It was predetermined that a minimum of 75% of nurses in both the PICU and the ED had to have successfully completed all components of the training program and passed the test before ‘going live’ with SCs.
Follow-up activities
A convenience sample of computer calculation sheets generated in the PICU during the month following the institution of SCs was compiled to assess the effect on volumes administered.
Medication error reports for the PICU were compiled for periods preceding and following the institution of SCs.
Ongoing education was carried out for staff who use SCs infrequently, and to maintain competency in backup procedures.
Staff were surveyed nine months following implementation of SCs to determine satisfaction and perceptions regarding patient safety and quality of care.
RESULTS
Projected effect of SCs on fluid volumes in lower-weight children
To examine the potential impact of SC infusions, details of 78 infusions received by 48 successive infants weighing less than 20 kg in the PICU were compiled. Sixteen of the 48 infants weighed less than 5 kg, and 36 of the 78 infusions were set at rates of less than 1 mL/h for a portion of the total therapy. The medication-related fluid volumes were compared with the volumes that would have been received had SCs been in use (Table 1). No significant increase was observed between the volume of infusions actually received and the volume that would have been delivered using SCs (the difference between the average projected and actual infusion volumes was −0.43 mL/kg/day; 95% CI −0.95 to 0.08; P=0.100).
TABLE 1.
Projected volume infused using standard concentrations compared with volume actually received using variable concentrations in infants weighing less than 20 kg
| Drug | n | Mean difference in volume (hypothetical – received), mL/kg/day (range) | % difference in volume (hypothetical – received) (95% CI [range]) |
|---|---|---|---|
| Total | 78 | –0.4 (−8.7 to 6.4) | –8.9 (−17 to 0.8) |
| Dopamine | 13 | –0.3 (−1.0 to 0.05) | –20.1 (−32.7 to −7.4) |
| Adrenaline | 7 | –1.6 (−8.7 to 0.06) | –8.7 (−35.9 to 18.5) |
| Fentanyl | 12 | –0.5 (−7.4 to 1.9) | 4.2 (−17.1 to 25.4) |
| Milrinone | 13 | –1.0 (−3.9 to 0.01) | –23.1 (−33.3 to −12.9) |
| Morphine | 30 | 0.2 (−6.0 to 6.4) | –1.2 (−18.8 to 16.4) |
| Vasopressin | 3 | –2.5 (−4.5 to −0.2) | –28 (−54.2 to −2.5) |
n Number of infusions
For a large majority (80%) of infusions, the difference in fluid volumes between the two dosing methods was less than 10% of the volume received (Table 2), with most of the deviations associated with morphine infusions.
TABLE 2.
Frequency that the projected difference in infusion volumes was less than 10% of the volume received in infants weighing less than 20 kg
| Drug | n | N | % (95% CI) |
|---|---|---|---|
| Total | 78 | 62 | 80 (69 to 87) |
| Dopamine | 13 | 13 | 100 (77 to 100) |
| Adrenaline | 7 | 6 | 86 (49 to 97) |
| Fentanyl | 12 | 11 | 92 (65 to 99) |
| Milrinone | 13 | 12 | 92 (67 to 99) |
| Morphine | 30 | 17 | 57 (39 to 73) |
| Vasopressin | 3 | 3 | 100 (44 to 100) |
n Number of infusions; N Number of infusions in which the projected difference in infusion volumes was less than 10% of the volume received
There was no correlation between patient weight and higher projected volumes using SCs for any of the medications examined (Table 3), thus the concern regarding excessive volumes in lower weight patients was not borne out. Pearson’s correlation (r) values were very low, much less than 0.8 – the level that would indicate a possible trend.
TABLE 3.
Correlation of patient weight versus projected difference in weight-adjusted infusion volumes between variable concentration and standard concentration medications in infants weighing less than 20 kg
| Drug | n | Pearson’s correlation (r) | P |
|---|---|---|---|
| Total | 78 | 0.160 | 0.163 |
| Dopamine | 13 | –0.295 | 0.328 |
| Adrenaline | 7 | 0.312 | 0.495 |
| Fentanyl | 12 | 0.120 | 0.711 |
| Milrinone | 13 | 0.350 | 0.241 |
| Morphine | 30 | 0.062 | 0.746 |
| Vasopressin | 3 | 0.107 | 0.932 |
r>0.8 signifies a significant correlation between patient weight and the difference in weight-based infusion volumes using standard concentrations versus variable concentrations. n Number of infusions
Based on these results, the number of proposed SCs for morphine was increased from two to four. The SCs finally selected (Table 4) were based on these results as well as physician consultation, availability of commercially premixed formulations in Canada and comparison with SCs used at other paediatric hospitals. Morphine concentrations are the only potentially confusing ‘look-alike’ concentrations that differ by a factor of 10.
TABLE 4.
Standard concentrations of intravenous medications
| Medication | Standard concentrations (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Dobutamine | 800 | 1600 | 3200 | 5000 | ||
| Dopamine | 800* | 1600* | 3200* | 5000* | ||
| Adrenaline | 6 | 12 | 25 | 50 | 100 | 200 |
| Fentanyl | 2 | 10 | 50* | |||
| Midazolam | 200 | 1000* | ||||
| Milrinone | 60 | 100 | 200 | 400 | ||
| Morphine | 50 | 100 | 500 | 1000* | ||
| Noradrenaline | 6 | 12 | 25 | 50 | 100 | 200 |
Commercially available premixed solutions
Computerized concentration selection and infusion rate determination
An Excel-based program for choosing the SC and calculating the patient-specific infusion rate that had previously been implemented in the NICU was modified and installed on the CHEONET intranet.
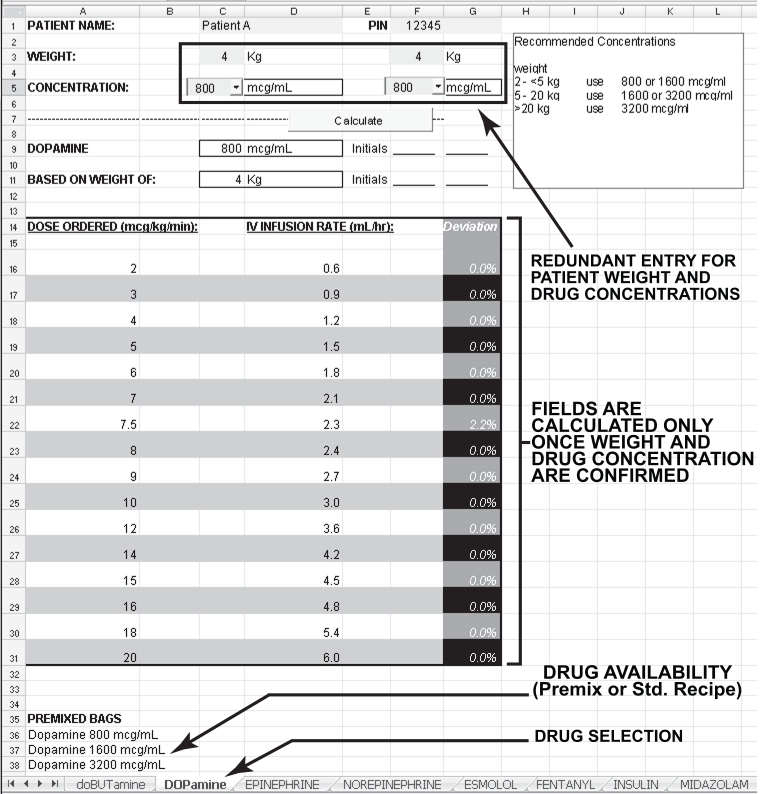
The program forces the sequence of data entry. It also ensures that data from only one patient is on the screen, avoiding possible confusion if the computer has been left unattended. The medication page is selected from the medications listed across the bottom. Figure 1 depicts the screen for dopamine. The patient’s weight is entered twice to decrease the risk of entry error. Possible SCs are then indicated on the screen, and one is selected from a drop-down menu (perfomed twice to reduce the risk of error). The selection process is guided by weight-based recommendations visible on the screen. The final selection is determined by the user, and is influenced by factors including patient fluid volume requirements and dose magnitude. A table then appears listing the possible infusion rates, and the deviation from the prescribed dose (infusion pump settings have increments of 0.1 mL/h, so that only a numerical coincidence would result in the exact prescribed dose). Patient information, selected drug concentration and infusion rate are then printed out, along with a step-by-step, fill-in-the-boxes manual dose verification (ie, back-calculation using the concentration and infusion rate to determine the dose). If the product is not available commercially, a standard recipe for preparation of a 50 mL syringe is also printed. Two nurses verify and sign this sheet, and proceed with the rest of the standard drug administration, as well as double-check safety procedure (correct drug, correct dose, correct rate, etc).
Figure 1.
Standard concentrations computer screen
The ‘deviation’ column, which shows the per cent difference between the prescribed dose and the dose proposed to be administered, was added following staff consultations. This feature has been well accepted because it helps to ensure reliability of drug dosing. The SCs have been selected so that the difference between the infused and prescribed doses will generally be less than 10%. A larger deviation is permitted if the physician deems it medically insignificant. This is most likely to happen at low doses when a patient is being weaned off a medication (eg, dopamine below 5 μg/kg/min).
A back-up manual was produced for use during computer failure.
Implementation
In the week before the ‘go live’ date, the SC program was promoted with e-mails to all users, and posters were displayed in the ED, the OR, the PICU and the pharmacy department. On the day the new system ‘went live,’ 76% of the ED nurses and 82% of the PICU nurses had successfully completed the in-service training, educational package and testing program. All new staff completed the same training process. The NICU also incorporated the new features of the SC program. Physicians were now required to give orders in the format of the computer program to eliminate possible errors in the conversion of units.
Post-hoc analyses
A convenience sample of 26 computer sheets generated in the PICU (55% of the 47 SC infusions ordered) during the first month after the institution of SCs were compiled to assess the effect on volumes administered. Comparison of the infusion volumes (data not shown) demonstrated no clinically significant differences compared with the volumes that would have resulted from the previous variable concentration approach. The largest increase in flow rate as a result of SCs was 18.9 mL/day (2.1 mL/kg/day for a 9 kg child receiving morphine), and the greatest decrease was 112.2 mL/day (5.1 mL/kg/day for a 22 kg child receiving adrenaline).
High-alert drugs are used less frequently in the ED and OR than in the PICU; to maintain competency, a bimonthly quiz is e-mailed by pharmacy staff to ED nursing staff and anesthesia assistants to complete and hand in for marking. Follow-up is carried out for any failed results.
Nurses, physicians, anesthesiologists and anesthesia assistants in the ED, the OR and the PICU were surveyed nine months after implementation. Among 43 respondents (29% response rate), 95% believed that SCs improved patient safety, 95% believed that SCs improved continuity of care and 77% believed that SCs decreased drug delivery time.
Throughout the hospital, before implementation of SCs, there had been no sentinel events with the SC medications. Incidents related to the targeted medications averaged 2.4 per year over the previous eight years. During 19 months postimplementation, three errors were reported. One implementation incident involved an infusion calculated with the ‘rule of six’ in the OR that was then switched to SCs in the PICU. Two incidents in the PICU involved changing the drug concentration without generating a new computer printout. None of the events resulted in patient harm.
In recognition that medication errors are under-reported (11,12), an institutional online system for incident reporting was initiated six months postimplementation of SCs. In the PICU, during the three-month period, from January to March in 2006 and 2007, with and without online reporting respectively, the total reported medication-related incidents doubled from 10 to 21. However, there was no corresponding increase in reported errors regarding high-alert medications provided as SCs, with only one incident reported during each time period.
DISCUSSION
SCs of high-risk infusions were successfully implemented across areas of critical care in a paediatric hospital, using a computer program that incorporated multiple safety features. This change of practice involved consultation; recognition of and addressing potential barriers to acceptance of the new practice; careful planning and education, and responding to feedback; follow-up education so that new skills were reinforced; and follow-up assessment of fluid volumes, errors and incidents, and staff perceptions.
A consistent method for intravenous medications was needed for the ED, the PICU and the OR, to promote continuity of care for patient transfer across these units. The SC program eliminated redundant preparation of solutions, and reduced the potential for error in provision of these potent drugs with narrow windows of efficacy and safety. This is one of the first reports of SCs being instituted across multiple areas of a paediatric institution, with a computer program to calculate the flow rate for syringe pump rather than relying on ‘smart pump’ technology (13,14).
Addressing the concern that SCs may lead to increased fluid volumes for critically ill children receiving multiple medications, it was found that infusion volumes would not be significantly increased. Rigorous analysis eliminated the potential barrier and opened up opportunities for dialogue, education and acceptance of the new practice.
Computers are powerful tools for improving patient safety because computerized physician order-entry systems may significantly reduce prescription and transcription errors (15–17). While computerized physician order-entry alone may not reduce errors in administration of medications (15), SCs address safety in this area.
SCs have been successfully implemented with ‘smart pump’ technology in a PICU in a community hospital (13) and across an entire paediatric hospital (14). The latter initiative resulted in significant reductions in overdoses and errors in preparation and administration of medications, and also demonstrated that proper selection of SCs could ensure appropriate fluid volumes, even in neonates. Even without the technology, instituting a list of SCs in a PICU resulted in significantly less use of nonstandard solutions and decreased errors in both medication concentration and dose (18).
Significant changes to the way in which an institution orders, delivers and stocks drugs may give rise to additional opportunities for problems or errors during the transition. In this case, staff were supportive and competent in the new practices before the implementation so that change occurred as planned in a systematic manner, with only a single incident of failure to use the new SC system.
A major goal in the implementation of SCs is to improve patient safety and care. In the present case, a low baseline rate of incidents was reported. It is encouraging that SC medication error reports did not increase in step with other medication errors, with implementation of the online reporting, and that the three errors reported were procedural, none of which resulted in patient harm. A survey demonstrated the staff perception that SCs improved medication safety and resulted in faster drug delivery to patients; however, these perceptions need to be verified in a more rigorous manner.
To maximize medication safety, it is recommended that hospitals use centrally prepared SCs. This recommendation poses challenges for hospitals operating without a 24 h pharmacy. The SC program addressed this concern by using premixed solutions where available. The preparation of nonpremixed solutions by nursing and anesthesia staff was facilitated by the provision of a standard recipe for the selected SC available on the computer printout. Compared with variable concentrations, the overall drug preparation workload was decreased due to the availability of the premixed SC solutions.
The SC program has recently been expanded with the addition of more drugs, with the goal of including approximately 40 high-alert continuous-infusion medications in 2008. To further consolidate medication information and administration, the ‘paediatric advanced life support’ program for bolus drugs has also been incorporated into the SCs computer program. Finally, the program has been modified to a wizard format that can be accessed via a Web-based interface.
CONCLUSION
SCs of high-alert drugs were successfully implemented across all critical care units in a Canadian paediatric hospital, following an analysis demonstrating that SCs would not result in increased infusion volumes for critically ill children. The computer program has multiple safety features to calculate the infusion rate for the selected SC, including a forced sequence of data entry to avoid confusion between patients or medications, duplicate entries, a ‘deviation’ column to indicate how closely the infused dose matches the prescribed dose, a printout to guide manual dose verification and a standard recipe if the medication provided is not premixed. The SC program contributes to continuity of care across critical care departments; easing transfer of patients between the ED, the OR and the PICU; and eliminating reformulation of medications on transfer. SCs with computer support, in lieu of smart pump technology, have reduced potential for error in the provision of high-risk drugs, and meet the requirements of the Canadian Council on Health Services Accreditation and the JCAHOs for SCs of high-alert intravenous medications using syringe pumps without ‘smart pump’ technology.
Acknowledgements
The authors thank the staff members who contributed their time, expertise and good will to this project, including Chris Sorfleet, Julie Wade and Claire Laframboise.
Footnotes
SOURCE: This study was funded internally by the Children’s Hospital of Eastern Ontario (Ottawa, Ontario).
REFERENCES
- 1.Levine SR, Cohen MR, Blanchard NR, et al. Guidelines for preventing medication errors in pediatrics. J Pediatr Pharmacol Ther. 2001;6:426–42. [Google Scholar]
- 2.Joint Commission Perspectives on Patient Safety. Maintaining safety: Reducing risk. < http://www.theschwartzcenter.org/news/news/Implementing.pdf> (Version current at April 18, 2008)
- 3.Brown M. Medication safety issues in the emergency department. Crit Care Nurs Clin North Am. 2005;17:65–9. xi. doi: 10.1016/j.ccell.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Cohen H, Mandrack MM. Application of the 80/20 rule in safeguarding the use of high-alert medications. Crit Care Nurs Clin North Am. 2002;14:369–74. doi: 10.1016/s0899-5885(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 5.JCAHO’s compliance expectations for standardized concentrations. Rule of Six in pediatrics does not meet requirements. Jt Comm Perspect. 2004;24:11. [PubMed] [Google Scholar]
- 6.Canadian Council on Health Services Accreditation. Patient Safety Goals and Required Organizational Practices. < http://www.cchsa.ca/default.aspx?page=113&cat=30> (Version current at April 18, 2008)
- 7.Siberry GK, Iannone R. The Harriet Lane Handbook, 15th edn. St Louis: Mosby; 1999. [Google Scholar]
- 8.Parshuram CS, Ng GY, Ho TK, et al. Discrepancies between ordered and delivered concentrations of opiate infusions in critical care. Crit Care Med. 2003;31:2483–7. doi: 10.1097/01.CCM.0000089638.83803.B2. [DOI] [PubMed] [Google Scholar]
- 9.Lesar TS. Errors in the use of medication dosage equations. Arch Pediatr Adolesc Med. 1998;152:340–4. doi: 10.1001/archpedi.152.4.340. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro JM, Mitchell AL, Lesar TS. Systematic steps to diminish multi-fold medication errors in neonates. J Pediatr Pharmacol Ther. 2003;8:266–73. doi: 10.5863/1551-6776-8.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal R. Using chart review to screen for medication errors and adverse drug events. Am J Health Syst Pharm. 2002;59:2323–5. doi: 10.1093/ajhp/59.23.2323. [DOI] [PubMed] [Google Scholar]
- 12.Kozer E, Scolnik D, Jarvis AD, Koren G. The effect of detection approaches on the reported incidence of tenfold errors. Drug Saf. 2006;29:169–74. doi: 10.2165/00002018-200629020-00007. [DOI] [PubMed] [Google Scholar]
- 13.Roman N. Innovative solutions: Standardized concentrations facilitate the use of continuous infusions for pediatric intensive care unit nurses at a community hospital. Dimens Crit Care Nurs. 2005;24:275–8. doi: 10.1097/00003465-200511000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Larsen GY, Parker HB, Cash J, O’Connell M, Grant MC. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics. 2005;116:e21–5. doi: 10.1542/peds.2004-2452. [DOI] [PubMed] [Google Scholar]
- 15.Wang JK, Herzog NS, Kaushal R, Park C, Mochizuki C, Weingarten SR. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics. 2007;119:e77–85. doi: 10.1542/peds.2006-0034. [DOI] [PubMed] [Google Scholar]
- 16.Potts AL, Barr FE, Gregory DF, Wright L, Patel NR. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics. 2004;113:59–63. doi: 10.1542/peds.113.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann CU, Kim GR, Gujral R, Veltri MA, Clark JS, Miller MR. Decreasing errors in pediatric continuous intravenous infusions. Pediatr Crit Care Med. 2006;7:225–30. doi: 10.1097/01.PCC.0000216415.12120.FF. [DOI] [PubMed] [Google Scholar]
- 18.Bullock J, Jordan D, Gawlinski A, Henneman EA. Standardizing IV infusion medication concentrations to reduce variability in medication errors. Crit Care Nurs Clin North Am. 2006;18:515–21. doi: 10.1016/j.ccell.2006.08.008. [DOI] [PubMed] [Google Scholar]