A 15-month-old boy presented to his doctor when his mother became concerned about his diet. Despite the introduction of solids at six months of age, he was almost exclusively breastfed and a ‘picky eater’. His parents were partial vegetarians, with little meat intake. His mother had noticed that he was pale with gait disturbance and occasional falls. His developmental history was otherwise unremarkable. His past medical history included a term delivery with no complications and an uneventful postnatal period. After initial bloodwork, he was referred to a paediatric hematologist.
On examination, he was pale. His weight and height were between the 5th to 10th and 15th percentiles, respectively. A systolic murmur grade 2/6 was present. The patient’s bloodwork included a complete blood count with white blood cell count 5.2×109/L (normal 5.3×109/L to 16×109/L), neutrophils 1×109/L (normal 1×109/L to 6.5×109/L), hemoglobin 83 g/L (normal 103 g/L to 135 g/L), mean corpuscular volume 107 fL (normal 70 fL to 86 fL) and platelet count 311×109/L (normal 200×109/L to 550×109/L). His reticulocyte count was 31×109/L (normal 40×109/L to 120×109/L), and his peripheral blood smear showed occasional oval macrocytes and hypersegmented neutrophils. A bone marrow aspirate was performed, and the images demonstrated classic findings of a particular diagnosis.
CASE 2 DIAGNOSIS: MEGALOBLASTIC ANEMIA DUE TO DIETARY COBALAMIN DEFICIENCY
Additional investigations for macrocytic anemia included serum folate 33.3 nmol/L (normal greater than 6.8 nmol/L), and vitamin B12 (cobalamin) 44 pmol/L (deficient range lower than 107 pmol/L). Based on the test results and the bone marrow aspirate findings of hypersegmented neutrophils, megaloblasts and giant metamyelocytes, a diagnosis of megaloblastic anemia (MA) was made.
Bloodwork was also performed on the mother. Serum folate was normal at 30.9 nmol/L (greater than 6.81 nmol/L) and serum vitamin B12 was low-normal at 209 pmol/L (normal 133 pmol/L to 675 pmol/L). Maternal hemoglobin level and mean corpuscular volume (MCV) were normal.
Given the history, the likely cause of MA and cobalamin deficiency was reduced dietary intake. The toddler was treated with intramuscular cyanocobalamin injections (20 μg daily for seven days, then 100 μg weekly for four weeks) and a dietician was consulted. Oral cobalamin therapy was also an option and would have been effective, but for convenience and to ensure compliance, the parenteral route was chosen.
Two months later, he had improved significantly with a normal hemoglobin level (107 g/L) and MCV (83 fl). He was more energetic and interactive. His gait had returned to normal and his falls had decreased. His diet had improved with increased intake of meats, eggs, milk, fruits and vegetables, supplemented with PediaSure (Abbott Nutrition, Canada). Eight months after presentation, his development was appropriate for age and he was not anemic, despite being off therapy for several months. Maintenance of normal counts after discontinuation of therapy and adherence to a balanced diet are consistent with a dietary etiology for MA.
MA describes a group of disorders characterized by defective DNA synthesis. Morphological hallmarks in the marrow are megaloblasts and giant metamyelocytes displaying nuclear to cytoplasmic asynchrony. A megaloblast has a nucleus that is immature relative to the cytoplasm because the nucleus has impaired DNA synthesis, but hemoglobinization, which is dependant on ribosomal function, continues normally in the cytoplasm. There are many causes of MA, some of which are listed in Table 1.
TABLE 1.
Causes of megaloblastic anemia
| Etiology | Cobalamin deficiency | Folate deficiency |
|---|---|---|
| Nutrition | Strict vegetarianism or vegan diets | Malnutrition in elderly, alcoholics, impoverished communities |
| Gastrointestinal abnormalities | Gastric atrophy: achlorhydria | Celiac disease |
| Intrinsic factor deficiency – congenital or acquired abnormality | Dermatitis herpetiformis | |
| Total or partial gastrectomy | Tropical sprue | |
| Bacterial overgrowth in the small bowel (achlorhydria, anatomical defects, impaired motility) | Extensive jejunal resection | |
| Terminal ileal resection | Crohn’s disease | |
| Crohn’s disease | ||
| Extensive celiac disease | ||
| Zollinger-Ellison syndrome | ||
| Pancreatic insufficiency | ||
| Fish tapeworm (Diphyllobothrium latum) | ||
| HIV | ||
| Congenital defects (eg, Imerslund-Gräsbeck syndrome) | ||
| Drugs | Proton pump inhibitors | Cytotoxics (eg, methotrexate) |
| Metformin | Antibiotics (eg, nitrofurantoin, tetracycline) | |
| Phenformin | Anticonvulsants (eg, phenytoin, carbamazepine) | |
| Anticonvulsants | ||
| Cytotoxic drugs | ||
| Increased utilization/loss | Pregnancy | Pregnancy |
| Chronic hemolysis | ||
| Exfoliative dermatitis | ||
| Metabolic abnormalities | Congenital transcobalamin II deficiency or functional abnormality | Congenital folate malabsorption |
| Congenital intrinsic factor deficiency | Dihydrofolate reductase deficiency |
The case described demonstrates MA in a paediatric patient who was a ‘picky eater’ and was found to have macrocytic anemia. The insidious onset can delay diagnosis. It is important to diagnose this condition early to avoid the symptoms of anemia, as well as the neurological sequelae, including loss of vibration sensation and potential progression to spastic ataxia due to demyelination of the dorsal and lateral columns of the spinal cord. An approach to the workup of suspected MA in paediatric patients is proposed.
History and physical examination
Many patients are asymptomatic, and a diagnosis of MA is made incidentally when macrocytosis is found on routine bloodwork. There may be a history of poor food intake, prolonged breastfeeding, and a maternal history of vegetarian and vegan diets or autoimmune disorders. Nonspecific symptoms include irritability, weight loss, diarrhea or constipation. The clinical features are primarily those of a classic ‘lemon yellow’ pallor because of the combination of anemia and jaundice. Severe cases may have marked anorexia, weight loss, glossitis and angular cheilosis. Neurological effects may be manifested by failure to reach developmental milestones and may include paresthesias, muscle weakness and impaired intellectual development.
Screening bloodwork
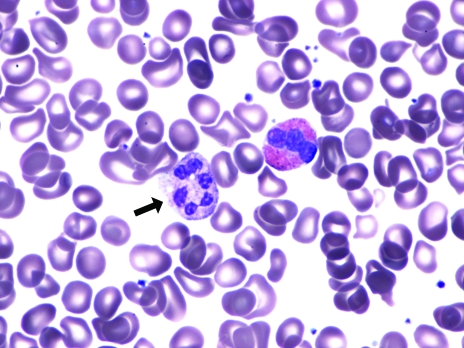
The complete blood count shows macrocytic anemia. The MCV can be normal when there is concomitant microcytosis (thalassemia trait or iron deficiency). The peripheral blood smear shows oval macrocytes and hypersegmented neutrophils (five or more lobes) (Figure 1). If a child is being breastfed, maternal bloodwork must be performed to exclude maternal vitamin deficiencies.
Figure 1.
Peripheral blood smear demonstrating a hypersegmented neutrophil
Diagnostic tests
Folate and cobalamin levels are critical diagnostic blood tests. Red blood cell folate levels may be a better indicator of body folate because recent changes in dietary intake and hemolysis of the specimen will interfere with serum folate levels. Patients deficient in folate have low assay results, but a significant proportion of cobalamin-deficient patients will also have low red cell folate assays because cobalamin is a cofactor in folate metabolism. Cobalamin assays have limitations when correlating clinical deficiency with low-normal assay levels in some patients. Concentrations of the cobalamin carrier protein transcobalamin 1 can influence serum levels of vitamin B12. The assays for folate and vitamin B12 levels are generally robust and convenient for diagnosing deficient states.
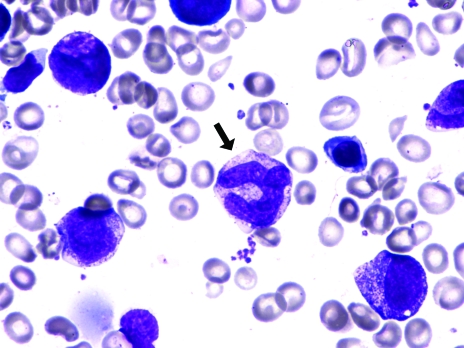
A bone marrow biopsy is an invasive procedure and less frequently performed with the availability of diagnostic blood tests, but it can be warranted to expedite diagnosis. The marrow can provide a morphological diagnosis within a matter of minutes; however, it requires skilled physician resources. A bone marrow aspirate in MA shows hypercel-lularity with ineffective hematopoiesis and megaloblastic erythropoiesis (Figure 2), giant myeloid precursors (giant metamyelocytes) (Figures 2 and 3), increased iron stores and, less commonly, hyperpolyploid megakaryocytes. When no explanation for cytopenias is found, bone marrow studies must be performed to exclude bone marrow failure, hematological malignancy or metastatic tumour.
Figure 2.
Bone marrow aspirate demonstrating megaloblastic hematopoiesis with a megaloblast (white arrow) and a giant metamye-locyte (black arrow)
Figure 3.
Bone marrow aspirate demonstrating megaloblastic hematopoiesis with a giant metamyelocyte (black arrow)
Additional tests and special tests
Schilling test:
When Addisonian pernicious anemia (PA) is suspected, a Schilling test may be performed to assess cobalamin absorption. The test measures urinary excretion of orally administered radioactive cobalamin, with and without added intrinsic factor. PA is rare in children. The Schilling test would be helpful, and should be performed when there is a need to distinguish PA from the rarer malabsorptive errors of cobalamin absorption that are listed in Table 1.
Total plasma homocysteine, serum methylmalonate and urinary excretion of methylmalonate:
Vitamin B12, but not folate, is required in methylmalonate (MMA) metabolism. Increased total plasma homocysteine and MMA levels are associated with cobalamin deficiency. Total plasma homocysteine level is elevated in both folate and vitamin B12 deficiency, and is less specific than MMA. These tests, however, may be useful in suspected presymptomatic deficiency when the patient is not anemic and cobalamin levels are in the low-normal range. Elevated total plasma homocysteine and MMA levels may signal functional vitamin B12 deficiency (1). Availability of these tests may limit their diagnostic application.
The etiology of folate or cobalamin deficiency must be determined because most causes are preventable or treatable. In developed countries, MA is more commonly caused by cobalamin rather than folate deficiency because many foods are folate-supplemented. Cobalamin deficiency must be ruled out before administering folic acid because treatment with folic acid alone, in the presence of cobalamin deficiency, can cause or exacerbate irreversible neurological damage.
MA can occur in children when there is a history of ‘picky eating’, poverty, chronic hemolysis such as hereditary spherocytosis, diets low in animal products or prolonged breastfeeding. Human milk cobalamin concentrations have been found to be lower in vegetarian mothers compared with omnivorous mothers (2). Breastfeeding mothers can have clinical (3) or subclinical cobalamin deficiency, the latter having low-normal cobalamin levels (1). As such, infants of cobalamin-deficient mothers are at risk of developing MA. Cobalamin deficiency may also occur in infants born to mothers with PA; however, this risk is largely theoretical because women with PA are usually infertile (4).
Early diagnosis of MA in childhood is imperative to prevent neurological consequences in infants who remain untreated. Increased awareness leading to early diagnosis and appropriate, timely therapy can prevent irreversible neurological effects for the child with MA. This is especially important during the critical period of neurodevelopment in early childhood.
CLINICAL PEARLS
Prolonged breastfed infants may be at risk of developing MA, especially if the mother consumes a vegetarian or vegan diet.
Cobalamin deficiency must be ruled out before instituting folate therapy.
Cobalamin deficiency may have neuropsychiatric as well as hematological manifestations, and early diagnosis is imperative to prevent irreversible neurological damage.
Measurement of serum MMA is important in the detection of presymptomatic cobalamin deficiency.
RECOMMENDED READING
- 1.Korenke GC, Hunneman DH, Eber S, Hanefeld F. Severe encephalopathy with epilepsy in an infant caused by subclinical maternal pernicious anaemia: Case report and review of the literature. Eur J Pediatr. 2004;163:196–201. doi: 10.1007/s00431-004-1402-4. [DOI] [PubMed] [Google Scholar]
- 2.Specker BL, Black A, Allen L, Morrow F. Vitamin B-12: Low milk concentrations are related to low serum concentrations in vegetarian women and to methylmalonic aciduria in their infants. Am J Clin Nutr. 1990;52:1073–6. doi: 10.1093/ajcn/52.6.1073. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Fogelman Y, Bennett M. Severe vitamin B12 deficiency in an infant associated with a maternal deficiency and a strict vegetarian diet. J Pediatr Hematol Oncol. 2004;26:270–1. doi: 10.1097/00043426-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Chanarin I. The Megaloblastic Anaemias. Oxford: Blackwell Scientific Publishers; 1979. p. 483. [Google Scholar]