Abstract
We applied recombinant forms of the Rho-related small guanosine triphosphatases (GTPases) Rac2 and Cdc42/G25K to permeabilized mast cells to test their ability to regulate exocytotic secretion. Mast cells permeabilized with streptolysin-O leak soluble (cytosol) proteins over a period of 5 min and become refractory to stimulation by Ca2+ and guanosine triphosphate (GTP)γS over about 20–30 min. This loss of sensitivity is likely to be due to loss of key regulatory proteins that are normally tethered at intracellular locations. Exogenous proteins that retard this loss of sensitivity to stimulation may be similar, if not identical, to those secretory regulators that are lost. Recombinant Rac and Cdc42/G25K, preactivated by binding GTPγS, retard the loss of sensitivity (run-down) and, more importantly, enable secretion to be stimulated by Ca2+ alone. Investigation of the concentration dependence of each of these two GTPases applied individually to the permeabilized cells, and of Cdc42/G25K applied in the presence of an optimal concentration of Rac2, has provided evidence for a shared effector pathway and also a second effector pathway activated by Cdc42/G25K alone. Dominant negative mutant (N17) forms of Rac2 and Cdc42/G25K inhibit secretion induced by Ca2+ and GTPγS. Our data suggest that Rac2 and Cdc42 should be considered as candidates for GE, GTPases that mediate exocytosis in cells of hematopoeitic origin.
INTRODUCTION
Although the events determining membrane fusion are likely to be the same, it is apparent that the upstream pathways regulating the commitment to fusion in exocytosis in different classes of cells vary widely. Depending on the class of secretory cell, cytosol Ca2+ (Knight and Baker, 1982), cAMP (Dormer and Ashcroft, 1974; McMillian et al., 1988), or activation of a guanosine triphosphate (GTP)-binding protein (so-called GE [Gomperts, 1990]) provides the main regulatory impetus. Most information regarding intracellular regulators for secretion has been derived from experiments with permeabilized cells that allow access to, and therefore manipulation of, the cytosol composition (Lindau and Gomperts, 1991). For myeloid and other cells of hematopoeitic origin including platelets (Athayde and Scrutton, 1990), T-lymphocytes (Mittrucker and Fleischer, 1992), neutrophils (Barrowman et al., 1986), eosinophils (Cromwell et al., 1991; Nusse et al., 1990), and mast cells (Lillie and Gomperts, 1992), secretory activity can be induced by nonhydrolyzable analogs of GTP such as GTPγS, but the precise identification of the guanosine triphosphatases (GTPases), GE, mediating secretion remains obscure. For mast cells there are now indications, depending on the nature of the stimulus, for involvement of the heterotrimer Gi3 (Aridor et al., 1993) and for a low Mr GTP-binding protein related to Rho (O’Sullivan et al., 1996).
In addition to the manipulation of low Mr regulators, individual proteins can be introduced into the cytosol of permeabilized cells and tested for their effects on the secretory mechanism. Secretory cells, permeabilized by reagents such as streptolysin-O (SL-O) or digitonin, leak endogenous proteins (monitored as lactate dehydrogenase) within minutes, but their propensity to respond to stimulation generally declines over a much longer time period. This so-called “run-down” has been ascribed to the loss of tethered proteins that may act as essential regulators, or even as components of the fusion mechanism leading to exocytosis (Howell and Gomperts, 1987; Ali and Burgoyne, 1990; Nishizaki et al., 1992; Matsuda et al., 1994; O’Sullivan et al., 1996). Exogenous proteins provided to the permeabilized cells that retard or accelerate the rate of run-down should be considered as candidates, or at least surrogates, for these regulators, capable of insinuating themselves into the pathway to replace others that have been lost by detachment and leakage. The complex of Rac1/RhoGDI (FOAD-II) isolated from bovine brain can retard, and RhoGDI applied alone can accelerate, run-down (Mariot et al., 1996; O’Sullivan et al., 1996), and this indicates that one (or more) of the Rho-related proteins is likely to be a GTPase-mediating exocytosis (GE) in these cells.
In this paper we demonstrate that recombinant forms of Rac and Cdc42/G25K (both members of the Rho family of GTPases) can act as regulators for secretion in mast cells.
MATERIALS AND METHODS
Human thrombin was obtained from Sigma Chemical (St. Louis, MO) as a frozen solution. [3H]-GTP and [3H]-guanosine diphosphate (GDP) solutions were obtained from DuPont NEN (Stevenage, United Kingdom). The G75 Superdex (preparation grade) and rapid desalting columns were obtained from Pharmacia (Uppsala, Sweden). SL-O was obtained from Murex Diagnostics (Dartford, Kent, UK). GTPγS was obtained as a frozen stock (100 mM) from Boehringer Mannheim (Mannheim, Germany). Anti-Cdc42/G25K antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal anti-Rac was from Upstate Biotechnology (Lake Placid, NY). HRP-coupled anti-rabbit IgG was from Pierce (Chester, United Kingdom), and HRP-coupled anti-mouse IgG was from Bio-Rad (Hemel Hempstead, United Kingdom). ECL detection kit was purchased from Amersham (Amersham, Buckinghamshire, United Kingdom). All other chemicals used were of the highest quality available from standard commercial sources.
Production of Recombinant Proteins
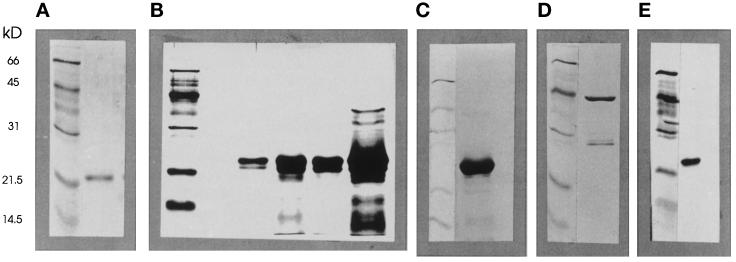
Recombinant proteins were expressed as GST fusion proteins in Escherichia coli. Recombinant Rac2 was purified essentially as described (Kwong et al., 1993). Recombinant Cdc42/G25K, Myc-tagged Cdc42/G25K, and N17-Cdc42/G25K were purified as described by Self and Hall (1995a). After removal of thrombin with p-aminobenzamidine agarose beads, all proteins were subjected to gel filtration (G75 Superdex) to remove any remaining impurities and high Mr aggregates. Proteins were eluted in the relevant purification buffer with added proteinase inhibitors (PMSF [0.1 mM], pepstatin A [1 μg ml−1], leupeptin [1 μg ml−1]). The purity of all recombinant proteins was assessed by electrophoretic separation on 12% SDS-polyacrylamide gels (Laemmli, 1970) and detection by silver staining (Morrissey, 1981) (see Figure 1).
Figure 1.
Analysis of recombinant GTPases. All recombinant proteins used in this work were analyzed for purity by electrophoresis in 12% SDS-polyacrylamide gels and detection by silver staining. The panels illustrate the positions of Mr markers as indicated together with (a) Rac2; (b) Cdc42/G25K (four preparations); (c) N-Myc-Cdc42/G25K; (d) N17-Rac2-GST with GST; (e) N17-Cdc42. Note the heterogeneity of Cdc42/G25K, variable between preparations (b) and the relative homogeneity of the N-Myc tagged Cdc42/G25K product (c).
The production of the N17-Rac2 mutant presented some problems because after induction by isopropyl-d-thiogalactopyranoside, most of the product was insoluble. Without induction, a low but sufficient level of constitutive synthesis of soluble GST-fusion protein was achieved, but after cleavage with thrombin this still adhered strongly and nonspecifically to many surfaces, including the glutathione Sepharose beads used in the purification, and could not then be eluted. N17-Rac2, was therefore purified as the GST fusion protein that remained strongly and nonspecifically adherent. SDS-PAGE analysis of this preparation (see Figure 1d) revealed the presence of two proteins, one being the fusion protein (52 kDa, 66%), the other being pure GST (28 kDa, 33%), and it was used in this form without further purification. Due to the adhesive properties of the thrombin-cleaved N17-Rac2, pure GST could be eluted from the glutathione Sepharose beads with 10 mM GSH, and then further purified by gel filtration. This was used as a control protein in secretion experiments.
Except for the N17-mutants of Rac2 and Cdc42/G25K, protein concentrations are expressed as their active concentrations, assessed according to their ability to bind [3H]-GTP and [3H]-GDP using a standard filter assay (Self and Hall, 1995a). The binding was measured at two different concentrations of protein, in triplicate (Y40C-Rac1 and F37A-Rac-1 in duplicate), and the experiments were repeated on three occasions (nontagged Cdc42/G25K, twice). In agreement with published results (Self and Hall, 1995a) approximately 10% of Rac2 and nontagged Cdc42, and 20% of N–Myc-Cdc42/G25K bind guanine nucleotide and are regarded as active. Total protein concentrations were determined by Coomassie blue binding assay (Bradford, 1976) using BSA as the calibration standard.
Preactivation of GTPases
Where indicated, proteins were “preactivated” by binding GTPγS in a buffer (pH 8) comprising Tris (20 mM), EDTA (3 mM), MgCl2 (0.16 mM: free Mg2+ = 2.75.10−8 M), DTT (1 mM), NaN3 (0.02%), in the presence of GTPγS (1 mM), for 10 min at 30°C. After this, MgCl2 was added to a final concentration of 4 mM (total), and the protein was immediately loaded onto a rapid desalting column (Pharmacia) and eluted with an iso-osmotic salts buffer (pH 6.8) comprising NaCl (137 mM), KCl (2.7 mM), MgCl2 (1 mM), piperazine-N,N′-bis(2-ethanesulfonic acid) (20 mM), supplemented with DTT (1 mM), EGTA (0.3 mM), and proteinase inhibitors (PMSF [0.1 mM], pepstatin A [1 μg ml−1], leupeptin [1 μg.ml−1]). This step served the dual purposes of removing unbound GTPγS and also exchanging the protein into an iso-osmotic buffer (pH 6.8) suitable for applying to permeabilized cells.
N17-Rac2 and N17-Cdc42/G25K were used without preactivation after dialysis against the isotonic pH 6.8 buffer for 15 h using a BRL Laboratories (Gaithersburg, MD) microdialyser (3 kDa Mr cut-off).
Secretion Measurements
Cells were obtained by peritoneal lavage of male Sprague Dawley rats (>300 g), and mast cells were purified to greater than 98% purity by centrifugation through Percoll as previously described (Tatham and Gomperts, 1990). Cells, suspended in the iso-osmotic salts buffer (pH 6.8) supplemented with BSA (1 mg ml−1), were incubated with metabolic inhibitors (2-deoxyglucose (0.6 mM), and antimycin A (10 μM)) for 5 min at 37°C, and then cooled to ice temperature and added to SL-O (1.6 IU.ml−1) in the presence of EGTA (0.1 mM) at 0°C. After 5 min, cells were washed free of unbound SL-O and contaminating impurities (Larbi and Gomperts, 1996) by dilution and centrifugation at 4°C. Permeabilization and hence rundown were initiated by transferring the cells to prewarmed (37°C) iso-osmotic salts buffer (see above) supplemented with Ca·EGTA (0.3 mM to regulate pCa8), Mg·ATP (1 mM), and proteins under test. After allowing predetermined times for rundown (generally between 5 and 20 min), the cells were stimulated to secrete by transfer to solutions (final volumes 60 μl) contained in the V-wells of microtiter plates (8×12 format) containing Ca·EGTA buffers (3 mM) formulated to regulate pCa5 (or pCa7 for controls) and GTPγS to a final concentration of 100 μM (or zero) with sufficient Mg·ATP to maintain the concentration at 1 mM. After 20 min to allow secretion to approach completion, the reactions were quenched by addition of ice-cold buffer supplemented with EGTA (10 mM), and the cells were sedimented by centrifugation. The supernatants were sampled for measurement of secreted hexosaminidase as previously described (Tatham and Gomperts, 1990; Gomperts and Tatham, 1992).
Calcium/EGTA buffers were prepared by mixing solutions of EGTA and end-point titrated Ca·EGTA, made up at identical concentrations and adjusted to pH 6.8, according to a computer program (Tatham and Gomperts, 1990).
Secretion is expressed as the percent of total cellular hexosaminidase released, calibrated by reference to appropriate reagent blanks and the total cell content released by 0.1% Triton X-100. All determinations were carried out in quadruplicate unless otherwise stated.
Leakage of Rac and Cdc42
Purified mast cells were treated with diisopropyl fluorophosphate (2 mM) for 10 min at room temperature. The cells were treated with SL-O at ice temperature as described above and then permeabilized by bringing the temperature to 37o. Samples of cells were removed at intervals and promptly sedimented and the supernatants were harvested. Ice-cold acetone was added to the supernatants to a final concentration of 80%, and the mixture was maintained at −20°C for 2 h after which the aggregated proteins were sedimented by centrifugation. These were taken up in Laemmli sample buffer and separated on 12% polyacrylamide gels. Proteins were transferred to nitrocellulose using a wet blot method and probed for Cdc42 and Rac using specific antibodies according to manufacturers’ instructions. Antibody binding was detected using appropriate HRP-linked secondary antibodies and an ECL detection kit.
Presentation of data
Least squares fitting (without weighting) was carried out using Origin (Origin Microcal, Northampton, MA) software.
RESULTS
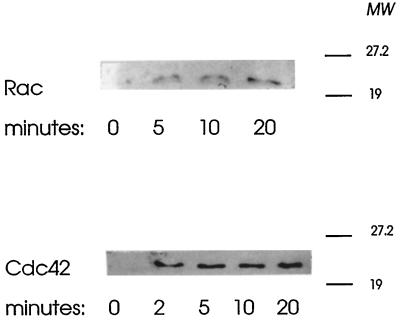
After permeabilization of mast cells with SL-O, the leakage of soluble proteins (measured as lactate dehydrogenase) is effectively complete within 5 min (Howell and Gomperts, 1987). RhoGDI leaks from permeabilized mast cells according to a similar rapid timecourse (O’Sullivan et al., 1996) and as shown in Figure 2, the monomeric GTPases Rac and Cdc42 also leak after permeabilization. These proteins both possess the consensus sequence for posttranslational modification leading to attachment of geranylgeranyl groups at their carboxy termini, and as a consequence they are likely to be either tethered at specific membrane locations (Didsbury et al., 1990) or components of soluble complexes with an escort protein such as RhoGDI. Regardless of this they leak rapidly from the permeabilized cells. We have investigated whether provision of recombinant Rac2 and Cdc42/G25K to permeabilized mast cells can retard the loss of sensitivity to stimulation for secretion (rundown) after permeabilization.
Figure 2.
Leakage of Rac and Cdc42 from mast cells after permeabilization by SL-O. The permeabilized cells were sampled at the times indicated and rapidly sedimented, and the proteins present in the supernatants were separated on 12% SDS-polyacrylamide gels. After transfer to nitrocellulose membranes, the proteins were detected by probing with specific antibodies followed by ECL.
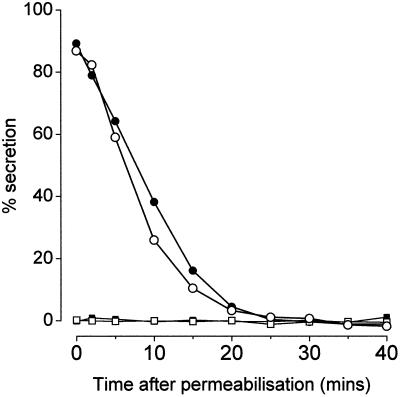
Figure 3 illustrates the extent of secretion elicited by application of a stimulus (pCa5 with 100 μM GTPγS) to mast cells either at the time of elevating the temperature to 37o (which allows the prebound SL-O to generate plasma membrane lesions) or at various times thereafter. Typically, these cells can respond to the stimulus by releasing close to 100% of their contained N-acetyl-β-glucosaminidase (hexosaminidase), but if the stimulus is delayed for a period of about 10 min, this declines to about 50% and then to zero at about 30 min. We refer to this decline in response to stimulation by effectors of low molecular weight as “rundown.” As shown in Figure 3, recombinant Rac2, applied to the permeabilized mast cells in its nonactivated form as isolated, has little effect on the rate of rundown. In contrast, Rac2, preactivated by binding of GTPγS (see Figure 4) (active protein 1.5 μg. ml−1, 60 nM), reduced the rate of rundown and enhanced the extent of secretion at all times beyond 10 min of incubation. In the presence of preactivated Rac2 the secretory mechanism then remained sensitive to stimulation by the combined stimulus (Ca2+ plus GTPγS) for an extended period such that it was still possible to induce 15% hexosaminidase secretion even 40 min after permeabilization.
Figure 3.
Time course of rundown of permeabilized mast cells in the presence and absence of recombinant Rac2. The cells were permeabilized with SL-O in a buffer (pH 6.8) containing Mg·ATP (1 mM) and sufficient EGTA (0.1 mM) to suppress Ca2+ to below pCa8, and in the presence and absence of Rac2 (14.8 μg ml−1). At various times (indicated), samples were removed and stimulated by transfer to solutions containing Ca·EGTA (3 mM) to regulate pCa5 plus GTPγS (100 μM). After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. ○ and •, Cells stimulated with Ca2+ (pCa5) and GTPγS (100 μM); □ and ▪, unstimulated cells; filled symbols indicate presence of Rac2. Similar results were obtained on three occasions.
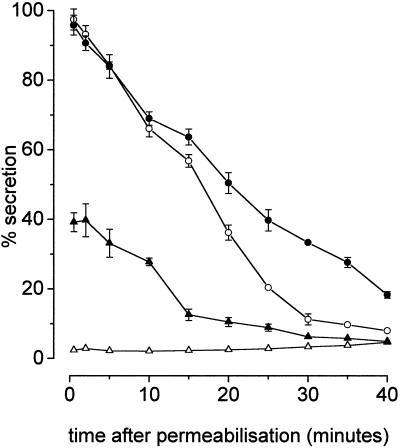
Figure 4.
Time course of rundown of permeabilized cells in the presence and absence of preactivated Rac2. The cells were permeabilized with SL-O in a buffer (pH 6.8) containing Mg·ATP and sufficient EGTA (0.1 mM) to suppress Ca2+ to below pCa8, and in the presence and absence of Rac2 (1.5 μg ml−1), which had been preactivated by binding GTPγS. At various times thereafter (indicated), samples were removed and stimulated by transfer to solutions containing Ca·EGTA (3 mM, ▵, ▴) to regulate pCa5 or Ca·EGTA (pCa5) plus GTPγS (100 μM, ○, •). After a further 20-min incubation, the cells were sedimented by centrifugation, and the supernatants were sampled for analysis of secreted hexosaminidase. Filled symbols indicate presence of preactivated Rac2. Similar results were obtained on four separate occasions.
More striking is the observation that for cells provided with preactivated Rac2, it becomes possible to induce exocytosis by provision of Ca2+ alone (see Figure 4). In the experiment illustrated, this caused 40% secretion when the Ca2+ stimulus (pCa5) was provided at the time of permeabilization, declining to zero when provided at times beyond 30 min. Clearly, the protein cannot have penetrated the cells at the time the immediate stimulus (Ca2+ alone) was applied, and so it is likely that the onset of Ca2+-induced secretion was delayed until the intracellular concentration of preactivated Rac2 had built up over its threshhold level for activation. However, we had to consider the alternative and trivial possibility that the secretion elicited by Ca2+ alone is due to the cooperation of free GTPγS liberated by detachment from the exogenous GTPase.
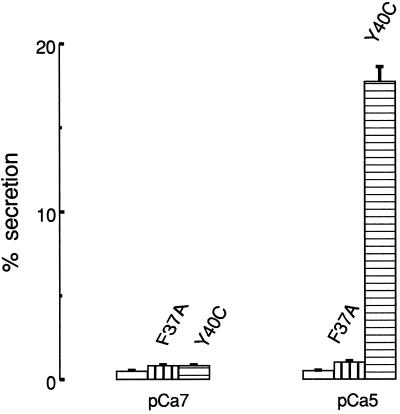
The experiment presented in Figure 5 shows the effects on secretion of two effector domain mutants of Rac1, both preactivated with GTPγS in the normal way. While one of these, Y40C-Rac1, provides a strong stimulus to secretion, the other, F37A-Rac1, is without effect even when presented at higher concentrations than those of Y40C-Rac1, which caused 17% secretion. The possibility that the secretion was evoked by Ca2+ acting in conjunction with free GTPγS liberated by detachment from the Rac2 is unsupported by our observations.
Figure 5.
Selective activation of exocytosis by effector mutants of Rac1. Rac1 mutants were preactivated with GTPγS as described and applied to permeabilized mast cells as outlined in the legend to Figure 3. After a period of 7 min, the cells were stimulated by addition of Ca2+ (pCa5), and the incubation was continued for a further 20 min. The mutant Y40C was applied at a concentration of 3.9 μg ml−1 activated protein, equivalent to 0.18 μM GTPγS, and the mutant F37A, which failed to induce secretion, was applied at 5.5 μg ml−1 activated protein, equivalent to 0.26 μM. Results are presented as means ± SEM (n = 4 determinations). Similar results were obtained on five separate occasions.
Concentration–effect relationships for the GTPγS-loaded Rac2 in the stimulation of secretion were established using Ca2+ alone and also Ca2+-plus-GTPγS as stimuli. Obviously, for these experiments it was important to delay the stimulus for a sufficient time for the proteins to diffuse into the cells and also to allow the secretory process to decline to a sufficient extent that the enhancement due to the exogenous protein would be apparent. When applying the Ca2+-only stimulus, the cells were allowed to run down for a relatively brief period (7 min), just sufficient to allow penetration of the cells by the exogenous protein, but when applying the much stronger dual stimulus (Ca2+ plus GTPγS) a longer period of run-down was allowed.
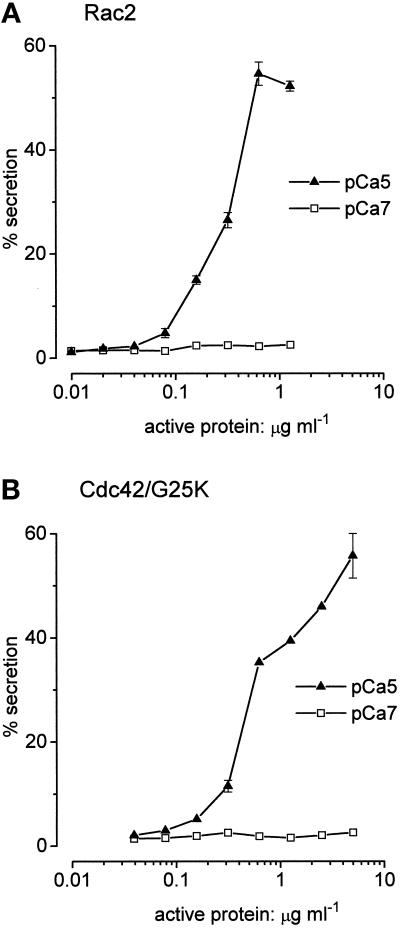
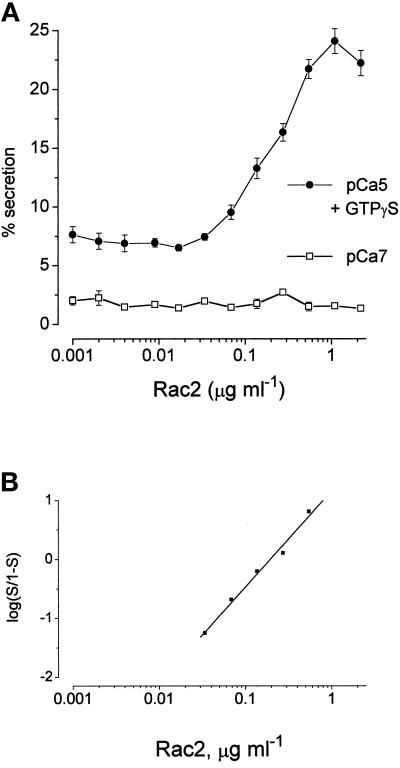
Figure 6a shows the effect of varying the concentration of preactivated Rac2 on secretion induced by Ca2+ alone (protein concentrations are given as the “active” concentrations as determined by guanine nucleotide-binding assays). In this experiment, the enhancement due to Rac2 became manifest at about 0.08 μg ml−1 and was optimal at about 0.6 μg ml−1 (0.025 μM), causing 60% of Ca2+-dependent secretion. The active concentration range (EC50 of about 0.33 μg ml−1, as drawn) encompasses about one decade. Analysis of the data from the experiment is illustrated in Figure 6a according to the logistic (Hill) expression, % secretion = 100*[Rac]P/([RacP] + KP), gives a value of p = 1.8 (least squares fitting for values of secretion lying between 5% and 95% gives r = 0.99; n = 3 data points). In two other experiments that we were able to analyze, we derived values of p = 1.9 and 2.3, and in two further experiments the slopes of the Hill plots were so steep that there were no data points within the useful range (5–95% secretion) for analysis. When the dual stimulus (Ca2+ plus GTPγS) was applied (see Figure 7), the range of concentrations of Rac2 enhancing secretion may have been somewhat extended. For the experiment illustrated, p = 1.6 (r = 0.99; n = 5 data points) but, in spite of the much longer period of time allowed for the run-down to occur, the midpoint concentration for preactivated Rac2 (EC50 0.23 μg ml−1, as drawn) was not significantly shifted with respect to stimulation by Ca2+ alone.
Figure 6.
Concentration–effect relationships for the enhancement by (a) Rac2 and (b) Cdc42/G25K (preactivated by binding of GTPγS) in mast cells stimulated by Ca2+ alone. In this experiment, the cells were run down for 7 min before the stimulus was applied. Protein concentrations, expressed as active protein binding [3H]-GTP, were applied according to a ×2 serial dilution scheme. Results are expressed as means ± SEM (n = 4). Similar results were obtained on four separate occasions (Rac) and on nine occasions (Cdc42). ▴, Cells stimulated with Ca2+ (pCa5); □, unstimulated cells.
Figure 7.
a) Concentration–effect relationships for the enhancement of secretion by Rac2 (preactivated by binding of GTPγS) in mast cells stimulated by Ca2+ plus GTPγS (100 μM). In this experiment, the cells were run down for 17 min before application of the stimulus. (a) Results are expressed as means ± SEM (n = 4). •, Cells stimulated with Ca2+ (pCa5) and GTPγS (100 μM); □, unstimulated cells. (b) Data of Figure 6a presented as a Hill plot. For secretion stimulated by Ca2+ plus GTPγS, slope p = 1.6 (r = 0.99, n = 5 data points). Similar results were obtained on four separate occasions.
The further augmentation of secretion due to the presence of free GTPγS suggests the involvement of at least one additional GTP-binding protein. Among possible candidates we should include the membrane-bound heterotrimeric G-protein Gi3, suggested to mediate signals due to receptor-mimetic agents such as mastoparan and compound 48/80 (Aridor et al., 1993). Also, in view of accelerated run-down and inhibition by RhoGDI (O’Sullivan et al., 1996; Mariot et al., 1996), we must include other small GTPases of the Rho-family. Since exocytosis can be induced by Rac, and since there exists a class of effector proteins (those possessing a so-called CRIB sequence, such as PAK [Burbelo et al., 1995]) that can be accessed by both Rac and Cdc42, but not by Rho, we have tested Cdc42/G25K as a possible activating GTPase for exocytosis.
Similar to Rac, the presence of preactivated Cdc42/G25K allows secretion to be stimulated by Ca2+ alone. In the experiment illustrated (Figure 6b), the effect of Cdc42/G25K first became apparent when applied to permeabilized cells at about 0.2 μg ml−1, and the extent of secretion was then progressively enhanced as the concentration of the protein was increased to 5 μg ml−1 and even above this. Unlike the response to Rac2, which is confined within a single decade range of protein concentration, the response to preactivated Cdc42/G25K extends over almost two decades.
We compared the secretory responses to preactivated Cdc42/G25K in cells stimulated by Ca2+ alone and by the combination of Ca2+ plus GTPγS (Figure 8). As with Rac2, the presence of free GTPγS enhances the extent of secretion over that achieved by Ca2+ alone but with the difference that this also enhances the sensitivity to the Cdc42/G25K GTPase (see Figures 5a and 6). For cells stimulated by Ca2+ alone the mean midpoint activating concentration of Cdc42/G25K (EC50) was 4.1 ± 1.2 μg ml−1 (n = four separate experiments) and when the combined stimulus was applied this declined to a mean value of 0.66 ± 0.9 μg ml−1 (the difference is significant at the p < 0.005 level). On the other hand, the mean values for the Hill coefficients are not significantly different (p = 1.07 ± 0.15 for secretion stimulated by Ca2+ alone and p = 0.73 ± 0.11 for stimulation by Ca2+ plus GTPγS).
Figure 8.
a) Concentration–effect relationship for the enhancement of secretion by preactivated Cdc42/G25K from mast cells stimulated by Ca2+ alone, or by Ca2+ plus GTPγS. In this experiment, the cells were run down for 7 (Ca2+ only) or 17 (Ca2+ plus GTPγS) minutes before application of the stimulus. Results are expressed as means ± SEM (n = 4). Similar results were obtained on four separate occasions. □, Cells stimulated with Ca2+ (pCa5) alone; •, cells stimulated by Ca2+ plus GTPγS (100 μM); ▴, unstimulated cells. (b) Data of Figure 7a presented as Hill plots. For secretion stimulated by Ca2+ alone, slope p = 0.9 (r = 0.98, n = 5 data points); for secretion stimulated by Ca2+ plus GTPγS, p = 0.6 (r = 0.97, n = 8 data points).
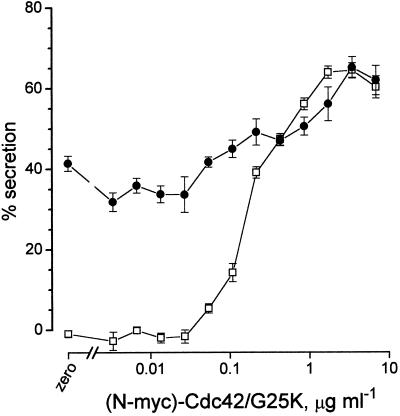
We should point out that although the extended (two decade) range of concentrations of Cdc42/G25K activating secretion was always observed, the actual concentrations (EC50) supporting secretion were subject to some variation, which appeared to be dictated by the quality of the various batches of protein. Routine analysis of each lot by SDS gel electrophoresis (see Figure 1b) revealed the presence of two or more polypeptides (on one occasion, four) running close together, indicative of small differences of molecular weight, possibly due to the action of bacterial endopeptidases. To obviate artefacts arising from this, we used a recombinant N-Myc–tagged Cdc42/G25K for all subsequent experiments in the hope that any endopeptidase activity would now damage the N-terminal tag while leaving the GTPase intact. After purification, analysis of the N-Myc–tagged Cdc42/G25K revealed only a single band at 25 kDa, and measurements of [3H]-GTP binding indicated that approximately 20% of this protein was active.
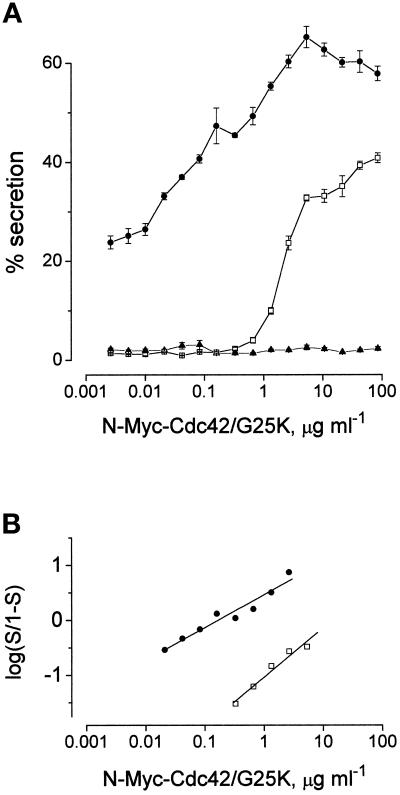
Figure 9 illustrates the results of an experiment designed to determine whether preactivated N-Myc-Cdc42/G25K can interact with a Rac2 effector in the stimulation of secretion. This protein also enhances secretion when applied over the extended (two decade) range of concentration (open symbols) characteristic of the untagged Cdc42/G25K. In the experiment illustrated, this extended from about 0.07 to 7 μg ml−1 (EC50 0.4 μg ml−1, as drawn) inducing a maximum of 65% secretion. In the same experiment, preactivated Rac2, applied at a concentration above its optimum (1 μg ml−1 active protein), elicited 40% secretion on stimulation with Ca2+ (pCa5) and when N-Myc-Cdc42/G25K was also provided at concentrations in the low end of its activation range (0.05–0.4 μg ml−1), it caused an additional increment of secretion. As the concentration of N-Myc-Cdc42/G25K was elevated above 0.4 μg ml−1, the simultaneous presence of the Rac2 was without apparent effect and the extent of secretion was about the same as that induced by N-Myc-Cdc42/G25K alone. As can be seen, even with the baseline of 40% secretion due to the presence of the Rac2, the activating effect of N-Myc-Cdc42/G25K is still expressed throughout its normal two-decade concentration range. This result is consistent with the idea that there may be two downstream effectors for these GTPases. While only one of these can be accessed by Rac2, Cdc42/G25K is able to access both.
Figure 9.
Concentration–effect relationships for preactivated N-Myc-Cdc42/G25K applied either alone or together with a saturating concentration of preactivated Rac2 (10 μg ml−1) in mast cells stimulated by Ca2+ (pCa5) alone. In this experiment the cells were allowed to run down for 7 min before application of the stimulus. Results are expressed as means ± SEM (n = 4). Open symbols indicate effect of N-Myc-Cdc42/G25K alone; closed symbols indicate effect of Rac2 (10 μg ml−1) and N-Myc-Cdc42/G25K.
The finding that RhoGDI can inhibit secretion permits the general conclusion that one or more members of the family of Rho GTPases act as regulators of secretion in mast cells (O’Sullivan et al., 1996). We have now shown that Rac2 and Cdc42/G25K are capable of replacing (at least in part) the role of free guanine nucleotide in this process. However, it does not follow from this that either of these GTPases constitutes the authentic GE mediating GTP-dependent secretion in these cells. Stronger evidence for a definitive role for Rac or Cdc42 (as opposed to another Rho-related protein) would be forthcoming if we could demonstrate inhibition of secretion by specific dominant negative mutant forms such as the T17N-mutants of Rac and Cdc42 (Takaishi et al., 1994; Kozma et al., 1995, 1996; Olson et al., 1995).
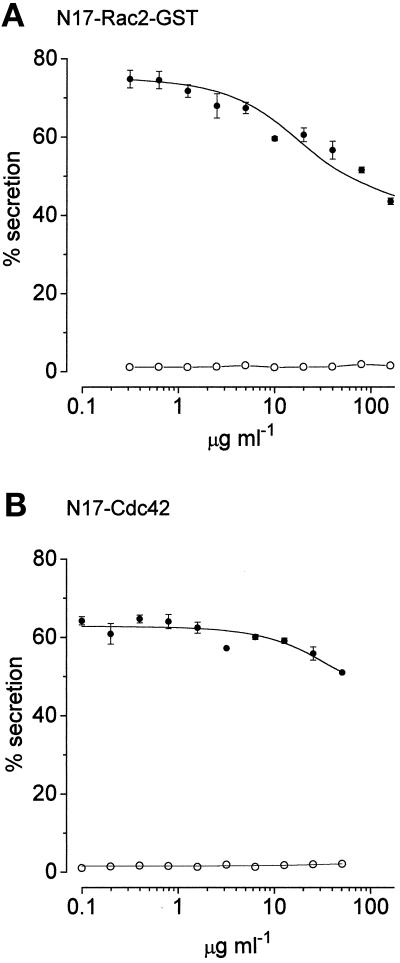
The experiments illustrated in Figure 10 demonstrate the effects of the dominant negative mutants, N17-Rac2-GST and N17-Cdc42/G25K, on secretion elicited by application of the dual stimulus (Ca2+ + GTPγS). Since we were unable to apply the standard measurements of guanine nucleotide binding to these proteins, the concentrations discussed and recorded in Figure 10 are totals, not estimates, of the amounts of active material. In these experiments, the cells were allowed to run down briefly (7 min), sufficient to allow the protein to penetrate the cells while still ensuring a high level of release. We found that secretion can be inhibited by N17-Rac2-GST as its concentration is elevated above 1 μg ml−1. Since this protein tends to adhere to plastic ware, we were unable to apply it above 160 μg ml−1 at which concentration the level of secretion was depressed by about 45%. GST, applied as a control, was without effect when applied at concentrations up to 66 μg ml−1 (not shown). Inhibition by N17-Cdc42/G25K was harder to discern. The availability of this protein was very limited (typical yield was 10 μg l−1 of Escherichia coli suspension) and the highest (total) concentration that we were able to apply was 50 μg ml−1. In the experiment illustrated, this concentration of protein caused 20% inhibition of secretion (p < 0.002; n = 4 determinations) and in two other experiments we measured 10% and 20% inhibition at the highest concentration applied.
Figure 10.
Inhibition of GTPγS-stimulated secretion by (a) N17-Rac2 and (b) N17-Cdc42/G25K. In this experiment the cells were allowed to run down for 7 min before stimulation by Ca2+ (pCa5) and GTPγS (100 μM). Note that the N17-Rac2 preparation is contaminated with about 33% of GST. Results are expressed as means ± SEM (n = 4). •, Cells stimulated with Ca2+ (pCa5) and GTPγS (100 μM); ○, unstimulated cells. Similar results have been obtained on three separate occasions.
DISCUSSION
Treatment of cells with SL-O generates membrane lesions of the order of 30 nm (diameter) (Bhakdi et al., 1993), which allow leakage from mast cells, within minutes, of soluble proteins such as lactate dehydrogenase (Howell and Gomperts, 1987), phosphoglycerate kinase (Gomperts et al., 1987), and actin (Koffer and Gomperts, 1989). In spite of this, it remains possible to induce extensive secretion by addition of appropriate stimuli even when these are presented some minutes after most of the readily soluble proteins have leaked from the cells. For permeabilized adrenal chromaffin cells (Brooks and Carmichael, 1988) and for eosinophils (Newman et al., 1996), ultrastructural examination indicates that such secretion occurs by an authentic exocytotic mechanism involving fusion of the secretory granule membranes with the plasma membrane. At later times, cells become refractory to stimulation, possibly due to the detachment and leakage of key protein regulators from their binding sites (Howell and Gomperts, 1987). Exogenous proteins that can retard such “rundown” must be considered as candidates, or at least analogs, for the authentic protein regulators that have been lost from the cells.
The finding that the complex of Rac with RhoGDI (FOAD-II) can retard run-down of permeabilized mast cells, and that RhoGDI accelerates run-down gave a strong indication that one (or more) of the Rho-related proteins might be natural regulators for secretion (O’Sullivan et al., 1996). RhoGDI also inhibits exocytosis in individual patch-clamped mast cells (Mariot et al., 1996) and since exocytosis does not run down after patch rupture (Oberhauser et al., 1992; Mariot et al., 1996), this strongly validates the reality of RhoGDI as an inhibitor of the exocytotic machinery. The report (Prepens et al., 1996) of inhibition of antigen-induced secretion from rat basophilic leukemia (RBL-2H3, a mast cell line) cells by Clostridium difficile toxin B again points to an action of Rho-related proteins although Rho itself is probably ruled out since Botulinum C-3 toxin, under conditions that caused 90% ADP-ribosylation of Rho, was without effect on secretion. Similarly, C-3 toxin is without effect on exocytosis from single mast cells in the whole cell patch-clamp configuration (Mariot and Tatham, unpublished observation).
In this work we have concentrated on Cdc42/G25K and Rac2 (the major form of Rac expressed in myeloid cells [Abo et al., 1994; Didsbury et al., 1989]) although our observations using Rac1 indicate that both subtypes of Rac activate secretion by identical or similar mechanisms. We found that to support secretion, Rac2 must be preactivated by binding GTPγS (data not shown). Although we have not tested non-GTPγS–bound Rac1 and Cdc42/G25K, we can think of no reason to believe that they differ in this respect since the recombinant proteins differ from native (posttranslationally modified) GTPases such as FOAD-II (the Rac1–RhoGDI complex isolated from bovine brain), which is able to interact with the upstream regulators dictating guanine nucleotide exchange (Didsbury et al., 1990; Ando et al., 1992; Heyworth et al., 1993).
Permeabilized mast cells loaded with preactivated Rac2 or Cdc42/G25K can undergo secretion in response to elevation of Ca2+ alone. Although GTPγS-induced (Ca2+-independent) secretion from these (Fernandez et al., 1984; Lillie and Gomperts, 1992; Larbi and Gomperts, 1996) and other cells of hematopoeitic origin (Barrowman et al., 1986; Nusse et al., 1990) is well established, we have not previously been able to induce secretion in the absence of a stimulating guanine nucleotide (Lillie and Gomperts, 1992). The finding of Ca2+-induced (guanine nucleotide-independent) secretion is therefore significant, but even so, when GTPγS is provided in addition to the activated protein, the extent of release is further increased. This enhancement by free GTPγS, which is manifest even when the cells are stimulated with a saturating concentration of preactivated Rac, points to the involvement of a second GTP-binding protein.
Since Ca2+-induced (guanine nucleotide independent) secretion becomes evident in cells stimulated at very early times after permeabilization, we have to question whether it arises not as a consequence of the activated GTPase but as a result of the liberation of free GTPγS and its interaction with an endogenous GTPase. The following observations are inconsistent with the second possibility:
1) The midpoints (EC50) for activation and the ranges of concentrations over which Rac and Cdc42/G25K induce secretion are quite different. These two proteins exhibit similar rates of guanine nucleotide exchange (Self and Hall, 1995b) and, therefore, if nucleotide leakage were occurring, one would expect the two proteins to have similar concentration–effect relationships. Clearly, this is not the case. Furthermore, the enhancing effects of preactivated Rac2 and Cdc42/G25K approach definite saturation points and above this, the extents of secretion frequently decline (this occurred in 7/11 experiments).
2) Of two Rac effector domain mutants, both of which retain the capacity to bind GTPγS, only one, Y40C-Rac1, was capable of inducing exocytosis. The other, F37A-Rac1, which is understood to react selectively with those effectors that contain a CRIB sequence (Lamarche et al., 1996), was without effect.
We are confident that our results represent specific actions of specific proteins and that both Rac2 and Cdc42/G25K can support secretion in run-down mast cells. These proteins themselves play a role or can substitute for the endogenous regulators mediating this process in intact cells. The demonstration of the presence of Cdc42 and Rac in these cells, and their leakage following permeabilization, suggests that these could be the actual GTPases regulating exocytosis.
Additional evidence supporting the idea that Rac2 and Cdc42 are native GTPases regulating exocytosis (GE) in mast cells is found in the inhibition of GTPγS-induced secretion by the dominant negative mutant proteins N17-Rac2 and N17-Cdc42/G25K. The extent of inhibition due to the Rac2 mutant is considerable. While we were never able to apply a saturating concentration, in the experiment illustrated, it reduced the secretion by about 30%, which represents a considerable proportion of the maximum that we were able to induce with the active form of this GTPase. The analogous mutations in Ras are known to form stable inactive complexes with exchange factors (Quilliam et al., 1994) and also to possess preferential affinity for GDP, owing to improper coordination of Mg2+ (Shinjo et al., 1990). Taken together, the demonstration of leakage of Rac and Cdc42 after permeabilization, stimulation of secretion by these GTPases after preactivation, and inhibition of GTPγS-elicited secretion by specific dominant negative mutants give a strong indication that these two proteins may be the actual (although not necessarily the exclusive) GTPases regulating secretion in mast cells.
Rac and Cdc42 are closely related. They exhibit about 70% identity (Shinjo et al., 1990) and share common effectors (Manser et al., 1994; Burbelo et al., 1995; Teo et al., 1995) and yet the secretion responses due to these two proteins are different. Not only is the maximal extent of secretion due to Cdc42/G25K invariably greater, but the effective ranges over which they stimulate secretion are different with activation by Rac confined within a single decade range of concentration, while that due to Cdc42/G25K extends over about two decades. In an attempt to find out whether activation by these two GTPases is due to the involvement of the same or separate downstream effectors, we tested the effect of titrating N-Myc-Cdc42/G25K over and above a maximal stimulus delivered by Rac2. Under these conditions, the extended concentration-effect relationship characteristic of Cdc42/G25K was still manifest. While Rac2 was without discernible effect when Cdc42/G25K was presented at concentrations in the high end of its activation range, there was a definite increase in the amount of secretion over that which could be elicited by either protein alone when it was presented at low concentrations. From this, it would appear that low concentrations of Cdc42/G25K are accessible to a high affinity effector not available to Rac, while at high concentrations they share a common lower affinity target. Alternatively, Rac and Cdc42 may address quite separate effectors but, if this is the case, these two effectors must converge onto a common pathway.
A further difference between the two GTPases is revealed by providing free GTPγS in addition to the preactivated protein. It increases the extent of secretion induced by Rac (Figure 7), but with Cdc42/G25K it also enhances the sensitivity by about sixfold (Figure 8) while leaving the slope of the concentration effect relationship (Hill coeffient) unaltered. We presume that this enhancement of the affinity for Cdc42/G25K by free GTPγS is due to the action of another GTPase, although this is unlikely to be Rac, which even when provided at a maximal activating concentration has no discernible effect on the concentration range for activation by Cdc42/G25K (Figure 9).
The most definitive evidence underlying a role for a Rho-related protein in the regulation of exocytosis must remain the inhibition by RhoGDI (Mariot et al., 1996). However, neither purified nor recombinant RhoGDI were ever capable of inhibiting secretion in the run-down cells beyond a limit of about 80%. For this reason, it is likely that there is yet another GTP-binding protein capable of inducing a small amount of secretion. In this paper, we have demonstrated that preactivated Rac and Cdc42/G25K can induce up to about 60% secretion, but the provision of additional GTPγS then enhances this further still. It is likely that the true nature of GE is expressed by the combined effects of more than one class of GTP-binding protein.
Acknowledgements
This work was supported by grants from the Wellcome Trust (034698) with further financial assistance from the Vandervell Foundation and the Gower Street Secretory Mechanisms Group. We thank Professor Alan Hall who read the script critically, provided much advice, and who, with Nathalie Lamarche, provided the bacteria expressing Cdc42/G25K, N-Myc-Cdc42/G25K, and N17-Cdc42/G25K. Also, Dr. Roberto Solari (Glaxo-Wellcome Medicines Research Centre) for provision of bacteria expressing Rac2. We thank Dr. Steve Moss and Shaun Donnelly for much help and practical advice in the expression of GST-fusion proteins. Anna Brown was the recipient of a Wellcome Prize Studentship.
Footnotes
Abbreviations used: GDI, GDP-dissociation inhibitor protein; GTPγS, guanosine 5′-(3-thiotriphosphate); hexosaminidase, N-acetyl β-d-glucosaminidase; SLO, streptolysin-O.
REFERENCES
- Abo A, Webb MR, Grogan A, Segal AW , Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem J. 1994;298:585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SM, Burgoyne RD. The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signalling. 1990;2:265–276. doi: 10.1016/0898-6568(90)90054-e. [DOI] [PubMed] [Google Scholar]
- Ando S, et al. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J Biol Chem. 1992;267:25709–25713. [PubMed] [Google Scholar]
- Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Athayde CM, Scrutton MC. Guanine nucleotides and Ca2+-dependent lysosomal secretion in electropermeabilised human platelets. Eur J Biochem. 1990;189:647–655. doi: 10.1111/j.1432-1033.1990.tb15533.x. [DOI] [PubMed] [Google Scholar]
- Barrowman MM, Cockcroft S, Gomperts BD. Two roles for guanine nucleotides in the stimulus secretion sequence of neutrophils. Nature. 1986;319:504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Weller U, Walev I, Martin E, Jonas D, Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol. 1993;182:167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Carmichael SW. Ultrastructural demonstration of exocytosis in intact and saponin-permeabilized cultured bovine chromaffin cells. Am J Anat. 1988;178:85–89. doi: 10.1002/aja.1001780111. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Cromwell O, Bennett JP, Kay AB, Hide I, Gomperts BD. Mechanisms of granule enzyme secretion from permeabilised guinea pig eosinophils: dependence on Ca2+ and guanine nucleotides. J Immunol. 1991;147:1905–1911. [PubMed] [Google Scholar]
- Didsbury JR, Uhing RJ, Snyderman R. Isoprenylation of the low molecular mass GTP-binding proteins rac 1 and rac 2: possible role in membrane localization. Biochem Biophys Res Commun. 1990;171:804–812. doi: 10.1016/0006-291x(90)91217-g. [DOI] [PubMed] [Google Scholar]
- Didsbury JR, Weber RF, Bokoch GM, Evans T, Snyderman R. rac. a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- Dormer RL, Ashcroft SJH. Studies on the role of calcium ions in the stimulation by adrenaline of amylase release from rat parotid. Biochem J. 1974;144:543–550. doi: 10.1042/bj1440543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Gomperts BD. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Gomperts BD, Cockcroft S, Howell TW, Nüsse O, Tatham PER. The dual effector system for exocytosis in mast cells: obligatory requirement for both Ca2+ and GTP. Biosci Rep. 1987;7:369–381. doi: 10.1007/BF01362501. [DOI] [PubMed] [Google Scholar]
- Gomperts BD, Tatham PER. Regulated exocytotic secretion from permeabilized cells. Methods Enzymol. 1992;219:178–189. doi: 10.1016/0076-6879(92)19020-7. [DOI] [PubMed] [Google Scholar]
- Heyworth PG, Knaus UG, Xu X, Uhlinger DJ, Conroy L, Bokoch GM, Curnutte JT. Requirement for posttranslational processing of Rac GTP-binding proteins for activation of human neutrophil NADPH oxidase. Mol Biol Cell. 1993;4:261–269. doi: 10.1091/mbc.4.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell TW, Gomperts BD. Rat mast cells permeabilised with streptolysin-O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim Biophys Acta. 1987;927:177–183. doi: 10.1016/0167-4889(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Knight DE, Baker PF. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membrane Biol. 1982;68:107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Koffer A, Gomperts BD. Soluble proteins as modulators of the exocytotic reaction of permeabilised rat mast cells. J Cell Sci. 1989;94:585–591. doi: 10.1242/jcs.94.3.585. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia. Mol Cell Biol. 1996;16:5069–5080. doi: 10.1128/mcb.16.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong CH, Malech HL, Rotrosen D, Leto TL. Regulation of the human neutrophil NADPH oxidase by rho-related G-proteins. Biochemistry. 1993;32:5711–5717. doi: 10.1021/bi00072a029. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Larbi KY, Gomperts BD. Practical considerations regarding the use of streptolysin-O as a permeabilising agent for cells in the investigation of exocytosis. Biosci Rep. 1996;16:11–21. doi: 10.1007/BF01200997. [DOI] [PubMed] [Google Scholar]
- Lillie THW, Gomperts BD. Guanine nucleotide is essential, Ca2+ is a modulator, in the exocytotic reaction of permeabilised rat mast cells. Biochem J. 1992;288:181–187. doi: 10.1042/bj2880181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Gomperts BD. Techniques and concepts in exocytosis: Focus on mast cells. Biochim Biophys Acta. 1991;1071:429–471. doi: 10.1016/0304-4157(91)90006-i. [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by cdc42 and rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Mariot P, O’Sullivan AJ, Brown AM, Tatham PER. Rho-guanine nucleotide dissociation inhibitor protein (Rho-GDI) inhibits exocytosis in mast cells. EMBO J. 1996;15:6476–6482. [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Okabe T, Sugimoto N, Senda T, Fujita H. Tetanus toxin and Clostridium perfringens enterotoxin as tools for the study of exocytosis. Ann NY Acad Sci. 1994;710:94–106. doi: 10.1111/j.1749-6632.1994.tb26617.x. [DOI] [PubMed] [Google Scholar]
- McMillian MK, Soltoff SP, Talamo BR. Mediation of norepinephrine effects on free cytosolic calcium in rat parotid acinar cells by α1 adrenergic receptors. Biochem Pharmacol. 1988;37:3790–3793. doi: 10.1016/0006-2952(88)90419-4. [DOI] [PubMed] [Google Scholar]
- Mittrucker H, Fleischer B. Functional localization of an exocytosis-triggering G-protein in human cytotoxic T lymphocytes. Immunology. 1992;76:610–615. [PMC free article] [PubMed] [Google Scholar]
- Morrissey JH. Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal Biochem, 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Newman TM, Tian M, Gomperts BD. Ultrastructural characterisation of tannic acid arrested degranulation of GTPgS stimulated guinea pig eosinophils. Eur J Cell Biol. 1996;70:209–220. [PubMed] [Google Scholar]
- Nishizaki T, Walent JH, Kowalchyk JA, Martin TFJ. A key role for a 145-kDa cytosolic protein in the stimulation of Ca2+-dependent secretion by protein kinase C. J Biol Chem. 1992;267:23972–23981. [PubMed] [Google Scholar]
- Nusse O, Lindau M, Cromwell O, Kay AB, Gomperts BD. Intracellular application of GTP-5′-O-(3-thiotriphosphate) induces exocytotic granule fusion in guinea pig eosinophils. J Exp Med. 1990;171:775–786. doi: 10.1084/jem.171.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan AJ, Brown AM, Freeman HNM, Gomperts BD , Purification and identification of FOAD-II, a cytosolic protein that regulates secretion in streptolysin-O permeabilised mast cells, as a rac/rhoGDI complex. Mol Biol Cell. 1996;7:397–408. doi: 10.1091/mbc.7.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Monck JR, Balch WE, Fernandez JM. Exocytotic fusion is activated by Rab3a peptides. Nature. 1992;360:270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Prepens U, Just I, von Eichel-Streiber C, Aktories K. Inhibition of Fc∈RI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase) J Biol Chem. 1996;271:7324–7329. doi: 10.1074/jbc.271.13.7324. [DOI] [PubMed] [Google Scholar]
- Quilliam LA, Kato K, Rabun KM, Hisaka MM, Huff SY, Campbell Burk S, Der CJ. Identification of residues critical for Ras(17N) growth-inhibitory phenotype and for Ras interaction with guanine nucleotide exchange factors. Mol Cell Biol. 1994;14:1113–1121. doi: 10.1128/mcb.14.2.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self AJ, Hall A. Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 1995a;256:3–10. doi: 10.1016/0076-6879(95)56003-3. [DOI] [PubMed] [Google Scholar]
- Self AJ, Hall A. Measurement of intrinsic nucleotide exchange and GTP hydrolysis rates. Methods Enzymol. 1995b;256:67–76. doi: 10.1016/0076-6879(95)56010-6. [DOI] [PubMed] [Google Scholar]
- Shinjo K, Koland JG, Hart MJ, Narasimhan V, Johnson DI, Evans T, Cerione RA. Molecular cloning of the gene for the human placental GTP-binding protein Gp (G25K): identification of this GTP-binding protein as the human homolog of the yeast cell-division-cycle protein CDC42. Proc Natl Acad Sci USA. 1990;87:9853–9857. doi: 10.1073/pnas.87.24.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Tatham PER, Gomperts BD. Cell permeabilisation. In: Siddle K, Hutton JC, editors. Peptide Hormones—A Practical Approach. Oxford, United Kingdom: IRL Press; 1990. pp. 257–269. [Google Scholar]
- Teo M, Manser E, Lim L. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J Biol Chem. 1995;270:26690–26697. doi: 10.1074/jbc.270.44.26690. [DOI] [PubMed] [Google Scholar]