Abstract
Lung disease is a leading cause of death and likely to become an epidemic given increases in pollution and smoking worldwide. Advances in stem cell therapy may alleviate many of the symptoms associated with lung disease and induce alveolar repair in adults. Concurrent with the ongoing search for stem cells applicable for human treatment, precise delivery and homing (to the site of disease) must be reassured for successful therapy. Here, I report that stem cells can safely be instilled via the trachea opening a non-stop route to the lung. This method involves a skin incision, caudal insertion of a cannula into and along the tracheal lumen, and injection of a stem cell vehicle mixture into airways of the lung. A broad range of media solutions and stabilizers can be instilled via tracheotomy, resulting in the ability to deliver a wider range of cell types. With alveolar epithelium confining these cells to the lumen, lung expansion and negative pressure during inhalation may also assist in stem cell integration. Tracheal delivery of stem cells, with a quick uptake and the ability to handle a large range of treatments, could accelerate the development of cell-based therapies, opening new avenues for treatment of lung disease.
Protocol
Pre-operative
Recipient mouse and donor cells
Operating platform with tape and suture thread
1cc syringe
23G needle
Anesthetic: avertin 25 mg/ml (alternatively ketamine 9 mg/ml and xylazin 1 mg/ml)
Hair clipper with #40 blade
Sterile gloves
Alcohol swabs
Iodine
Sterilized surgical instruments
Suture thread
22G catheter
200 ul pipette (sterile)
Sterilized pipette tips
Antibiotic glue ointment
Sharpie container
Operative
Aseptic techniques are used throughout this procedure.
Weigh mouse.
Intraperitoneal injection of anesthetic (250 mg/kg avertin or alternatively ketamine 90 mg/kg and xylazine 10 mg/kg) at doses sufficient to allow spontaneous breathing, but provide surgical-concentration anesthesia. For surgery to commence the toe pinch was used as an indicator of deep pain with no response from the anesthetized animal.
Lay mouse with incisors tucked in suture thread and tape tail to stabilize.
Clip hair using a #40 clipper blade.
Change gloves (continue with sterile gloves).
Disinfect twice with alcohol prior to skin incision.
Sterilize area with an iodophore.
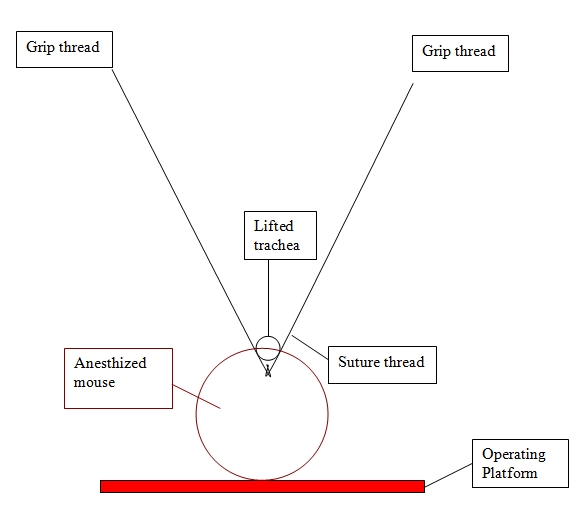
Using sterile instruments make a small incision (<1 cm) parallel to the trachea.
Carefully separate tissue from cartilage.
A tie is inserted underneath the trachea.
Gently raise tie upward and insert needle into exposed trachea.
Remove needle and insert the tube smoothly, yet rapidly, until feeling slight resistance.
Pipette cells (up to 50 microliters volume) directly into the catheter.
Immediately remove tube and tie while liberating mouse from operating apparatus.
If needed gently hold mouse upright (vertical) by clinging his anterior paws and move gently.
Close the wound with one suture or antibiotic glue.
Post-operative
Keep mouse warm.
Buprenorphine (0.05 mg/kg) should be given as post-operative analgesia every 12 hours for the first 24 hours following this procedure.