Abstract
The hepatocarcinogen and toxicant, furan, requires metabolic activation to elicit its toxic effects. The available experimental evidence indicate that the overall metabolism of furan is initiated via cytochrome P450 catalyzed oxidation to cis-2-butene-1,4-dial. This α,β-unsaturated dialdehyde reacts in vitro with protein and DNA nucleophiles. To determine if this compound is an in vivo intermediate in the metabolism of furan, rats were treated with either [12C4]furan or [13C4]furan and urine was collected for 24 h. Capillary LC/MS/MS analysis of the urine indicated that one of the metabolites was a mono-glutathione conjugate of cis-2-butene-1,4-dial. These results indicate that glutathione conjugation of the reactive metabolite of furan occurs in vivo. This metabolite may serve as a useful marker for furan exposure and metabolism in risk assessment studies.
Keywords: furan, metabolism, stable isotope methods, capillary LC-MS, urinary metabolites
Introduction
Furan is an important industrial chemical that is also present in the environment (1). It is both hepatoxic and carcinogenic after oral administration in mice and rats (2,3). Based on these results and the large potential for human exposure, furan has been listed as a possible human carcinogen (Group 2B) by the National Toxicology Program and the International Agency for Research on Cancer (1,4). However, risk assessment has been hampered by the lack of effective biomarkers for furan uptake and metabolism.
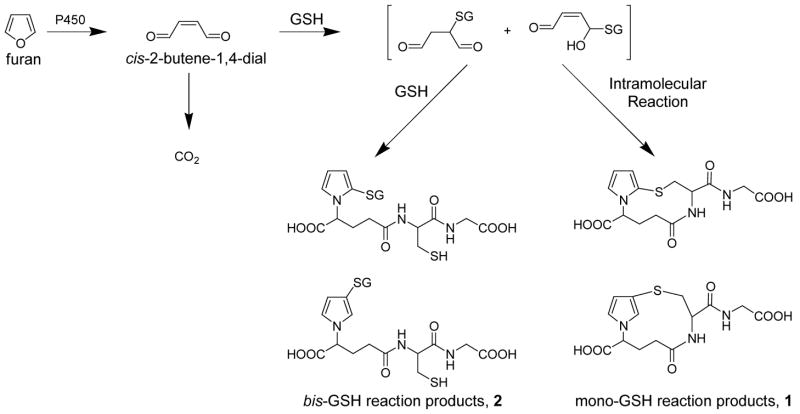
The mechanism of furan induced toxicity and carcinogenicity is unknown. The available experimental data indicate that the toxic effects of furan are initiated by a cytochrome P-450 catalyzed oxidation of furan. Cytochrome P-450 inducers increase furan metabolism and its associated toxic effects such as furan derived protein binding, glutathione (GSH) depletion and cytotoxicity (5-9). Similarly, these toxic effects are reduced in the presence of cytochrome P-450 inhibitors (5–9). The initial product of microsomal furan oxidation is cis-2-butene-1,4-dial (Scheme 1) (10–12). This reactive α,β-unsaturated dialdehyde is likely to be a candidate for the ultimate toxic and carcinogenic properties of furan since it readily reacts with protein and DNA nucleophiles (11,13,14) and is a bacterial mutagen (15). The formation and fate of this molecule in vivo has not been investigated.
Scheme 1.
Proposed in vivo metabolic pathways for furan.
Furan is extensively metabolized in rats since only 14% of an 8 mg/kg oral dose of [14C]furan is eliminated unchanged (5). The major metabolite is carbon dioxide, accounting for 26% of the dose. A majority of the remainder is excreted in urine (22%) or feces(20%). Since the uptake of furan is a single saturable process that can be blocked by the cytochrome P450 inhibitor pyrazole (9), a cytochrome P450 catalyzed reaction is thought to initiate the in vivo metabolism of furan. Therefore, the observed microsomal metabolite, cis-2-butene-1,4-dial, is likely to be an important intermediate in the conversion of furan to carbon dioxide and other excreted metabolites (Scheme 1). The urinary and fecal metabolites of furan have not been chemically characterized. However, HPLC analysis of urine from [14C]furan-treated rats indicated the presence of several polar metabolites that were proposed to be GSH or mercapturic acid conjugates of furan metabolites (5).
In this report, we describe studies designed to characterize the urinary metabolites of furan. cis-2-Butene-1,4-dial reacts rapidly with GSH to form mono- and bis-GSH reaction products (1 and 2, respectively, Scheme 1) (10–12). Therefore, we developed stable isotope mass spectral methods to detect the GSH conjugates of cis-2-butene-1,4-dial and their subsequent metabolites (Scheme 1 and 2) in the urine of furan-treated rats. These studies are the first step in the development of markers which can be used to assess furan exposure and metabolism in humans.
Scheme 2.
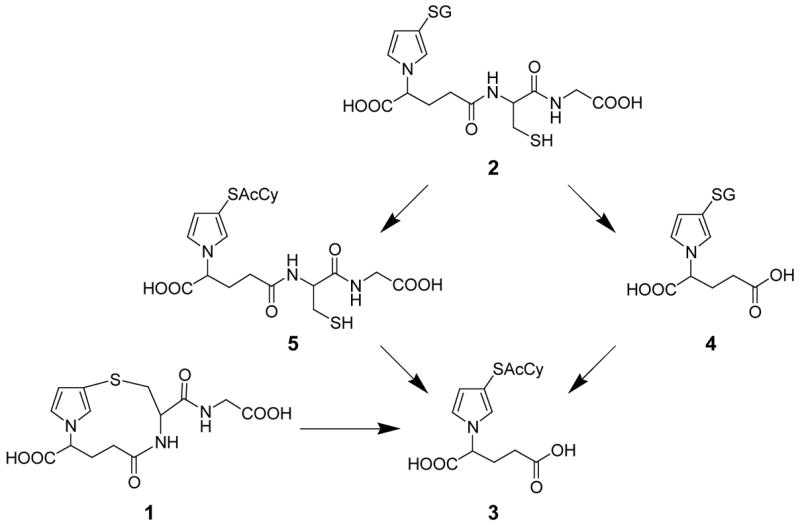
Further metabolism of the conjugates of cis-2-butene-1,4-dial. The 2-substituted isomer will generate structurally similar metabolites. GS, glutathione; AcCy, N-acetylcysteine.
Methods
Caution
cis-2-Butene-1,4-dial is toxic and mutagenic in cell systems. Furan is toxic and carcinogenic in laboratory animals. Both chemicals should be handled with proper safety equipment and precautions.
Furan and trifluoroacetic acid were purchased from Acros Organics (Pittsburgh, PA). [13C4]Furan, cis-2-butene-1,4-dial, and the mono- and bis-GSH conjugates of cis-2-butene-1,4-dial were prepared as previously described (11,12,14,16). Furan was distilled before use and its purity was confirmed by NMR. Corn oil was purchased from Sigma Chemical Co. (St. Louis, MO). tris-(Carboxyethyl)phosphine (TCEP) was purchased from Strem Chemicals (Newburyport, MA). All other chemicals were reagent grade.
2-{3-[S-(N-Acetylcysteinyl)]-1H-pyrrol-1-yl}pentanedioic acid and 2-{2-[S-(N-acetylcysteinyl)]-1H-pyrrol-1-yl}pentanedioic acid
N-Acetylcysteine (26 mg, 0.16 mmol) was added to a solution of cis-2-butene-1,4-dial (0.16 mmol) in 1 M sodium phosphate, pH 7.4 (total volume: 0.7 mL). Fifteen minutes later, glutamate (23 mg, 0.16 mmol) was added to the reaction. After 1 hr at room temperature, the reaction products were isolated by preparative HPLC. The Phenomenex Bondclone C18 column (250 × 10 mm, 10 micron) was eluted with a 15 min linear gradient from 20 mM NH4OAc, pH 4.9, to 20 mM NH4OAc, pH 4.9, containing 25% acetonitrile (flow rate: 3 mL/min). Absorbance was monitored at 222 and 254 nm. The reaction products eluted at 13.3 and 15.5 min.
2-{3-[S-(N-Acetylcysteinyl)]-1H-pyrrol-1-yl}pentanedioic acid (3)
1H NMR (300 MHz, D2O) δ: 6.78 (s, 1H, H2′), 6.66 (s, 1H, H5′), 6.08 (s, 1H, H4′), 4.33 (m, 1H, H2), 4.12 (m, 1H, Cys αCH), 3.0 (m, 1H, Cys β CHa), 2.75 (m, 1H, Cys β CHb), 2.17 (m, 1H, H3a), 1.99 (m, 1H, H3b), 1.91 (m, 2H, H4), 1.84 (s, 3H, N-CH3). MS m/z: 359 [M+H].
2-{2-[S-(N-Acetylcysteinyl)]-1H-pyrrol-1-yl}pentanedioic acid
1H NMR (300 MHz, D2O) δ: 6.87 (s, 1H, H5′), 6.26 (s, 1H, H3′), 6.07 (s, 1H, H4′), 4.88 (m, 1H, H2), 4.09 (m, 1H, Cys αCH), 2.83 (m, 1H, Cys β CHa), 2.68 (m, 1H, Cys β CHb), 2.26 (m, 1H, H3a), 2.04 (m, 1H, H3b), 1.90 (m, 2H, H4), 1.84 (s, 3H, N-CH3). MS m/z: 359 [M+H].
2-{3-[S-(Glutathionyl)]-1H-pyrrol-1-yl}pentanedioic acid (4)
GSH (65 mg, 0.2 mmol) and cis-2-butene-1,4-dial (0.2 mmol) were combined in 150 mM sodium phosphate, pH 7.4 (total volume: 7 mL). Glutamate (61 mg, 0.2 mmol) was added after 15 minutes. The desired product was isolated from a complex mixture by preparative HPLC. The HPLC column was eluted with a 19 min linear gradient from 100 mM ammonium acetate to 100 mM ammonium acetate containing 10% acetonitrile. 1H NMR (300 MHz, D2O) δ: 6.75 (s, 1H, H2′), 6.65 (s, 1H, H5′), 6.05 (s, 1H, H4′), 4.32 (m, 1H, H2), 4.20 (m, 1H, Cys αCH), 3.60 (t, 1H, Glu αCH), 3.5 (s, 2H, Gly CH2), 3.0 (dd, 1H, Cys β CHa), 2.68 (dd, 1H, Cys β CHb), 2.30 (m, 2H, Glu γ CH2), 1.90-2.25 (m, 6H, Glu β CH2, H3, H4). MS m/z: 503 [M+H].
N-{4-Carboxy-4-[3-S-(N-acetylcysteinyl)-1H-pyrrol-1-yl]-1-oxobutyl}-L-cysteinylglycine (5)
N-Acetylcysteine (260 mg, 1.6 mmol) was combined with cis-2-butene-1,4-dial (1.6 mmol) in water (total volume: 6.8 mL). After overnight at room temperature, GSH (480 mg, 1.6 mmol) was added to the reaction. One and a half h later, the reaction mixture was purified by preparative HPLC. A Phenomenex Synergi 4μ Hydro-RP 80A column (250 × 10 mm, 4μ) was isocratically eluted with 20 mM NH4OAc, pH 4.9, containing 4% acetonitrile (flow rate = 3 mL/min). Absorbance was monitored at 222 and 254 nm. The major reaction product eluted at 11.0 minutes: 1H NMR (300 MHz, D2O) δ: 6.89 (s, 1H, H2′), 6.81 (s, 1H, H5′), 6.23 (s, 1H, H4′), 4.51 (m, 1H, H4), 4.47 (m, 1H, Cys α CH), 4.21 (m, H, AcCys α CH), 3.72 (s, 2H, Gly CH2), 3.15 (m, 1H, H- AcCys β CHa), 2.93 (m, 1H, Cys β CHa), 2.85 (m, 1H, Cys β CHb), 2.82 (m, 1H, AcCys β CHb), 2.41 (m, 1H, H2a), 2.26 (m, 1H, H2b), 2.24 (m, 1H, H3a), 2.2 (m, 1H, H3b), 1.82 (s, 3H, N-CH3). MS m/z: 519 [M+H].
N-{4-Carboxy-4-[3-S-(N-acetylcysteinyl)-1H-pyrrol-1-yl]-1-oxobutyl}-L-cysteinylglycine disulfide
N-Acetylcysteine (25 mg, 0.15 mmol) and cis-2-butene-1,4-dial (0.15 mmol) were combined in 115 mM sodium phosphate, pH 7.4 (total volume: 2.6 mL). Oxidized GSH (47 mg, 0.077 mmol) was added after 30 minutes at room temperature. Thirty min later, the reaction product was purified by preparative HPLC. A Phenomenex Synergi 4μ Hydro-RP 80A column (250 × 10 mm, 4μ) was eluted with a 15 min linear gradient from 20 mM NH4OAc to 20 mM NH4OAc containing 15% acetonitrile. Fractions containing the product were concentrated under reduced pressure (95 mg, 60%). Compound 5 can be generated directly from this material by reacting it with TCEP. 1H NMR (300 MHz, D2O) δ: 6.71 (s, 1H, H2′), 6.62 (s, 1H, H5′), 6.03 (s, 1H, H4′), 4.52 (m, 1H, Cys α CH), 4.26 (m, 1H, H4), 3.98 (m, H, AcCys α CH), 3.55 (s, 2H, Gly CH2), 3.05 (m, 1H, H- Cys β CHa), 2.93 (m, 1H, AcCys β CHa), 2.74 (m, 2H, Cys β CHb, AcCys β CHb), 2.30 (m, 1H, H2a), 2.00 (m, 3H, H2b, H3), 1.84 (s, 3H, N-CH3). MS m/z: 1035 [M+H].
Furan Treatment of Rats
F344 male rats weighing 200–300 g were purchased from Charles River Laboratories (Kingston, NY). Groups of three rats were treated with 8 mg/kg [12C4]- or [13C4]furan in 5 ml/kg corn oil by gavage. A control group received only corn oil. Immediately after treatment, rats were transferred to individual metabolism cages. Urine was collected on dry ice for 24 hours. It was stored in glass tubes at −80 °C.
LC-MS Analysis of Urine
Urine was acidified with TFA to a final concentration of 2%, centrifuged to remove any particulate matter and filtered through a 0.45 μm syringe filter. The samples were stored at −20° C in glass autosampler vials until analysis. In some cases, the urine was treated with TCEP to reduce any disulfide bonds (17,18).
Urine (8 μL) was eluted from an Agilent (Palo Alto, CA) Zorbax C18 capillary column (0.5 × 150 mm; 5 micron) with 50 mM ammonium formate, pH 2.8. After a 10 min isocratic period, the column was eluted with a 26 min linear gradient to 37.5 mM ammonium formate, pH 2.8 containing 12.5% acetonitrile. This, in turn, was followed by a 10 min gradient to 25 mM ammonium formate, pH 2.8, containing 25% acetonitrile (retention times: 1, 24 min; 4, 33 min; 2, 35 min, 3, 39 min, and 5, 42 min). The column was washed with water containing 50% acetonitrile prior to returning to initial conditions. The HPLC was coupled to either an Agilent 1100 series LC/MSD Trap SL mass spectrometer or a ThermoFinnigan Quantum triple quadrapole mass spectrometer with an electrospray ionization source in positive ion mode. Initial analyses were performed with the mass spectrometer set to full scanning mode with a scan range of 100–750 m/z. Urine from individual animals treated with [12C4]- or [13C4]furan were analyzed separately as well as a 1:1 mixture. In some cases, the samples were treated with TCEP prior to MS analysis to reduce any disulfide bonds (12). The resulting data were searched for compounds that were present in the samples from furan-treated rats, absent in the samples from control rats and shifted by four amu in the urine from [13C4]furan-treated treated rats. The samples were then reanalyzed with the mass spectrometer set in the autoMS(n) mode to obtain fragmentation patterns for each of the metabolites.
The entire volume of urine from one furan-treated rat was treated with TFA as described above (total volume = ~4 mL), filtered and fractionated by HPLC. The urinary metabolites were eluted from a Phenomenex (Torrance, CA) Bondclone semi-prep scale column (250 × 10 mm, 10 micron) with 50 mM ammonium formate, pH 2.8. A 10 min isocratic period was followed by a 40 min linear gradient to 37.5 mM ammonium formation, pH 2.8, containing 12.5% acetonitrile and then a 10 min gradient to water containing 50% acetonitrile (flow rate = 4 mL/min). The UV absorbance was monitored at 254 nm. The fraction containing the monoGSH conjugate was used to obtain high resolution mass spectral information. This data was collected on a ThermoFinnigan Quantum triple quadrapole mass spectrometer with an electrospray ionization source in positive ion mode.
Results and Discussion
We have shown that cis-2-butene-1,4-dial reacts rapidly with GSH to form both mono- and bis-GSH reaction products in microsomes (1 and 2, respectively, Scheme 1) (11,12). Therefore, if cis-2-butene-1,4-dial is formed in vivo, we expected that it would be trapped by GSH. We also expected that these reaction products may serve as substrates for the enzymes involved in processing GSH conjugates to mercapturic acid derivatives. The mono-glutathione conjugate of cis-2-butene-1,4-dial (1) would ultimately be converted to 2-{3-[S-(N-acetylcysteinyl)]-1H-pyrrol-1-yl}pentanedioic acid (3), in the case of the 3-substituted pyrrole isomer (Scheme 2). Compound 3 might also be the product resulting from processing of both GSH moieties in the bis-GSH-cis-2-butene-1,4-dial-reaction product (2). Two intermediates in the conversion of 2 to 3 are 2-{3-[S-(glutathionyl)]-1H-pyrrol-1-yl}pentanedioic acid (4) or N-{4-carboxy-4-[3-S-(N-acetylcysteinyl)-1H-pyrrol-1-yl]-1-oxobutyl}-L-cysteinylglycine (5). These compounds (3–5) were prepared by adapting the procedure for preparing the cis-2-butene-1,4-dial-GSH reaction products (1 and 2) (11,12) and characterized by NMR and mass spectrometry. All these standards were then employed to develop chromatographic methods for their separation by capillary LC (Figure 1).
Figure 1.
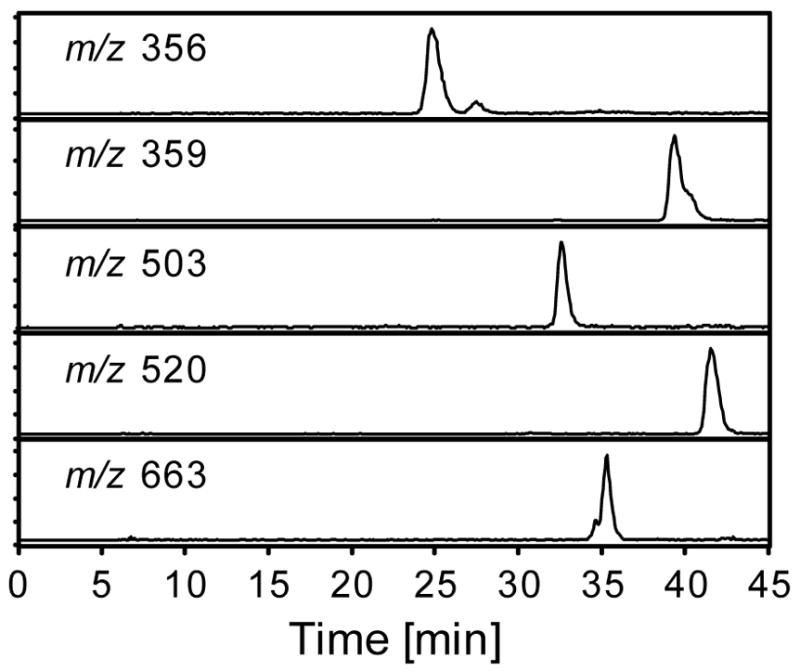
Extracted chromatograms for the expected GSH reaction products and their metabolites. The molecular ions for the metabolites are as follows: 1, m/z 356; 3, m/z 359; 4, m/z 503; 5, m/z 520 and 2, m/z 663.
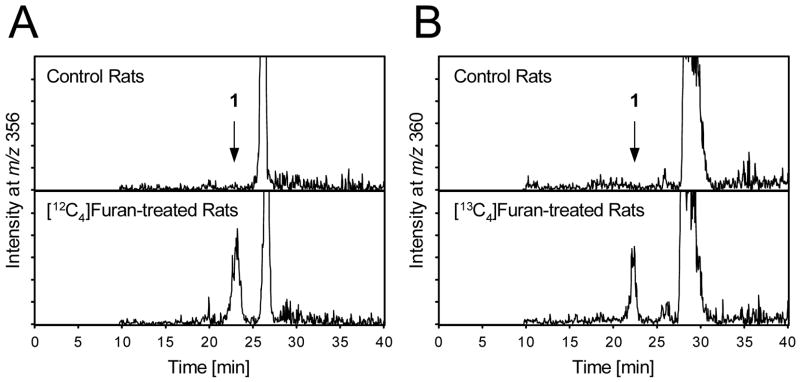
To determine the urinary metabolites of furan, groups of three F344 rats were treated with either [12C4]- or [13C4]furan (8 mg/kg, p.o.; in corn oil). A control group received vehicle alone. The animals were housed in metabolism cages for the collection of urine for 24 h. The urine was acidified with trifluoroacetic acid with the precipitate removed by centrifugation. The samples were then subjected to C18 capillary LC/MS/MS analysis with the mass spectrometer operating in full scanning mode. Data for the GSH and mercapturic acid derivatives of cis-2-butene-1,4-dial were extracted from the total ion current.
The only predicted metabolite detected in the urine from treated rats was the mono-GSH conjugate of cis-2-butene-1,4-dial. A peak was observed for m/z 356 in the urine of furan treated animals that had the same retention time as the standard for the mono-GSH conjugates (Figure 2A). A similar peak was observed at m/z 360 in the urine from [13C4]furan-treated rats (Figure 2B). The fragmentation pattern for the urinary metabolite is identical to the synthetic standard and the masses are shifted by 4 a.m.u. in the metabolite derived from [13C4]furan (Figure 3). In addition, high resolution mass spectral analysis is consistent with the proposed structure (accurate mass for C14H18N3O6S = 356.0916; mass of synthetic standard = 356.0894; mass of metabolite = 356.0904).
Figure 2.
Extracted mass chromatograms obtained at A) m/z 356 for urine from untreated or [12C4]furan-treated rats and B) m/z 360 for urine from untreated or [13C4]furan-treated rats.
Figure 3.
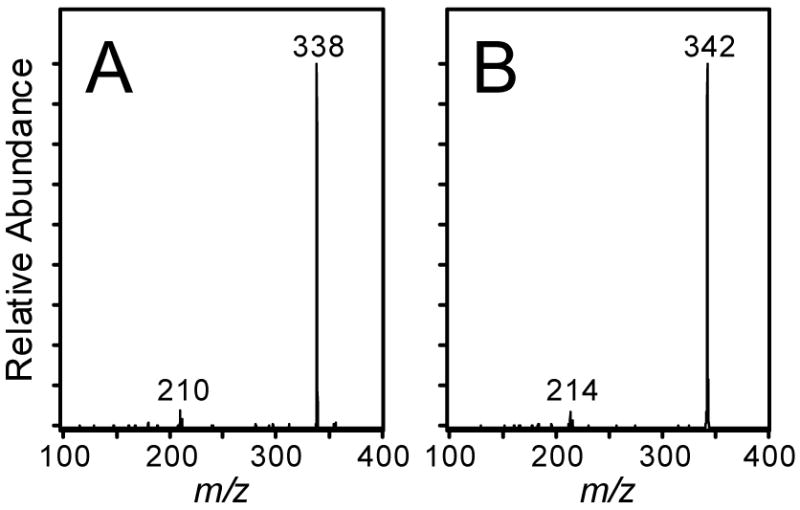
Collision induced mass spectra of the monoGSH conjugate detected in urine from rats treated with A) [12C4]furan or B) [13C4]furan.
The observation of this metabolite in the urine of furan-treated rats indicates that cis-2-butene-1,4-dial is formed and trapped with GSH in vivo. The major reaction product formed between cis-2-butene-1,4-dial and GSH in vitro are the bis-GSH conjugates (11,12). Their absence does not mean that they are not formed in vivo. Given their size, they may be preferentially eliminated via the bile as has been observed for the GSH conjugates of structurally related 4-ipomeanol (19). We are investigating whether these compounds and their metabolites are presented in the feces of furan-treated rats.
At least 18 other furan-derived urinary metabolites were also detected (data not shown). Preliminary analysis of the mass spectral data indicates that they are conjugates and that the furan-derived portion of the molecule results from further metabolism of cis-2-butene-1,4-dial. We are currently isolating the individual metabolites for more in-depth chemical characterization.
In conclusion, the mono-GSH reaction product of cis-2-butene-1,4-dial was observed in urine of furan treated rats. The presence of this metabolite indicates that cis-2-butene-1,4-dial is formed in vivo. Preliminary data provide evidence that this reactive metabolite of furan also undergoes further biochemical transformation to other compounds. These metabolites will be explored for their suitability as markers for the uptake and metabolism of furan in human risk assessment studies.
Acknowledgments
We thank Patrick Kinney, Dr. Fekadu Kassie, and Dr. Michael Byrns for their assistance with the animal studies and Dr. Peter Villalta for his assistance with the high resolution mass spectral analysis. The mass spectral analyses were performed in the Analytical Biochemical Core at the Cancer Center, University of Minnesota, which is funded by National Cancer Institute Center Grant CA-77598. This research was funded by ES-10577 from the National Institutes of Health.
Abbreviations
- GSH
glutathione
- TCEP
tris-(2-carboxyethyl)phosphine
Reference List
- 1.National Toxicology Program. 11th Report on Carcinogens. US Department of Health and Human Services; Washington DC: 2005. [Google Scholar]
- 2.National Toxicology Program. Toxicology and carcinogenesis studies of furan in F344/N rats and B6C3F1 mice vol. NTP Technical Report No. 402. US Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 1993. [Google Scholar]
- 3.Elmore LW, Sirica AE. “Intestinal-type” of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Res. 1993;53:254–259. [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. Vol. 53. IARC; Lyon: 1995. Furan; p. 393. [Google Scholar]
- 5.Burka LT, Washburn KD, Irwin RD. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health. 1991;34:245–257. doi: 10.1080/15287399109531564. [DOI] [PubMed] [Google Scholar]
- 6.Parmar D, Burka LT. Studies on the interaction of furan with hepatic cytochrome P-450. J Biochem Toxicol. 1993;8:1–9. doi: 10.1002/jbt.2570080103. [DOI] [PubMed] [Google Scholar]
- 7.Carfagna MA, Held SD, Kedderis GL. Furan-induced cytolethality in isolated rat hepatocytes: Correspondence with in vivo dosimetry. Toxicol Appl Pharmacol. 1993;123:265–273. doi: 10.1006/taap.1993.1245. [DOI] [PubMed] [Google Scholar]
- 8.Mugford CA, Carfagna MA, Kedderis GL. Furan-mediated uncoupling of hepatic oxidative phosphorylation in Fischer-344 rats: an early event in cell death. Toxicol Appl Pharmacol. 1997;144:1–11. doi: 10.1006/taap.1997.8121. [DOI] [PubMed] [Google Scholar]
- 9.Kedderis GL, Carfagna MA, Held SD, Batra R, Murphy JE, Gargas ML. Kinetic analysis of furan biotransformation by F-344 rats in vivo and in vitro. Toxicol Appl Pharmacol. 1993;123:274–282. doi: 10.1006/taap.1993.1246. [DOI] [PubMed] [Google Scholar]
- 10.Chen LJ, Hecht SS, Peterson LA. Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem Res Toxicol. 1995;8:903–906. doi: 10.1021/tx00049a001. [DOI] [PubMed] [Google Scholar]
- 11.Chen LJ, Hecht SS, Peterson LA. Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 1997;10:866–874. doi: 10.1021/tx9700174. [DOI] [PubMed] [Google Scholar]
- 12.Peterson LA, Cummings ME, Vu CC, Matter BA. Glutathione trapping to measure microsomal oxidation of furan to cis-2-butene-1,4-dial. Drug Metab Dispos. 2005;33:1453–1458. doi: 10.1124/dmd.105.004432. [DOI] [PubMed] [Google Scholar]
- 13.Byrns MC, Predecki DP, Peterson LA. Characterization of nucleoside adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 2002;15:373–379. doi: 10.1021/tx0101402. [DOI] [PubMed] [Google Scholar]
- 14.Byrns MC, Vu CC, Peterson LA. The formation of substituted 1, N6-etheno-2′-deoxyadenosine and 1, N2-etheno-2′-deoxyguanosine adducts by cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 2004;17:1607–1613. doi: 10.1021/tx049866z. [DOI] [PubMed] [Google Scholar]
- 15.Peterson LA, Naruko KC, Predecki D. A reactive metabolite of furan, cis-2-butene-1,4-dial, is mutagenic in the Ames assay. Chem Res Toxicol. 2000;13:531–534. doi: 10.1021/tx000065f. [DOI] [PubMed] [Google Scholar]
- 16.Vu CC, Peterson LA. Synthesis of [13C4]furan. J Label Compounds Radiopharm. 2005;48:117–121. [Google Scholar]
- 17.Burns JA, Butler JC, Moran J, Whitesides GM. Selective reduction of disulfides by tris(2-carboxyethyl)phosphine. J Org Chem. 1991;56:2648–2650. [Google Scholar]
- 18.Krijt J, Vackova M, Kozich V. Measurement of homocysteine and other aminothiols in plasma: Advantages of using tris(2-carboxyethyl)phosphine as reductant compared with tri-n-butylphosphine. Clin Chem. 2001;47:1821–1828. [PubMed] [Google Scholar]
- 19.Alvarez-Diez TM, Zheng J. Detection of glutathione conjugates derived from 4-ipomeanol metabolism in bile of rats by liquid chromatography-tandem mass spectrometry. Drug Metab Dispos. 2004;32:1345–1350. doi: 10.1124/dmd.104.000406. [DOI] [PubMed] [Google Scholar]