Abstract

Reaction of bisacetoxy pyranone 9 with Et3N gave 3-oxidopyrylium ylide 10, which underwent a stereo- and regiospecific [5 + 2] cycloaddition with α-methylenebutyrolactone to afford 16 (34%). Treatment of 16 with Cs2CO3 resulted in hydrolysis of the lactone and acetate and conjugate addition of the hydroxyethyl group to the enone. Lactonization on acidification afforded 4 (57%), completing a two-step, formal synthesis of polygalolides A and B.
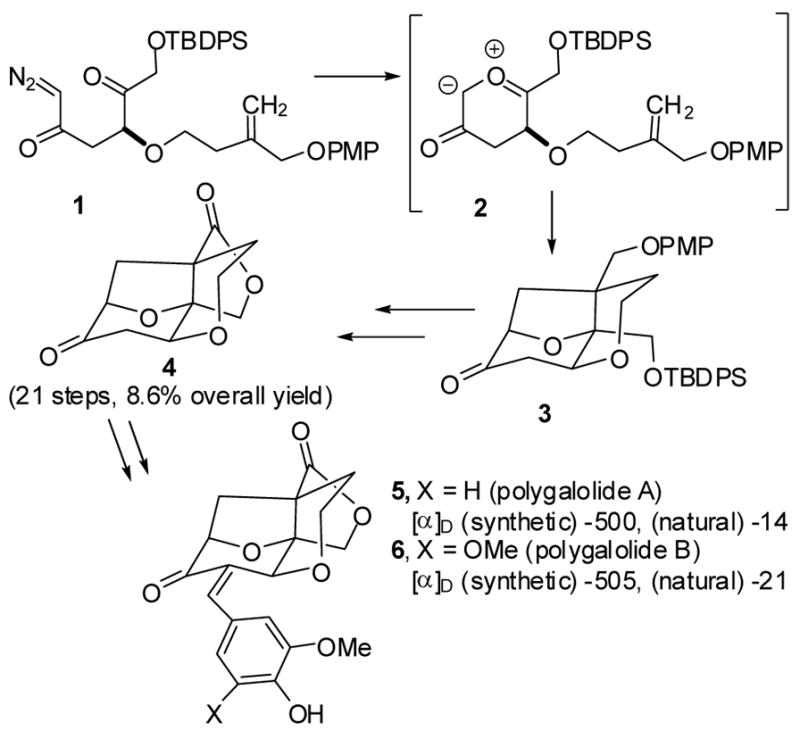
Some of us (S. N. and S. H.) recently reported the synthesis of polygalolides A (5) and B (6).1 The key step was the rhodium-catalyzed conversion of diazo ketone 1 to carbonyl ylide 2, which underwent an intramolecular 1,3-dipolar cycloaddition to give 3 in 73% yield. Further elaboration gave key intermediate 4, which was converted in 4 steps to polygalolides A (5) and B (6). This route afforded optically pure 4 in 8.6% overall yield in 21 steps. Remarkably, synthetic polygalolides A (5) and B (6) had much higher rotations, [α]D -500 and -505, respectively, than the natural products, [α]D -14 and -21, respectively.2 These values indicate that the enantiomeric excess of natural polygalolides A and B is only 3–4%.
One of us (B. B. S.) recently disclosed biomimetic syntheses of descurainin (13b) and cartorimine (14).3 5-Acetoxymethylfurfural (7), which is derived from fructose, was reduced to the alcohol, which was treated with bromine in methanol to give pyranone 8 in 80% yield. Acetylation afforded bisacetoxy pyranone 9. Treatment of 9 with Et3N in CH2Cl2 at 25 °C afforded 3-oxidopyrylium ylide 10, which underwent stereospecific [5 + 2] cycloadditions with styrenes 11a and 11b to give cyclo-adducts 12a (26%) and 12b (31%), respectively. Deprotection afforded the natural products 13a and descurainin (13b), respectively. Cycloaddition of 10, generated thermally from 9, with methyl p-acetoxycinnamate afforded cartorimine (14) after deprotection.3
The natural products 13a, descurainin (13b), and cartorimine (14) are probably biosynthesized by similar [5 + 2] cycloadditions of achiral compounds, but were isolated with small [α]D (+1.7 for 13b, −2.6 for 14) and Δε (+0.01 for 13a) indicating that they are not completely racemic. However, a [5 + 2] cycloaddition in a chiral environment could lead to an optically enriched product. A similar intermolecular [5 + 2] cycloaddition of an 3-oxidopyrylium ylide lacking the acetate protecting group of 10 with an isoprenoid fragment analogous to 15 might be involved in the biosynthesis of polygalolides A and B, which were isolated in only 3–4% enantiomeric excess.1
This biomimetic route can be carried out very efficiently providing a short route to the late polygalolide intermediate 4. We were surprised and delighted to find that treatment of bisacetoxy pyranone 9 with 2.5 equiv of Et3N and 6 equiv4 of α-methylenebutyrolactone (15) in CH2Cl2 for 16 h at 25 °C provided the desired [5 + 2] cycloadduct 16 with ~90% selectivity in 34% yield.5 We had not expected such a high degree of stereocontrol based on the limited literature precedent.6 The regio-chemistry of the cycloaddition follows from the 8.6 Hz coupling constant between Ha and Hb. The stereochemistry was established by the NOE between Hc and Hd.
Conversion of 16 to the polygalolide intermediate 4 requires hydrolysis of the lactone and acetate, conjugate addition of the hydroxyethyl side chain to the enone, and lactonization of the hydroxy acid. This was accomplished by stirring 16 with Cs2CO3 in 1:1 THF/H2O at 50 °C for 7 h, cooling to 25 °C, acidification to pH 1, and stirring for 3 h to effect lactonization giving 4 (57%) with 1H and 13C NMR spectra identical to those previously described.1 The analogous reaction in 1:1 acetone/H2O7 at 25 °C for 20 h provided 4 in 50% yield. Reaction of 16 with KOH or K2CO3 in MeOH/H2O afforded 4 in only 20–30% yield.
This sequence converts 9 and 15 to the late intermediate 4 in the polygalolides A and B synthesis in only two steps and 19.4% overall yield, making these natural products readily available. The efficiency and stereo-selectivity of this sequence supports the suggestion, originally based on the low enantiomeric excess of the natural products,1 that these natural products are biosynthesized by an analogous [5 + 2] cycloaddition.
Supplementary Material
Full experimental details and copies of 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Scheme 1.
Synthesis of Polygalolides A and B
Scheme 2.

Syntheses of Descurainin and Cartorimine
Scheme 3.

Biomimetic Two-step Synthesis of 4
Acknowledgments
We thank the National Institutes of Health (GM-50151) for generous financial support.
References
- 1.Nakamura S, Sugano Y, Kikuchi F, Hashimoto S. Angew Chem, Int Ed. 2006;45:6532–6535. doi: 10.1002/anie.200602030. [DOI] [PubMed] [Google Scholar]
- 2.Ma W, Wei X, Ling T, Xie H, Zhou W. J Nat Prod. 2003;66:441–443. doi: 10.1021/np020467i. [DOI] [PubMed] [Google Scholar]
- 3.(a) Snider BB, Grabowski JF. Tetrahedron Lett. 2005;46:823–825. [Google Scholar]; (b) Snider BB, Grabowksi JF. Tetrahedron. 2006;62:5171–5177. [Google Scholar]
- 4.The yield decreased to 24% and 9% with 3 and 1 equiv of 15, respectively
- 5.The minor impurity could not be separated or characterized.
- 6.See the Supporting Information for references to [5 + 2] cycloadditions of 3-oxidopyrylium ylides.
- 7.Luppi G, Tomasini C. Synlett. 2003:797–800. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental details and copies of 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.


