Abstract
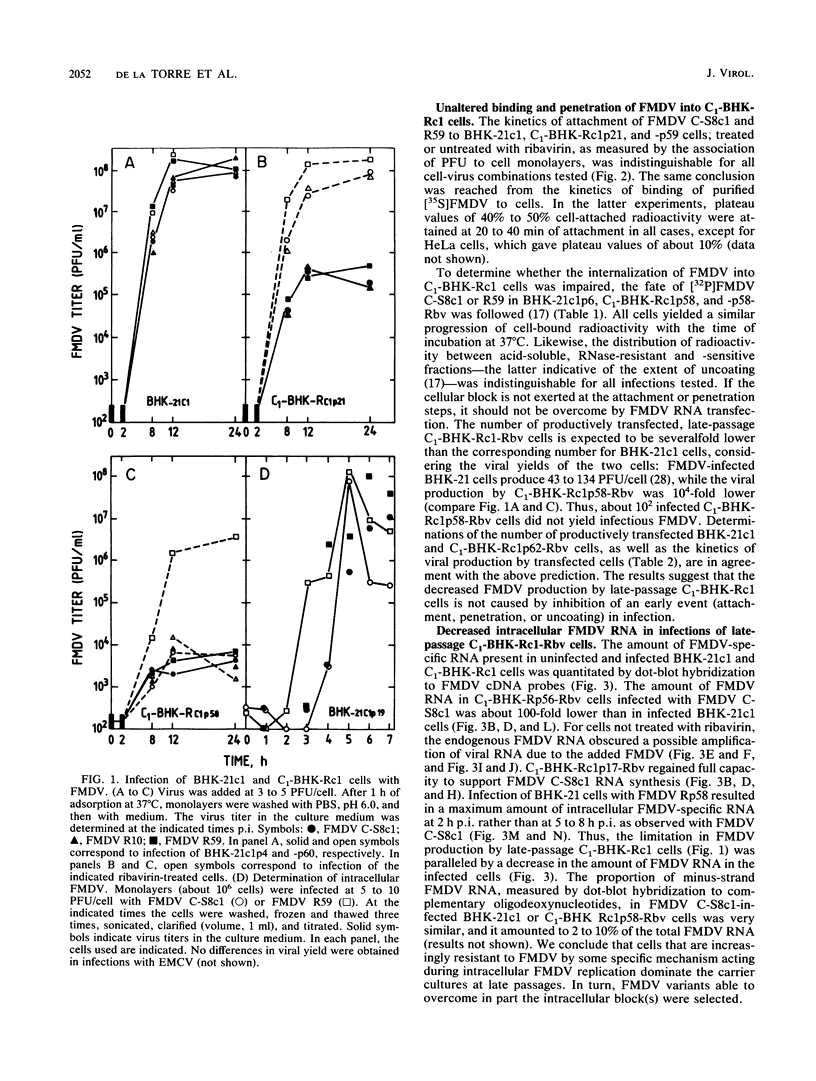
Virus and cells evolve during serial passage of cloned BHK-21 cells persistently infected with foot-and-mouth disease virus (FMDV). These carrier cells, termed C1-BHK-Rc1 (J.C. de la Torre, M. Dávila, F. Sobrino, J. Ortín, and E. Domingo, Virology 145:24-35, 1985), become constitutively resistant to the parental FMDV C-S8c1. Curing of late-passage C1-BHK-Rc1 cells of FMDV by ribavirin treatment (J.C. de la Torre, B. Alarcón, E. Martínez-Salas, L. Carrasco, and E. Domingo, J. Virol. 61:233-235, 1987) did not restore sensitivity to FMDV C-S8c1. The resistance of C1-BHK-Rc1 cells to FMDV C-S8c1 was not due to an impairment of attachment, penetration, or uncoating of the particles but to some intracellular block that resulted in a 100-fold decrease in the amount of FMDV RNA in the infected cells. FMDV R59, the virus isolated from late-passage carrier cells, partly overcame the cellular block and was more cytolytic than FMDV C-S8c1 for BHK-21 cells. Sequencing of the VP1 gene from nine viral clones from C1-BHK-Rc1 cells showed genetic heterogeneity of 5 X 10(-4) substitutions per nucleotide. Mutations were sequentially fixed during persistence. In addition to resistance to FMDV C-S8c1, C1-BHK-Rc1 cells showed a characteristic round cell morphology, and compared with BHK-21 cells, they grew faster in liquid culture, were less subject to contact inhibition of growth, and had an increased ability to form colonies in semisolid agar. Reconstitution of a persistent infection was readily attained with late-passage C1-BHK-Rc1 cells and FMDV C-S8c1 or FMDV R59. The results suggest that coevolution of BHK-21 cells and FMDV contributes to the maintenance of persistence in cell culture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Clarke J. B., Spier R. E. An investigation into causes of resistance of a cloned line of BHK cells to a strain of foot-and-mouth disease virus. Vet Microbiol. 1983 Jun;8(3):259–270. doi: 10.1016/0378-1135(83)90078-0. [DOI] [PubMed] [Google Scholar]
- Clarke J. B. Transformation and foot and mouth disease virus (FMDV) productivity of some BHK cell lines. Acta Virol. 1983 Nov;27(6):534–534. [PubMed] [Google Scholar]
- DeBorde D. C., Naeve C. W., Herlocher M. L., Maassab H. F. Resolution of a common RNA sequencing ambiguity by terminal deoxynucleotidyl transferase. Anal Biochem. 1986 Sep;157(2):275–282. doi: 10.1016/0003-2697(86)90626-3. [DOI] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Gibson J. P., Righthand V. F. Persistence of echovirus 6 in cloned human cells. J Virol. 1985 Apr;54(1):219–223. doi: 10.1128/jvi.54.1.219-223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas E., Ortín J., Domingo E. Sequence of the viral replicase gene from foot-and-mouth disease virus C1-Santa Pau (C-S8). Gene. 1985;35(1-2):55–61. doi: 10.1016/0378-1119(85)90157-x. [DOI] [PubMed] [Google Scholar]
- Newton S. E., Carroll A. R., Campbell R. O., Clarke B. E., Rowlands D. J. The sequence of foot-and-mouth disease virus RNA to the 5' side of the poly(C) tract. Gene. 1985;40(2-3):331–336. doi: 10.1016/0378-1119(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Ron D., Tal J. Coevolution of cells and virus as a mechanism for the persistence of lymphotropic minute virus of mice in L-cells. J Virol. 1985 Aug;55(2):424–430. doi: 10.1128/jvi.55.2.424-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Tal J. Spontaneous curing of a minute virus of mice carrier state by selection of cells with an intracellular block of viral replication. J Virol. 1986 Apr;58(1):26–30. doi: 10.1128/jvi.58.1.26-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino F., Dávila M., Ortín J., Domingo E. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology. 1983 Jul 30;128(2):310–318. doi: 10.1016/0042-6822(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Palma E. L., Beck E., Dávila M., de la Torre J. C., Negro P., Villanueva N., Ortín J., Domingo E. Fixation of mutations in the viral genome during an outbreak of foot-and-mouth disease: heterogeneity and rate variations. Gene. 1986;50(1-3):149–159. doi: 10.1016/0378-1119(86)90320-3. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., HABEL K. Virus-cell relationship in a carrier culture of HeLa cells and Coxsackie A9 virus. Virology. 1959 Jan;7(1):28–44. doi: 10.1016/0042-6822(59)90175-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- Vallbracht A., Hofmann L., Wurster K. G., Flehmig B. Persistent infection of human fibroblasts by hepatitis A virus. J Gen Virol. 1984 Mar;65(Pt 3):609–615. doi: 10.1099/0022-1317-65-3-609. [DOI] [PubMed] [Google Scholar]
- Villanueva N., Dávila M., Ortín J., Domingo E. Molecular cloning of cDNA from foot-and-mouth disease virus C1-Santa Pau (C-S8). Sequence of protein-VP1-coding segment. Gene. 1983 Aug;23(2):185–194. doi: 10.1016/0378-1119(83)90050-1. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Alarcón B., Martínez-Salas E., Carrasco L., Domingo E. Ribavirin cures cells of a persistent infection with foot-and-mouth disease virus in vitro. J Virol. 1987 Jan;61(1):233–235. doi: 10.1128/jvi.61.1.233-235.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Dávila M., Sobrino F., Ortín J., Domingo E. Establishment of cell lines persistently infected with foot-and-mouth disease virus. Virology. 1985 Aug;145(1):24–35. doi: 10.1016/0042-6822(85)90198-9. [DOI] [PubMed] [Google Scholar]





