Abstract
Although depression is often associated with a reduction in cellular immune responses, other types of emotional disturbance and psychopathology can activate certain aspects of immunity. Activation markers on T cells, in particular, have been found to be elevated in post-traumatic stress states. However, little is known about the relationship between the severity of PTSD symptoms and the degree of change in T cell phenotypes, or about the potential role of neuroendocrine factors in mediating the association. Twenty-four women with a history of sexual trauma during childhood, including 11 who met diagnostic criteria for PTSD, were compared to 12 age-matched, healthy women without a history of maltreatment. The women provided fasted blood samples for enumeration of cell subsets by immunofluorescence and 24-hour urine samples for analysis of catecholamine and cortisol levels. The percent of T cells expressing CD45RA, an early activation marker, was higher in the PTSD diagnosed women, and the levels correlated positively with intrusive symptoms and negatively with avoidant symptoms. These alterations in cell surface markers did not appear to be mediated by norepinephrine (NE) or cortisol, making them a distinctive and independent biomarker of arousal and disturbance in PTSD.
1. Introduction
Post-traumatic stress disorder (PTSD) is an anxiety disorder characterized by intrusive recall of a prior trauma, active avoidance of reminders of the traumatic events, and heightened autonomic arousal (American Psychiatric Association, 1994). Affected individuals vacillate between periods of high arousal, accompanied by flashbacks or unwanted memories, and behavioral efforts to avoid reminders of the original trauma (Horowitz, 1976; van der Kolk, 1987). By its very nature, PTSD is experienced as a dynamic and highly individualized disorder. Two patients with similar overall symptom severity may present quite differently: one showing a preponderance of intrusive symptoms, while another manifests more avoidant symptoms. Thus, some have argued that a greater appreciation of the individual variation in these psychological features is critical for understanding the PTSD patient (Foa et al., 1995). We hypothesized further that variability in presentation might shed light on the neuroendocrine and immune correlates of PTSD.
Many researchers have reported that individuals with PTSD have a unique neuroendocrine profile, which is often characterized by elevated norepinephrine (NE) in conjunction with low or slightly below normal cortisol levels, although some also have found elevated cortisol (Carrion et al., 2002; Heim et al., 2001; Mason et al., 1988; Oquendo et al., 2003; Yehuda et al., 1994). A growing literature also indicates that a chronic disturbance of immune regulation can accompany PTSD, including abnormal numbers and types of T lymphocytes in circulation. For example, Woods et al. (2005a) found that PTSD symptoms induced by interpersonal violence were correlated with the absolute number of CD4+ and CD8+ cells in a symptom-specific manner. Intrusive symptoms were positively associated with CD4+ numbers, but not CD8+ cells, whereas avoidance was positively linked to both CD4+ and CD8+ cells.
In addition, a history of childhood sexual abuse may continue to affect T cell number and activity in adulthood via the persistence of PTSD symptoms. The severity of the PTSD appears to drive the relationship between an abuse history and the increased production of IFN-gamma by T cells (Woods et al., 2005a). Moreover, a study of 10 adult women with a current diagnosis of PTSD secondary to childhood sexual trauma found an elevated ratio of CD45RO+/CD45RA+ lymphocytes, suggestive of chronic immune activation (Wilson et al., 1999). In general, these immune findings concur with other surveys of larger samples, which indicated that a history of childhood trauma was associated with higher lymphocyte counts later in adulthood (Surtees et al., 2003). One possible functional significance for these phenotypic changes was suggested by the fact that women with PTSD related to childhood sexual abuse evince larger delayed type hypersensitivity responses (DTH) (Altemus et al., 2003), a cutaneous reaction involving both T regulatory and effector cells (Li et al., 1994; Werfel et al., 1995).
The number of T cells in circulation has been determined in several aroused and traumatized populations both with and without PTSD diagnoses (Ironson et al., 2007). For example, an elevated CD4/CD8 cell ratio -- both at baseline and following a laboratory challenge -- was seen in women experiencing post-traumatic stress symptoms related to a diagnosis of cancer in their children (Glover et al., 2005). In contrast, following sustained exposure to an environmental disaster, both CD4+ and CD8+ lymphocytes were reduced in a community survey after Hurricane Andrew when compared to normative values in the laboratory control subjects (Ironson et al., 1997). Immune evaluations of Vietnam veterans with a current PTSD diagnosis have yielded more mixed results, with some investigators reporting lower lymphocyte counts and a tendency for reduced CD4+ and CD8+ cell percentages (Boscarino and Chang, 1999), while others failed to replicate those results (Laudenslager et al., 1996). Given the mixed results, it seemed of value to continue investigating how these immune alterations generalize across different types of trauma, and especially to consider the relationship to the profile of symptoms.
There have actually been very few studies that simultaneously examined diagnostic category, symptom presentation, and the mediating role of neuroendocrine factors on the immune disturbance. Our primary aim was to examine the relative contribution of intrusive thought, distress, and avoidance, important components of PTSD in women with a history of childhood maltreatment (Antelman et al., 1997; Horowitz, 1976), and to replicate the evidence for chronic immune activation (Wilson et. al., 1999). A secondary goal was to examine the potential neuroendocrine mediation. Intrusion was hypothesized a priori to be an important negative predictor on the basis of its effects on number of natural killer cells (NK) (Ironson et al., 1997) and expression of IL-2 receptors on lymphocytes (La Via et al., 1996). It was also important to determine whether concurrent changes in neuroendocrine activity, specifically, abnormal levels of NE or cortisol, accounted for the aberrant lymphocyte profile. Other conditions that increase autonomic activity, including vigorous exercise and preeclampsia during pregnancy, lead to increases in T cell activation markers, including naïve CD45RA+, memory CD45RO+, and the transitional CD45RA+CD45RO+ cells (Chaiworapongsa et al., 2002; Chavance et al., 1993; Gabriel et al., 1993; Hong et al., 2004; Kryzywkowski et al., 2001; Sondergaard et al., 2000). A mediating role for cortisol has also been implicated in Vietnam veterans with PTSD, who were found to have high levels of glucocorticoid receptors (GR) on their lymphocytes, suggestive of an upregulation in response to chronically low cortisol (Yehuda et al., 1991). However, attempts to replicate that result after the Bosnian conflict revealed that Croatian combat veterans with PTSD actually had the opposite profile with elevated cortisol levels and reduced GR receptors on lymphocytes (Gotovac et al., 2003). These inconsistencies may be explained by recent research indicating that both hormone and immune effects vary with the type of the trauma, the passage of time since the traumatic event, as well as the severity of symptoms (Kawamura et al., 2001; Yehuda, 2003). It seemed imperative to determine if symptom presentation and severity helped to explain the degree of lymphocyte activation, and to verify if the immune changes were dependent upon abnormal neuroendocrine activity.
2. Methods
2.1. Participants
Thirty-six women with or without a history of childhood maltreatment participated in this study. Participants were recruited through advertisements placed in local newspapers or announcements in specialized treatment centers. The study was described in all advertisements as a study of “the effects of women’s early experiences on adult mental and physical health”. Phone interviews were conducted to screen for eligibility. The newspaper advertisements for control and abused participants indicated we were looking for women who were between 18-40 years of age, menstruating regularly, non-medicated and willing to provide blood and urine samples. When recruiting non-abused controls, the callers were informed that we sought to evaluate women who had never experienced any unwanted sexual contact prior to 18 years of age either within or outside of the home. The only difference in the advertisement for controls was the absence of the words “sexual abuse survivors” in the title. Both advertisements also indicated that an examination by medical staff would be included. After being screened as a likely candidate meeting criteria, all participants completed the PTSD Symptom Scale, a validated and standardized interview that assesses 17 DSM-III-R symptoms of PTSD (Foa et al., 1993; Foa et al., 1995).
A total of 72 women were initially screened (mean age = 30.3, SD = 6.4 years). Half were not included because of rigorous exclusion criteria, including the presence of any current physical disease or use of any medication with immune-modulating effects, as determined by self-report and a review of the medical interview by a physician (MC). All participants were completely medication free at the time of testing. Any woman who had experienced a traumatic event or major life stressor (e.g., divorce, death of a close family member, crime victimization, major medical illness, job loss) within the previous 12 months was also excluded. None of the participants were pregnant, within one year of giving birth, or lactating. After being provided with a complete description of the study, each woman signed a consent form following procedures approved by the Institutional Review Board of the University of Wisconsin Health Sciences Center, and a subsequent visit was scheduled. The women received a small financial remuneration for their participation.
The presence of Axis I diagnoses of PTSD and major depressive disorder was obtained through a computerized version of the NIMH Diagnostic Interview Scale (DIS) (Marcus et al., 1991) followed by a clinical interview during the initial visit. Any participant with a questionable diagnosis of any Axis I disorder was further interviewed and case notes reviewed by a Licensed Psychologist (AML) in consultation with a physician (MC) to ensure accuracy of all diagnoses1. The presence of a psychotic disorder, bipolar disorder or eating disorder disqualified the subjects from continued participation. One subject was excluded on the basis of psychotic symptoms and referred to a local psychiatrist for assistance. Final diagnostic categorizations were made after study completion only after full examination of all available data on the participant, including the phone interview material, IES scores, and clinical interview, by both the psychologist and physician. The Symptom Checklist-90 Revised (SCL-90-R) was administered and the resulting scores for depressive symptom severity used in the regression analyses. Eligible participants were categorized initially into two groups comprised of controls who did not report a history of childhood sexual abuse or treatment for other psychiatric conditions (n = 12, mean age = 31.17 ± 1.7, range = 20 - 40 years) and 24 women who self-reported a history of childhood sexual abuse. Approximately half of the latter women met criteria for a DSM-IV PTSD diagnosis (PTSD+: n = 11, mean age = 28.2 ± 2.1, range = 18 - 40 years; and PTSD-: n = 13, mean age = 31.3 ± 1.8, range = 21-42 years of age, respectively). The 3 groups did not differ significantly in age, ethnicity, or annual income.
2.2. Immunological Measures
Blood samples were processed on the morning of collection. Aliquots of heparinized blood (100 μl) were used for enumerating the cell subsets using standard immunofluorescence and two-color flow cytometric techniques (Fac-Scan, Becton-Dickinson). Briefly, samples were incubated with 20 μl of the appropriate antibodies for 30 min at room temperature. Red blood cells were then lysed, membranes were stabilized with a 2% formalin solution, and cells washed in Dulbecco’s phosphate buffered saline (D-PBS). Surface proteins were stained with the following monoclonal antibodies (MoAB): CD3 (pan T cell) phycoerythrin conjugated, CD4 (helper T cell) phycoerythrin conjugated, CD8 (cytotoxic T cell) both phycoerythrin and FITC-conjugated, CD45RA phycoerythrin conjugated, and CD45RO FITC conjugated (Immunotech, New York). Cell counts were analyzed both as a percentage of gated lymphocytes and the total number of lymphocytes.
2.3. Psychological instruments
Each participant completed the Impact of Events Scale (IES) to delineate PTSD symptoms (Horowitz et al., 1979). To generate comparable responses from the control participants, they were asked to “think about the most upsetting or traumatic event during your childhood.” Examples of stressful events within the control group included death of family members, divorce, life-threatening accidents, or witnessing the traumatic death of a pet. In addition, the Symptom Checklist-90-Revised was used as a means to evaluate depressive symptoms (Derogatis, 1977). Both the IES and the SCL-90-R have demonstrated excellent reliability and validity, and have been used extensively in a wide variety of trauma populations, both those with and without PTSD (Foa et al., 1993; Lemieux and Coe, 1995; Neal et al., 1994; Popiel and Susskind, 1985; Solomon, 1989; Zilberg et al., 1982). All abused subjects also completed the Incest History Questionnaire (IHQ) (Courtois, 1988). Finally, the Survey of Immunological and General Health (SIGH) served as a self-report measure of physical health (Kang et al., 1991). The SIGH also included questions on age, weight, and demographic background and has been used successfully across a wide variety of studies (Kang et al., 1991; Lemieux and Coe, 1995; Strauman et al., 1993; Strauman et al., 2004).
2.4. Neuroendocrine measures
Adreno-medullary and -cortical activity were assessed via analysis of the 24-hr urine specimens. The clinical laboratory at UW Hospital quantified catecholamines (NE, epinephrine, and dopamine) using high pressure liquid chromatography (HPLC), and urinary free cortisol via radioimmunoassay. Creatinine levels were also determined, but not used to adjust hormone values. The very high correlation with urine volume verified complete collection of the daily voids, and thus it was not necessary to transform the actual hormone concentrations by the excretion of creatinine.
2.5. Procedures
Once accepted into the study, the women were evaluated individually at the General Clinical Research Center (GCRC) of the University of Wisconsin Hospital and Clinics. Each subject completed two sessions: an initial session and a sample collection session. During the initial session the DIS, physical exam, clinical interview, IHQ, SCL-90-R, and orientation/consent to the project occurred. During the physical examination, strict adherence to the medication exclusion criteria was explained to the subjects and all women again confirmed they were not using any medications currently, either prescribed or over-the-counter. This included hormonal drugs for contraception; in addition, a detailed description of both oral and topical medications was discussed with each participant. Any participant who reported a change in medication status was either rescheduled or dropped from the study (n = 3). Given the age range of 18-40 years, it was not expected that any women were peri-menopausal, but this fact was verified by the physician during the clinical interview. None of the women were postpartum. The initial sessions occurred at any time, while the sample collection session was specifically scheduled to occur during the mid-follicular phase of the menstrual cycle (7 - 10 days after menses). At the initial session each subject was given urine collection materials and the IES questionnaire, and explicitly instructed to time the collection to her menstrual cycle. The women were also told not to complete the IES until they were “in a safe and comfortable environment” during the 24 hr of urine collection. This scheduling was intended to minimize any effect of a potentially distressing clinical interview on the urine and blood results.
At the end of the 24-hr urine collection period, the subject returned to the GCRC unit and blood sampled between 0700-0900 to control for diurnal variation. In this way, urine and blood samples, as well as the assessment of PTSD symptoms, were obtained as concurrently as possible. An 8 ml blood sample was obtained, and utilized for a Complete Blood Count with differential and immunophenotyping of the lymphocytes. Subjects were also asked to refrain from consuming alcohol the night before, provided a fasted sample, and again verified on interview that they were not using any prescription medications. Adherence to procedures for obtaining complete urine voids was encouraged via telephone contact and self-report about the collection procedures.
2.6. Data analyses
Demographic, abuse, and psychological variables were analyzed as between-subjects factors. Fisher’s exact test was used for the nominal data, rather than chi square, because of expected frequencies below 5. Continuous data were analyzed using analysis of variance (ANOVA) or t test. Regression analyses were conducted to assess the relationships between the psychological, neuroendocrine, and immune measures. Distributions for each residual were tested for normality using the Shapiro-Wilks W test of normality. In all cases, except for numbers of CD45RO+ cells, the residuals were normal. Analysis of the CD45RO distribution indicated that one outlier accounted for this discrepancy, and this score was not included in subsequent analyses. Because age can have a significant influence on many immune variables, it was included as a covariate in all analyses. Given the potential for a comorbid diagnosis of depression, a careful examination of the effects of depression, including the issue of collinearity, was conducted. Statistical diagnostics were conducted (SPSS v14), and indicators such as critical condition indices, variance inflation factors (VIF), and tolerance indicators in the regression models were examined (see results section. The primary dependent variables were percentage and number of cells with the following surface antigen designations: CD4+CD45RA+ (early activation, T helper/inducer cells), CD8+CD45RA+ (early activation T suppressor/cytotoxic cells), and CD45RO+ (late activation or memory cells). Predictors of immune activity included the cardinal symptoms of PTSD (i.e., intrusion and avoidance), depression severity on the SCL-90-R, urinary NE and cortisol.
3. Results
3.1. PTSD diagnosis and overall group differences
To verify that the rigid exclusion criteria did not differentially affect one group, a Chi Square analysis was conducted to compare those who had been accepted or rejected to the eventual group composition. Forty-seven percent of the women classified as controls were accepted into the study, 32% of the women later classified as PTSD+ were accepted, and 48% of the women eventually classified as PTSD - were included. The Chi Square analysis indicated showed that there had not been preferential loss of subjects and that exclusion criteria had a comparable effect across groups [X2(2) = 1.36, p > .05]. The sample was predominantly Caucasian [1 Other in the Control group, 1 African American and 1 in the abused women with PTSD, and 1 African American in the abused women without PTSD; X2(2) = .80, p > .10]. Age was similar across all groups based on eventual diagnostic status [F(2, 33) = .85, p > .10].
Based on the clinical interview and the DIS, 11 of the 24 women with a history of childhood abuse met criteria for PTSD (Table 1), although the score range for individual items showed considerable overlap across groups. Consistent with the diagnosis, the PTSD+ women scored significantly higher on the intrusive and avoidance subscales of the IES as compared to PTSD negative (PTSD-) and control women [F(2,33) = 15.35, p = .01; F(2,33) = 17.56, p = .01, respectively]. Similarly, their total symptom scores were significantly higher [F(2,33) = 23.35, p = .01]. The values were comparable to published reports for hospitalized male combat veterans with PTSD (Yehuda et al., 1992).
Table 1.
Psychological and abuse characteristics of the 3 groups of women
| Controls | PTSD- | PTSD+ | |
|---|---|---|---|
| N=12 | N=13 | N=11 | |
| IES Intrusiona | 2.5 (2.9) | 6.9 (6.6) | 17.5 (9.17)*** |
| Range: | 0 - 8 | 0 - 19 | 6 - 33 |
| IES Avoidancea | 4.3 (5.5) | 12.0 (11.8) | 24.7 (5.7)*** |
| Range: | 0 - 15 | 0 - 36 | 16 - 34 |
| IES Totala | 6.8 (7.8) | 18.9 (16.9) | 42.3 (10.7)*** |
| Range: | 0 - 21 | 0 - 55 | 25 - 59 |
| Age at entry into studya | 31.2 (6.0) | 31.3 (6.5) | 28.2 (6.9) |
| Ethnicityb | 92% | 82% | 92% |
| Alcohol use per weeka | 2.5 (3.3) | 1.3 (1.6) | 0.9 (1.4) |
| Age at abuse onset (yrs) | NA | 7.2 (2.8) | 9.3 (4.0) |
| Duration (years) | NA | 1.5 (2.1) | 4.0 (1.9)** |
| Perpetrators | NA | 1.1 (0.5) | 1.8 (1.1)# |
| Father abuser | NA | 1 | 5* |
| Life threat | NA | 1 | 6* |
| Penetration | NA | 7 | 8 |
| Predictability | NA | 7 | 4 |
| Weapons | NA | 0 | 1 |
Note: Data presented as means (standard deviations) followed by range.
Ethinicity expressed as percent Caucasian.
p =.07
p < .05
p < .01
p < .001
Women with a PTSD diagnosis had experienced a more severe form of sexual abuse than did the other 12 previously maltreated women who did not qualify for a current diagnosis (Table 1). They were more likely to have been abused by their fathers [Fisher’s Exact test = .048], felt a threat to life during the abuse [Fisher’s Exact test = 0.018], and had experienced abuse for longer periods [t(18) = 2.82, p = .01]. The PTSD+ women also evinced a nonsignificant trend for a higher number of abusers [t(19) = 1.94, p = .07]. Other factors, such as mean age at onset of abuse, vaginal penetration, ability of the victim to predict each occurrence, and the use of weapons for intimidation, did not differ between the PTSD+ and PTSD- groups. Although most of the PTSD- women were free of a current psychiatric diagnosis, the DIS indicated that 46% of them might have qualified for a PTSD diagnosis at some point earlier in their lives.
As expected, depression as defined by the DIS and clinical evaluation was the most frequent comorbid condition in the PTSD+ group (73%), and it was the most common primary diagnosis in the PTSD- group (23%). Only one subject had a history of smoking (PTSD+), but she did not smoke at the time of the study. None of the subjects had current or lifetime alcohol abuse or addiction diagnoses as determined by the DIS. One PTSD+ participant had a history of cannabis use, but she had been drug-free for the 6 months prior to participation. Average weekly use of alcoholic beverages (defined simply as “drinks per week”) was the lowest in the PTSD+ women, highest in the controls, while the PTSD- women were intermediate [M = 0.9, SD = 1.43, M = 2.5, SD = 3.28, M = 1.3, SD = 1.57, respectively], though these differences were not significant (F(2, 33) = 1.53, p > .10). Recent sleep reported on the SIGH in average number of hours per night over the last 5 nights and quality of sleep (rated as excellent, good, difficult, poor or extremely disruptive) did not differ across the groups.
3.2. Group differences in immune and endocrine measures
In keeping with the prevailing PTSD literature, group comparisons were conducted initially to examine the possibility of differences in neuroendocrine and immune values, which laid the groundwork for the primary regression analyses with symptoms. Overall number and percentages of leukocytes and T lymphocytes did not differ between the 3 groups of women (Table 2). However, participants with abuse-related PTSD had a significantly higher percentage of CD8+ lymphocytes expressing the early activation marker (CD45RA+) than did both the controls or PTSD- women [F(2,29) = 5.24, p < .05]. Post hoc testing with Scheffe procedures confirmed that the PTSD+ women were significantly different from controls (Mean difference = -5.53, p = .03) and PTSD- women (Mean difference = -5.58, p = .025). There was also a similar tendency for more CD4+CD45RA+ cells in the PTSD+ women, but this difference did not reach statistical significance. Conversely, the PTSD+ women showed a marginal tendency for an opposite effect on lymphocytes expressing CD45RO, with lower percentages seen only in women experiencing PTSD related to their history of abuse [F(2,31) = 2.67, p = .08].
Table 2.
Neuroendocrine and immune status of control and previously abused women
| Controls | PTSD- | PTSD+ | |
|---|---|---|---|
| NE μg/day (n = 36)a | 32.4 (9.6) | 31.2 (14.6) | 36.7 (13.0) |
| Cortisol μg/day (n = 35)a | 82.2 (34.4) | 48.8 (17.1) | 22.2 (9.8) |
| CD4+ (n = 35) | 42.53 (13.13) | 44.95 (10.85) | 44.62 (11.09) |
| CD8+ (n = 35) | 24.17 (8.06) | 23.98 (6.68) | 22.99 (6.97) |
| CD4+CD45RA+ (early activation Th/I, n = 32)b | 17.7 (2.31) | 17.9 (2.21) | 23.97 (2.56) |
| CD8+CD45RA+ (early activation Tc/s, n = 32)b | 13.6 (1.32) | 13.6 (1.26) | 19.2 (1.45)* |
| CD45RO+ (memory T cells, n = 35)b | 40.2 (2.68) | 41.1 (2.47) | 32.7 (2.68)# |
Note: Data presented as total daily excretion not corrected by creatinine.
Data presented as percentage of lymphocyte subsets.
Post hoc Scheffe comparisons indicate significant differences between controls and PTSD+ as well as PTSD+ and PTSD- (p < .05), but not PTSD- and controls.
Overall effect of group comparison was marginal at F(2, 31) = 2.68, p = .085. Sample size differences reflect samples lost due to technical errors.
As seen in Table 2, none of the groups excreted significantly more NE or less cortisol than the other two groups (Table 2; F(2, 35) = .62, p >.10 and F(2, 34) = 2.53, p > .05 respectively), although the mean differences were in the expected direction based on previous PTSD studies. One cortisol specimen was lost due to technical error, thus accounting for the smaller sample size in the cortisol analysis. Further exploration of neuroendocrine activity in terms of the NE/cortisol ratio also did not reveal the abnormal hormone phenotype described for some other PTSD populations [F(2,34) = 0.16, NS]. As expected from the completeness of the urine voids, this negative result was found consistently with both creatinine-corrected and uncorrected values. The uncorrected values are presented in Table 2 to permit the interested reader to see the actual urinary concentrations. Finally, a regression analysis in which the continuous measures of psychological distress were entered into a hierarchical regression indicated that none of the psychological measures (SCL-90-R based estimates of depression, IES intrusion scores, and IES avoidance scores) predicted the 24-hr cortisol, NE or the ratio of NE/cortisol. Thus, neither diagnostic category nor individual differences in symptom presentation were related to urinary catecholamine and cortisol excretion levels.
3.3. Regression analyses between neuroendocrine and immune variables
Despite the absence of significant hormone differences, PTSD symptom severity was related to the T cell profile. Fig. 1 portrays a scatterplot of the IES Intrusion scores, regressed against one of the activation markers on CD4+ cells, indicating that symptom severity was associated with CD45RA+ expression across both the PTSD- and PTSD+ groups. Regression models with age as a covariate entered first, followed group status as step 2 and by intrusion and avoidance scores as step 3, were predictive of both the number and percentages of CD45RA+ and CD45RO+ cells. The age covariate was significant at step 1 for only the percentage of CD4+CD45RA+ subset (adjusted R2 = .10, p < .05) but not numbers of CD4+CD45RA+ cells (adjusted R2 = .09, p > .10). With both age and group status in the model, the regression model was again not significant for either number or percentage of CD4+CD45RA+ cells (adjusted R2 = .03, p > .05 and adjusted R2 = .07, p > .05). Addition of symptom severity in the second block of predictors raised the total model from non-significant R2 values near zero to significance for both the number and percentage of CD4+CD45RA+ (adjusted R2 = .25, p < .05 and adjusted R2 = .44, p < .001, respectively). Inclusion of symptom severity enabled the models to predict a total 44% of the variance in the %CD4+CD45RA+ cells. Similarly, significant final models using the same predictors emerged for the relationships with the percentage of CD8+CD45RA+ (R2 = .12, p < .05), and the percentage, but not number, of CD45RO+ cell subsets (R2 = .11, p < .05). Table 3 provides a comparison of these regression models for the percentages of CD4+CD45TA+, CD8+CD45RA+ and CD45RO+ cell subsets. The results from similar analyses of cell subset numbers have not been shown to limit redundancy in the presentation.
Fig. 1.
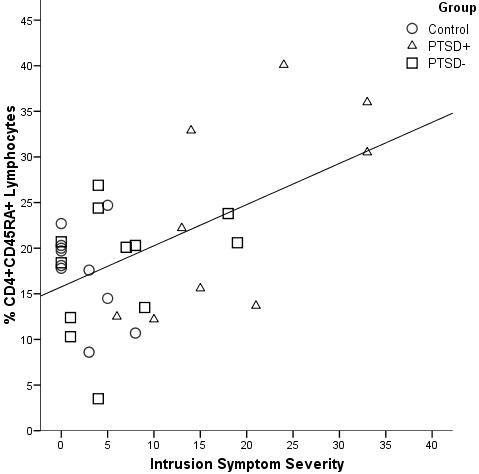
This scatterplot shows the association between intrusion scores and T cell activation (% CD4+45RA+) obtained in the first regression analysis (See Table 3), standardized β = .84, p < .01.To facilitate the subsequent discussion of the statistical analyses, the post hoc group designations are shown, which illustrates the overlap in intrusion scores across the three groups. Control women are indicated by circles, PTSD- women by squares, and PTSD+ women by the triangles.
Table 3.
Regression analyses of the comparison of group and PTSD symptom severity
| % CD4+CD45RA+ | % CD8+CD45RA+ | % CD45RO+ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Ba | SE B | β | t | Bb | SE B | β | t | Bc | SE B | β | t | |
| Step 1 | Age | -.485 | .227 | -.364 | 2.139* | -.157 | .148 | -.190 | -1.057 | .244 | .248 | .171 | .981 |
| Step 2 | Age | -.486 | .231 | -.365 | -2.106* | -.160 | .140 | -.193 | -1.141 | .244 | .253 | .171 | .967 |
| Group | -.167 | 1.597 | -.018 | -.105 | -.768 | .997 | -.134 | -.770 | .084 | 1.930 | .008 | .043 | |
| Step 3 | Age | -.475 | .180 | -.357 | -2.646** | -.160 | .140 | -.193 | -1.141 | .233 | .236 | .163 | .985 |
| Group | -.533 | 1.279 | -.058 | -.416 | -.768 | .997 | -.134 | -.770 | 1.004 | 1.847 | .092 | .544 | |
| Intrusion (IES) | .707 | .160 | .837 | 4.424*** | -.067 | .125 | -.128 | -.537 | -.484 | .232 | -.490 | -2.083* | |
| Avoidance (IES) | -.296 | .133 | -.427 | -2.223* | .229 | .104 | .532 | 2.206* | .059 | .184 | .076 | .322 | |
Note: For step 1, % CD4+CD45RA+ equation adjusted R2 = .103, ΔR2 = .132, p < .05; for step 2, % CD4+CD45RA+ equation adjusted R2 = .073, ΔR2 = .000, p >.05; for step 3, % CD4+CD45RA+ equation adjusted R2 = .439, ΔR2 = .379, p > .001.
For step 1, % CD8+CD45RA+ equation adjusted R2 = .004, ΔR2 = .036, p > .05; for step 2, % CD8+CD45RA+ equation adjusted R2 = -.030, ΔR2 = .001, p > .05; for step 3, % CD8+CD45RA+ equation adjusted R2 = .115, ΔR2 = .193, p < .05.
For step 1, % CD45RO+ equation adjusted R2 = -.001, ΔR2 = .029, p > .05; for step 2, % CD45RO+ equation adjusted R2 = -.033, Δ2 = .000, p > .05; for step 3, % CD45RO+ equation adjusted R2 = .106, ΔR2 = .185, p < .05.
Subsequent analyses considered the possible predictive value of the two neuroendocrine factors in addition to the symptom severity. As the inclusion of group designation based on PTSD diagnosis was not a significant predictor in the first regression analysis, it was dropped in favor of the symptom severity measures (see Table 3). Age was entered as a covariate, and then NE and cortisol as a block of variables, and psychological symptoms (depression symptoms on the SCL-90-R, intrusion, and avoidance) as a final block of variables. Due to the potential confound of depression and PTSD symptoms showing collinearity, a careful examination of the collinearity diagnostics was conducted (SPSS v14). Since none of the critical condition indices exceeded 15, collinearity did not seem evident. Further analyses supported this conclusion. All variance inflation factors (VIF) were well below 5, which are much lower than recommended “red flag” criteria of ≥ 10, and no tolerance indicators in the collinearity statistics were below 0.2. The full model predicting the percentage of CD4+CD45RA+ cells was significant and accounted for 55% of the variance [F(6, 23) = 4.72, p < .01; R2 = .55]. An examination of standardized beta coefficients showed that within this model only age (β = -.31, p < .05) and intrusive symptoms (β = .74, p < .001) were significant predictors of the percent of CD4+CD45RA+ cells. The level of avoidance symptoms had a marginal negative relationship with CD4+CD45RA percentages (β = -.49, p < .07). Neither the NE or cortisol levels were significant predictors in these models (all p > .10, see Table 4). Additional examination of the regression models was conducted to examine whether depressive symptoms was a primary factor accounting for either the cell numbers or hormone levels. The strongest predictors of CD4+CD45RA+ cells remained intrusive and avoidant symptoms, and these relationships did not appear to be mediated through depression (R2 change = .026, F(1,23) = 1.33, p > .10) or neuroendocrine activity (R2 change = .032, F(2,23) = 0.89, p > .10). Similar modeling attempts for the number of CD4+CD45RA+, and the number and percent of CD8+CD45RA+ cells yielded regression models that failed to attain significance (all p’s > .10).
Table 4.
Regression analyses of age, neuroendocrine values and PTSD symptoms in predicting the % CD4+CD45RA+, % CD8+CD45RA+ and % CD45RO+ subsets
| % CD4+CD45RA+ | CD8+CD45RA+ | CD45RO+ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Ba | SE B | β | t | Bb | SE B | β | t | Bc | SE B | β | t | |
| Step 1 | Age | -.505 | .234 | -.377 | -2.156* | -.183 | .132 | -.253 | -1.384 | .236 | .255 | .167 | .926 |
| Step 2 | Age | -.449 | .232 | -.335 | -1.933+ | -.150 | .132 | -.207 | -1.133 | .226 | .266 | .159 | .849 |
| Norepinephrine | -.192 | .122 | -.269 | -1.575 | -.094 | .069 | -.244 | -1.358 | -.142 | .175 | -.150 | -.812 | |
| Cortisol | -.060 | .056 | -.184 | -1.061 | -.037 | .032 | -.213 | -1.166 | .024 | .071 | .064 | .340 | |
| Step 3 | Age | -.410 | .196 | -.306 | -2.088* | -.101 | .125 | -.139 | -.807 | .256 | .252 | .181 | 1.016 |
| Norepinephrine | -.157 | .108 | -.219 | -1.450 | -.153 | .069 | -.397 | -2.231* | -.175 | .167 | -.185 | -1.049 | |
| Cortisol | -.006 | .049 | -.019 | -.126 | -.027 | .031 | -.153 | -.857 | -.031 | .068 | -.082 | -.454 | |
| SCL-90-R Depreesion | 2.148 | 1.861 | .246 | 1.154 | 2.068 | 1.186 | .438 | 1.743 | -.283 | 2.486 | -.028 | -.114 | |
| Intrusion (IES) | .622 | .190 | .743 | 3.282** | -.063 | .121 | -.139 | -.520 | -.688 | .258 | -.705 | -2.664* | |
| Avoidance (IES) | -.346 | .185 | -.489 | -1.864 | .067 | .118 | .174 | .564 | .259 | .233 | .331 | 1.114 | |
Note: For step 1, % CD4+CD45RA+ equation adjusted R2 = .122, ΔR2 = .142, p < .05; for step 2, % CD4+CD45RA+ equation adjusted R2 = .157, ΔR2 = .102, p >.05; for step 3, % CD4+CD45RA+ equation adjusted R2 = .435, ΔR2 = .308, p > .01.
For step 1, % CD8+CD45RA+ equation adjusted R2 = .031, ΔR2 = .064, p > .05; for step 2, % CD8+CD45RA+ equation adjusted R2 = .067, ΔR2 = .100, p > .05; for step 3, % CD8+CD45RA+ equation adjusted R2 = .215, ΔR2 = .214, p = .074.
For step 1, % CD45RO+ equation adjusted R2 = -.005, ΔR2 = .0028, p > .05; for step 2, % CD45RO+ equation adjusted R2 = .045, ΔR2 = .029, p > .05; for step 3, % CD45RO+ equation adjusted R2 = .131, ΔR2 = .243, p =.056.
Given the effect size reported above, a sample size analysis was conducted to verify that the study was not underpowered. An online sample size calculator was used (Soper, D. S., 2007; http://www.damielsoper.com/statcalc). An alpha level of 0.05 was entered with 7 predictors (full model with covariates), and an R2 = .55 as the effect size (f2 = 1.22), with a desired power level of 0.9. This analysis indicated a minimum required sample size would be 24. Because our study was based on 32 participants, the findings were not derived from an underpowered model. In addition, a post hoc statistical power calculation indicated that the observed power for a study with 7 predictors and our observed R2 of 0.55 was 0.99.
4. Discussion
This study provides further support for a significant and enduring relationship between PTSD and the percentages and types of T cell subsets in circulation. At a general level, these findings concur with a prior report indicating a relationship between severity of traumatic stress symptoms and numbers of T helper and T cytotoxic cells in the parents of pediatric cancer patients (Glover et al., 2005). Likewise, the current results generally support a very large recent study of childhood maltreatment, which indicated that early trauma during childhood can result in a proinflammatory physiological bias later in adulthood, although the influence of specific PTSD-related symptoms was not examined in those analyses (Danese et al., 2007). Furthermore, our results showed that at least in our sample of otherwise healthy women with a history of sexual abuse, neither NE nor cortisol were significant predictors of the effects on T cell subsets.
It is this documentation of a specific influence of PTSD-related symptoms, in the absence of neuroendocrine mediation, that makes our report unique. PTSD symptoms were sufficient to distinguish between women still suffering and those not currently manifesting symptoms or who had never been maltreated, and to predict the enumeration of the CD4+ CD45RA+ cell subset. This linkage occurred despite the lack of significance in a simpler factorial approach involving general diagnostic category. The findings thus concur with the individualistic and dynamic nature of the PTSD diagnosis. It was the analysis of the continuum of symptoms that most revealing beyond just meeting the criteria for a PTSD diagnosis. A relationship between the profile of PTSD symptoms and immunity concurs with the conclusions of Wilson et al. (1999), although the direction of their effect differed. They highlighted the importance of the ratio of CD45RO+ to CD45RA+ cells. The apparent discrepancy between their study and ours could be due to the small sample sizes, but more likely may reflect the absence of non-psychiatric controls and a possible influence of not including individual covariates in their statistical modeling. Their paper also did not detail whether one could predict CD45RA+ cell numbers based on diagnostic category alone. It is also important to note that the developmental age at the time of trauma and the extent of the abuse differed between the two studies. Finally, while our results differ from a large study of abused women that found elevated numbers of lymphocyte across several subsets, both reports found positive correlations with intrusion and avoidance (Woods et al., 2005b), Several methodical differences in that study should be noted, including the fact that their community sample included a high percent of minority participants, and that the blood specimens for immune analyses were collected 40 minutes after the completion of possibly arousing questionnaires.
The lymphocyte subset differences in the present study occurred in the absence of systematic differences in the total white cell count, which might be more likely to occur when PTSD+ participants also differ in their neuroendocrine activity. The regression analyses indicated that the relationship between intrusive symptoms and cell subsets was not mediated by either NE or cortisol. In general, the field of research on PTSD has been challenged by the marked variation in hormone levels across the populations under study (Bierer et al., 2006; Yehuda et al., 1994). So, while early studies suggested that a profile of elevated NE and low cortisol would be a reliable biological index of PTSD, further research on a wider range of traumas has indicated that the disturbance in neuroendocrine activity is quite variable (Yehuda et al., 2006). Even across populations of male combat veterans, different research teams found both low and high cortisol levels, which correlated with either increased or decreased GR numbers on lymphocytes (Gotovac et al., 2003). Thus, it may not be too surprising that a study of women who had experienced a very different type of trauma at a young age in childhood yielded results that don’t concur exactly with results on PTSD+ men. It is also now known that the co-occurrence of depression and PTSD can influence whether cortisol differences are found (Oquendo et al., 2003).
It should also be reiterated that our rigorous exclusion criteria prevented any women with physical illnesses, currently using prescription or over-the-counter medication, or postpartum and lactating from participating. This methodological issue is extremely important because many subclinical illnesses and psychological disorders can create a proinflammatory physiological state, which could drive the activation of lymphocytes (Maes et al., 1992). The issue of whether or not to include individuals with minor clinical conditions or using medications is a methodological challenge for any project of this type. In our case, we opted to exclude all participants using medications or reporting even minor health problems, which would certainly influence the likelihood of finding immune differences. There was not a differential rate of rejection or acceptance across groups indicating that overall the restrictions affected all groups in a comparable manner. The findings, do, however, provide a rare glimpse into the relatively pure effect of psychological state in a healthy sample free of the pharmacological effects of medication. Future studies will be needed to replicate these immune findings in other samples of PTSD patients who are medication free.
Finally, childhood sexual abuse can be a significant risk factor for early onset of alcohol consumption (ages 12-13), although the rate of conversion from use to dependence appears to be similar to the general population (Sartor et al., 2007). Thus the observed low rate of alcohol use by these women is a critical feature of the rigorous inclusion/exclusion criteria of the present study. Drinking was actually moderately higher in the controls, but still estimated by self-report as averaging only 2.5 alcoholic beverages per week. Given the relationship between alcohol dependence, sleep disturbance and immune function, especially in the recently abstinent African American participants (Irwin et al., 2002; Irwin et al., 2004), there is a considerable need for future studies to consider the potential immunological influence of alcohol use in women with abuse histories. The present study was not intended to examine this important issue and did not have detailed longitudinal design required to appropriately address this concern (c.f. Sartor et al., 2007). The very low rate of alcohol use documented in our sample, however, suggests that our rigid inclusion criteria were successful in providing us with a sample of women who were both physically healthy and had relatively healthy lifestyles.
It is also important to emphasize that our modeling indicated that the relationships between PTSD symptoms and T cell activation were not driven by depression. As reported in most other PTSD studies, the psychological evaluations indicated that current or past depression was common in those with a history of maltreatment. While other papers have reported that that major depressive disorder can affect the CD4+CD45RA+ cell subset (Maes et al., 1992), the current results were not secondary to depressive symptoms. Moreover, the regression models indicated that intrusion even more than avoidance was related to the cell activation. Thus, the effects were mediated more by the psychological distress and arousal than by dysphoria. The importance of intrusion has also been highlighted in analyses on NK cell numbers and IL-2 receptor levels, where the influence was a suppressive one (Ironson et al., 1997); (La Via et al., 1996). A prior report on patients with panic disorder indicated they showed a trend for higher percentages of CD45RA+ cells, which was positively predicted by global severity of symptoms and, marginally, by anxious symptoms (Manfro et al., 2000). T cell numbers, in general, increase in the circulation immediately following an acute stressor, often due to increased sympathetic activity and changes in adhesion molecules that serve to release cells from the blood vessel wall (Mills et al., 1997).
A potential, but untested, factor that could account for the increased CD45RA+ cells is neuropeptide Y (NPY). Low levels of NPY have been linked to childhood abuse (Seedat et al., 2003), and NPY increases with successful resolution of PTSD symptoms (Yehuda et al., 2006). The cognitive state of dissociation, which is common in PTSD, has also been negatively correlated with NPY during periods of acute interpersonal stress (Morgan et al., 2000). Given that NPY is co-localized with NE on sympathetic terminals, and plays a role in the differentiation and proliferation of T cells, and it could stimulate an increase in T helper cells (Bedoui et al., 2001). NPY may thus be another important physiological mediator to explore in the future.
In summary, women with PTSD were found to have a distinct T cell profile, which was related more to the severity of intrusive symptoms than to their avoidant behavior. These differences in naïve T helper cells did not appear to be due to altered NE or cortisol activity and thus may provide an independent biomarker for this disorder. Given that many studies have now found that victims of childhood abuse are over-represented later among many adult disease populations, including those with gastrointestinal disorders, chronic pain conditions, and even some types of cancer (Drossman, 1994; Felitti, 1991; Springer et al., 2007), more studies on the predictive significance of this immune and inflammatory dysregulation are warranted. We would argue further that a failure to account for individual differences in specific psychological symptoms may help to account for inconsistencies in the disparate PTSD literature, which has been based primarily on group differences in broad diagnostic categories.
Acknowledgments
This research was conducted at the University of Wisconsin General Clinical Research Center (GCRC), which was supported by M01 RR03186 from the National Center for Research Resources, NIH. Dr. Lemieux was also supported by NIMH Dissertation Grant MH10377. Dr. Carnes’ was supported by the General Clinical Research Center Grant M01 RR03186; and the Geriatric Research, Education, and Clinical Center of the Madison Veteran’s Hospital, Madison, WI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular response in women with PTSD related to childhood abuse. American Journal of Psychiatry. 2003;160:1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, D. C.: 1994. [Google Scholar]
- Antelman SM, Caggiula AR, Gershon S, Edwards DJ. Stressor-induced oscillation: A possible model of the bidirectional symptoms in PTSD. In: Yehuda R, McFarlane A, editors. Psychobiology of Posttraumatic Stress Disorder. New York Academy of Sciences; New York: 1997. p. 550. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Kuhlmann S, Nave H, Drube J, Pabst R, von Horsten S. Differential effects of neuropeptide Y (NPY) on leukocyte subsets in the blood: Mobilization of B-1 like B-lymphocytes and activated monocytes. Journal of Neuroimmunology. 2001;117:125–132. doi: 10.1016/s0165-5728(01)00328-9. [DOI] [PubMed] [Google Scholar]
- Bierer LM, Tischler L, Labinsky E, Cahill S, Foa E, Yehuda R. Clinical correlates of 24-H cortisol and norepinephrine excretion among subjects seeking treatment following the World Trade Center Attacks on 9/11. Annals of the New York Academy of Sciences. 2006;1071:514–520. doi: 10.1196/annals.1364.055. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: Research and clinical implications. Psychosomatic Medicine. 1999;61:378–386. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Gervasi M, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. American Journal of Obstetrics and Gynecology. 2002;187:889–893. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- Chavance M, Perrot JY, Annesi I. Smoking, CD45RO+ (Memory), and CD45RA (Naive) CD4+ T cells. American Review of Respiratory Diseases. 1993;148:237–240. doi: 10.1164/ajrccm/148.1.237. [DOI] [PubMed] [Google Scholar]
- Courtois CA. Healing the incest wound: Adult survivors in therapy. Norton, NY: 1988. [Google Scholar]
- Derogatis LR. SCL-90-R: Administration, scoring, and procedures manual I for the revised version. Johns Hopkins University, Clinical Psychometrics Research Unit; Baltimore: 1977. [Google Scholar]
- Drossman DA. Physical and sexual abuse and gastrointestinal illness: What is the link? American Journal of Medicine. 1994;97:105–107. doi: 10.1016/0002-9343(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Felitti VJ. Long-term medical consequences of incest, rape, and molestation. Southern Medical Journal. 1991;84:328–331. doi: 10.1097/00007611-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. [Google Scholar]
- Foa EB, Riggs DS, Gershuny BS. Arousal, numbing, and intrusion: Symptom structure of PTSD following assault. American Journal of Psychiatry. 1995;152:116–120. doi: 10.1176/ajp.152.1.116. [DOI] [PubMed] [Google Scholar]
- Gabriel H, Schmitt B, Urhausen A, Kindermann W. Increased CD45RA+CD45RO+ cells indicated activated T cells after endurance exercise. Medicine and Science in Sports and Exercise. 1993;25:1352–1357. [PubMed] [Google Scholar]
- Glover DA, Steele AC, Stuber ML, Fahey JL. Preliminary evidence for lymphocyte distribution differences at rest and after acute psychological stress in PTSD-symptomatic women. Brain, Behavior, & Immunity. 2005;19:243–251. doi: 10.1016/j.bbi.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotovac K, Sabioncello A, Rabatic S, Berk T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: Lower quantity of GCR in patients with post-traumatic stress disorder (PTSD) Clinical and Experimental Immunology. 2003;131:335–339. doi: 10.1046/j.1365-2249.2003.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–181. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hong S, Farag NH, Nelesen RA, Ziegler MG, Mills PJ. Effects of regular exercise on lymphocyte subsets and CD62L after psychological vs. physical stress. Journal of Psychosomatic Research. 2004;56:363–370. doi: 10.1016/S0022-3999(03)00134-X. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner N, Alvarez W. Impact of event scale: A measure of subjective stress. Psychosomatic Medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ. Stress response syndromes. Jason Aronson; New York: 1976. [Google Scholar]
- Ironson G, Cruess D, Kumar M. Immune and neuroendocrine alterations in post-traumatic stress disorder. In: Ader R, editor. Psychoneuroimmunology. Elsevier Academic Press; New York, New York: 2007. pp. 531–547. [Google Scholar]
- Ironson G, Wynings C, Schneiderman N, Baum A, Rodriguez M, Greenwood D, Benight CC, Antoni M, LaPerriere A, Huang H, Klimas N, Fletcher MA. Posttraumatic stress symptoms, intrusive thoughts, loss and immune function after Hurricane Andrew. Psychosomatic Medicine. 1997;59:128–141. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biological Psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American Alcoholics. Brain, Behavior, and Immunity. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kang D, Davidson RJ, Coe CL, Wheeler RE, Tomarken AJ, Erschler WB. Frontal brain asymmetry and immune function. Behavioral Neuroscience. 1991;105:860–869. doi: 10.1037//0735-7044.105.6.860. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Kim Y, Asuki N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. American Journal of Psychiatry. 2001;158:484–486. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- Kryzywkowski K, Petersen EW, Ostrowski K, Kristensen JH, Boza J, Pedersen BK. Effect of glutamine supplementation on exercise induced changes in lymphocyte function. American Journal of Physiology: Cell Physiology. 2001;281:C1259–1265. doi: 10.1152/ajpcell.2001.281.4.C1259. [DOI] [PubMed] [Google Scholar]
- La Via MF, Munno I, Lydiard RB, Workman EW, Hubbard JR, Michel U, Paulling E. The influence of stress intrusion on immunodepression in generalized anxiety disorder patients and controls. Psychosomatic Medicine. 1996;58:138–142. doi: 10.1097/00006842-199603000-00007. [DOI] [PubMed] [Google Scholar]
- Laudenslager M, Berger C, Reite M, Wilkins RT, Zweig L. Elevated cytotoxicity in combat veterans with long-term post-traumatic stress disorder: Preliminary observations. Brain, Behavior, & Immunity. 1996;12:74–79. doi: 10.1006/brbi.1997.0513. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related post-traumatic stress disorder: Evidence of chronic neuroendocrine activation in women. Psychosomatic Medicine. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Li L, Elliott JF, Mosmann TR. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. Journal of Immunology. 1994;153:3967–3978. [PubMed] [Google Scholar]
- Maes M, Stevens W, DeClerck L, Bridts C, Peeter D, Schotte C, Cosyns P. Immune disorders in depression: Higher T helper/T supressor-cytotoxic cell ratio. Acta Psychiatrica Scandinavica. 1992;86:423–431. doi: 10.1111/j.1600-0447.1992.tb03292.x. [DOI] [PubMed] [Google Scholar]
- Manfro GG, Pollack MH, Otto MW, Worthington JJ, Rosenbaum JF, Scott EL, Kradin RL. Cell-surface expression of L-selectin (CD62L) by blood lymphocytes: Correlates with affective parameters and severity of panic disorder. Depression and Anxiety. 2000;11:31–37. [PubMed] [Google Scholar]
- Marcus S, Robins LN, Bucholz K. Quick Diagnostic Interview Schedule III-R. Washington University; St. Louis, MO: 1991. [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Harkness L. Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. Journal of Nervous and Mental Disease. 1988;176:498–502. doi: 10.1097/00005053-198808000-00008. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Karnik RS, Dillon E. L-selectin expression affects T-cell circulation following isoproterenol infusion in humans. Brain, Behavior and Immunity. 1997;11:333–342. doi: 10.1006/brbi.1997.0500. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Wang S, Southwick SM, Rasmusson A, Hazlett G, Hauger RL, Charney DS. Plasma neuropeptide-Y concentration in humans exposed to military survival training. Biological Psychiatry. 2000;47:902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Neal LA, Busuttil W, Rollins JW, Herepath R, Strike P, Turnbull GJ. Convergent validity of measures of post-traumatic stress disorder in a mixed military and civilian population. Journal of Traumatic Stress. 1994;7:447–455. doi: 10.1007/BF02102789. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Echavarria GF, Galfalby HC, Grunebaum MF, Burke A, Barrera A, Cooper TB, Malone KM, John Mann J. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- Popiel DA, Susskind EC. The impact of rape: Social support as a moderator of stress. American Journal of Community Psychology. 1985;13:645–677. doi: 10.1007/BF00929794. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, McCutcheon VV, Nelson EC, Waldron M, Heath AC. Childhood sexual abuse and the course of alcohol dependence development: Findings from a female twin sample. Drug and Alcohol Dependence. 2007;89:139–144. doi: 10.1016/j.drugalcdep.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedat S, Stein MB, Kennedy CM, Hauger RL. Plasma cortisol and neuropeptide Y in female victims of intimate partner violence. Psychoneuroendocrinology. 2003;28:796–808. doi: 10.1016/s0306-4530(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Solomon Z. Characteristic psychiatric symptomatology of post-traumatic stress disorder in veterans: A three year follow-up. Psychological Medicine. 1989;19:927–936. doi: 10.1017/s003329170000564x. [DOI] [PubMed] [Google Scholar]
- Sondergaard SR, Lepri AC, Wiis HU, Hermann CK, Laursen SB, Qvist J, Gerstoft J, Skinhoj P, Pedersen BK. Adrenaline-induced mobilization of T cells in HIV-infected patients. Clinical & Experimental Immunology. 2000;119:115–122. doi: 10.1046/j.1365-2249.2000.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental consequences of childhood physical abuse: Results from a large population-based sample of men and women. Child Abuse and Neglect. 2007 doi: 10.1016/j.chiabu.2007.01.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauman TJ, Lemieux AM, Coe CL. Self-discrepancies and natural killer cell activity: Immunological consequences of negative self-evaluation. Journal of Personality and Social Psychology. 1993;64:1042–1052. doi: 10.1037//0022-3514.64.6.1042. [DOI] [PubMed] [Google Scholar]
- Strauman TJ, Woods TE, Schneider KL, Kwapil L, Coe CL. Self-regulatory cognition and immune reactivity: Idiographic success and failure feedback effects on the natural killer cell. Brain, Behavior, and Immunity. 2004;18:544–554. doi: 10.1016/j.bbi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Surtees P, Wainwright N, Day N, Brayne C, Luben R, Khaw K. Adverse experience in childhood as a developmental risk factor for altered immune status in adulthood. International Journal of Behavioral Medicine. 2003;10:251–268. doi: 10.1207/s15327558ijbm1003_05. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA, Kolk B.A.v.d. Psychological Trauma. American Psychiatric Press, Inc.; Washington, D. C.: 1987. The psychological consequences of overwhelming life experiences; pp. 1–30. [Google Scholar]
- Werfel S, Massey W, Lichtenstein LM, Bochner BS. Preferential recruitment of activated, memory T lymphocytes into skin chamber fluids during human cutaneous late-phase allergic reactions. Journal of Allergy and Clinical Immunology. 1995;96:57–65. doi: 10.1016/s0091-6749(95)70033-1. [DOI] [PubMed] [Google Scholar]
- Wilson S, van der Kolk B, Burbridge J, Fisler R, Kradin R. Phenotype of blood lymphocytes in PTSD suggests chronic immune activation. Psychosomatics. 1999;40:222–225. doi: 10.1016/S0033-3182(99)71238-7. [DOI] [PubMed] [Google Scholar]
- Woods AB, Page GG, O’Campo P, Pugh LC, Ford D, Campbell JC. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-[gamma] levels. American Journal of Community Psychology. 2005a;36:159–176. doi: 10.1007/s10464-005-6240-7. [DOI] [PubMed] [Google Scholar]
- Woods AB, Wineman M, Page GG, Hall RJ, Alexander TS, Campbell JC. Predicting immune status in women from PTSD and childhood and adult violence. Advances in Nursing Science. 2005b;28:306–319. doi: 10.1097/00012272-200510000-00003. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Hypothalamic-pituitary-adrenal alterations in PTSD: Are they relevant to understanding cortisol alterations in cancer? Brain, Behavior, & Immunity. 2003;17:S73–S84. doi: 10.1016/s0889-1591(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang R. Plasma neuropeptide Y concentrations in combat exposed veterans: Relationship to trauma exposure, recovery from PTSD, and coping. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.08.027. In press. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Giller EL, Southwick SM, Kahana B, Boisoneau D, Ma X, Mason JW. Relationship between catecholamine excretion and PTSD symptoms in Vietnam combat veterans and holocaust survivors. In: Murburg MM, editor. Catecholamine function in posttraumatic stress disorder: Emerging concepts. American Psychiatric Press; Washington, D. C.: 1994. pp. 203–219. [Google Scholar]
- Yehuda R, Lowry MT, Southwick SM, Shaffer D, Giller EL. Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. American Journal of Psychiatry. 1991;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. Journal of Nervous and Mental Disease. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Zilberg NJ, Weiss DS, Horowitz MJ. Impact of events scale: A cross-validation study and some empirical evidence supporting a conceptual model of stress response syndromes. Journal of Consulting and Clinical psychology. 1982;50:407–414. doi: 10.1037//0022-006x.50.3.407. [DOI] [PubMed] [Google Scholar]


