Abstract
Clinical manifestations of Q fever infection are fever, productive cough, decrease in exercise tolerance and chills. Cardiovascular involvement is well recognized and usually presents as endocarditis and infection of an aneurysm or vascular graft. Myocarditis has only rarely been described as a manifestation of acute Q fever infection. In this report we describe a case of a young adult who presented with angina-like symptoms and ECG and biochemical markers indicative of acute coronary syndrome. The diagnosis of myocarditis was ultimately made based on the results of a normal coronary angiography and increased anti-Coxiella burnetii antibody titer. The patient has not developed dilated cardiomyopathy after two years of follow up.
Keywords: Coxiella burnetii, Q fever, myocarditis, antibody titer
Case Report
A 30 year old male patient presented in the Emergency Department of our hospital with substernal pain beginning three hours before his arrival. The pain was squeezing in nature, radiated to his left arm and was accompanied by diaphoresis and nausea. The patient mentioned two other episodes of similar nature in the last 24 hours. The duration of each episode was approximately 3 hours. The patient had been well until 3 days before admission when he developed fever (up to 38.3 ℃), accompanied by chills, which persisted until admission to our hospital. No other concomitant symptoms were documented. The patient was in frequent contact with pigeons in the last few years as he owned a pigeonhole. No other exposure to animals or pets was reported. On examination, the temperature was 37.1℃, the blood pressure 110/55 mm Hg, and the pulse 78 beats per minute. No cardiac murmur or pericardial rub was detected on auscultation, and the lungs were clear. The abdomen was nontender, without organomegaly.
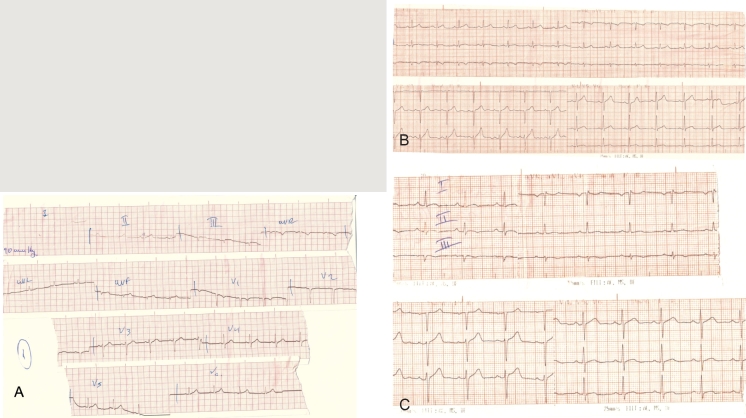
An electrocardiogram, obtained in a nearby health institution, 24 hours before admission to our hospital, during the first episode of pain, revealed ST elevations in leads II, III and aVF (Figure 1-A). On admission, the electrocardiogram revealed complete resolution of the ST-elevations in the above mentioned leads. However small sized, nonspecific negative T waves were evident in leads III and aVF (figure 1 - B).
Figure 1. ECG of the patient at: A: 24 hours before his admission - ST elevations are evident in leads II, III and aVF. B: on admission, and C: discharge - small sized negative T waves are seen in leads II, III, aVF, V5, V6.
The patient was admitted to the Coronary Care Unit. A diagnosis of acute coronary syndrome was assumed and he was treated with morphine, nitrates, aspirin, clopidogrel, ACEI and statin with prompt relief of his pain. Thereafter, the patient remained free of symptoms during his hospital stay. Moreover, during hospitalization no recurrences of fever were documented.
Results of laboratory tests showed a normal leukocyte count but an increase of the serum cardiac markers was observed. More specifically:
CPK: 474-582-530-432-240-112-81, (normal values 55-170U/lt),
CK-MB: 43-52-54-33-17-17-10, (normal values 0- 12U/lt),
SGOT: 48-57-58-51-35-21-44, (normal values 11- 47U/lt),
SGPT: 30-30-30-26, (normal values 7-53U/lt),
LDH: 475-705-537-515-537, (normal values 45- 90U/lt),
troponin I: 9.88, (normal values 0-1ng/ml),
myoglobin: 500, (normal values 0-107ng/ml).
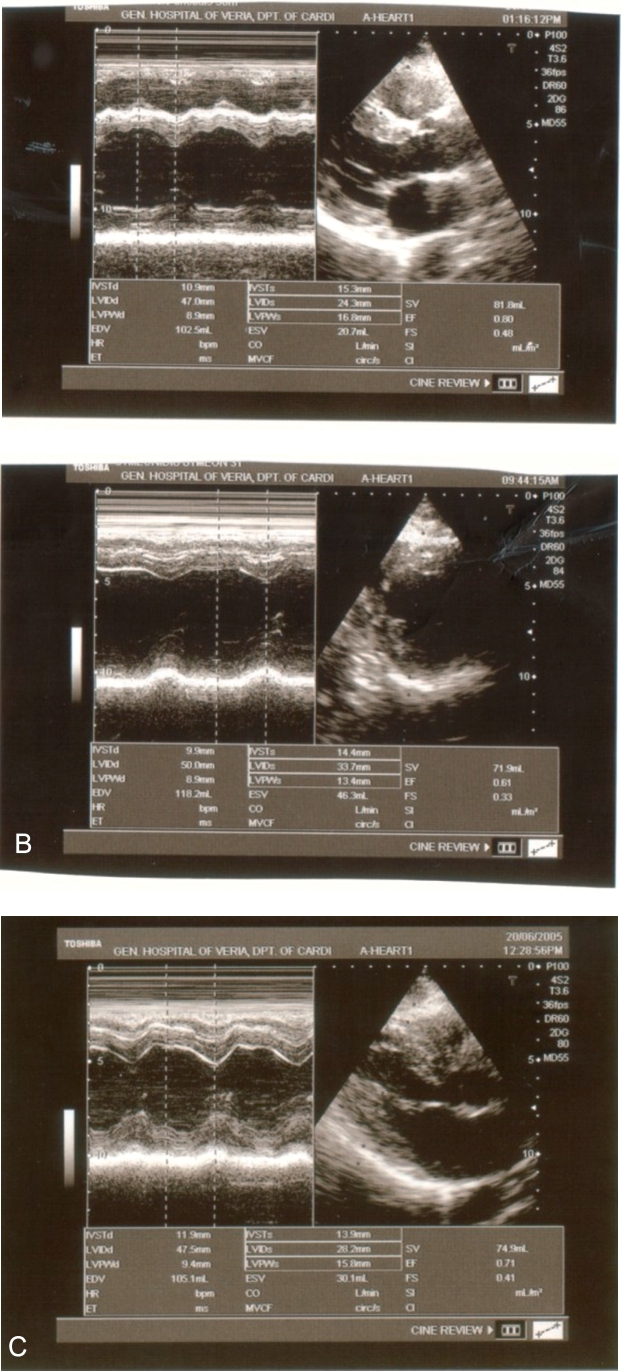
Echocardiography revealed normal wall thickness and contractile function of the left ventricle. No wall motion abnormalities were detected and there was no sign of pericardial effusion (figure 2). A predischarge exercise test (modified Bruce) was conducted which demonstrated no findings indicative of myocardial ischemia. The duration of hospitalization was seven days and as already has been stated no complications occurred. On discharge ECG, small sized negative T waves were evident in leads II, III, aVF, V5 and V6. Coronary angiography, that followed, revealed normal coronary arteries.
Figure 2. Echocardiographic examination of the patient. A: on admission, B: at discharge and C: one year later. The dimension and systolic function of the left ventricle remains normal.
In order to exclude acute myocarditis as the cause of our patient's symptoms (the suspicion was quite high due to the presence of fever before admission) two serologic tests were conducted, one month apart from each other. The serologic examination revealed acute infection by Coxiella burnetii. Specifically, the serologic test obtained during hospitalization revealed titers of 1:200 and 1:50 for IgG and IgM antibodies against phase II Coxiella burnetii, respectively (with IgG and IgM titers against phase I Coxiella burnetii being 1:50 and 0 respectively). Moreover a fourfold rise in antibody titers was observed, based on the results one month later.
Based on the above results doxycycline at a dose of 200 mg/day for 3 weeks was added to patients therapy immediately when the results of the first serologic test were obtained (6th day of hospitalization). It must be noticed that the patient's fever and symptoms resolved before initiating therapy with doxycycline. We speculated that Coxiella burnetii was transferred to the patient from the pigeons. A sample of 15 pigeons was sent to the Faculty of Veterinary studies of the Aristoteles University of Thessaloniki, in order to be tested. The specific serologic tests were not conducted though, due to technical problems. The patient is being followed up since then (approximately 2 years). He is free of symptoms. On periodic echocardiographic examinations no signs of dilated cardiomyopathy have been manifested so far (Figure 2).
Discussion
Q fever is an infectious disease of worldwide significance caused by the obligate intracellular bacterium Coxiella burnetii (the smallest in size of the Rickettsia group). It was first reported in 1935 in Australia and it was denominated "Query fever", as the pathogenic agent remained unknown until 1937. Q fever is a zoonosis. The primary sources of human infection are infected sheep, goats and cattle. However, the extensive wildlife reservoir for Coxiella burnetii includes mammals, birds and ticks. In humans, infection results by inhalation of contaminated aerosols from the amniotic fluid, placenta, or contaminated wool after direct or indirect contact with infected animals or by ingestion of unpasteurized milk from infected farm animals1. The frequency of Q fever is believed to be underestimated despite its worldwide distribution, while in many cases the primary animal source, is not identified. Men and adults are infected more frequently2.
The incubation period of Q fever varies from 9 to 28 days (with an average duration of 18 to 21 days) and 60% of the affected patients have an asymptomatic course. Clinically the disease is polymorphic and nonspecific and may present in an acute or chronic form3. The acute form of Q fever is commonly manifested as a self-limited flu-like syndrome ie high-grade fever (which can persist for up to three weeks) accompanied by chills, weakness and myalgias. A nonspecific skin rash may be evident in some patients. Other clinical presentations include pneumonia, hepatitis, pericarditis, myocarditis and meningoencephalitis1,4. Less than 1% of infected patients will develop the chronic form of the disease months or years after the acute illness. The chronic form of disease usually occurs in patients with a history of valvular heart disease, vascular aneurysm, immunosuppression or chronic renal insufficiency. The most common clinical manifestation is that of endocarditis (with negative blood cultures). Rarely, infection of an aneurysm or vascular graft or osteoarthritis may occur1,3.
According to recent research by the authors5, the possibility of acute myocarditis should not be omitted from the differential diagnosis of procardial pain with angina-like features, particularly in young people, that have no predisposing factors for coronary artery disease. The incidence of myocarditis in this particular group of patients seems to be significantly higher than in the general population and diagnostic tests toward this end should be performed.
The ECG changes observed in patients with acute myocarditis are believed to occur due to abnormalities of the repolarization phase of atrial and ventricular myocardium, as a result of epicardial inflammation. Thus variations of the shape of PR interval, ST complex and T wave are the most common ECG changes in acute myocarditis. The depolarization phase is usually unaffected and rarely changes of P wave and QRS complex are recorded6. Typically the ECG of a patient with acute myocarditis reveals sequential changes and four distinct phases are described based on the observed abnormalities of the ST complex and T wave. In the first phase, which is recorded during the first few hours from the manifestation of symptoms, ST elevation (usually diffuse) is seen. In the second phase (few days to several weeks), ST resolution and flattening of the T wave in the same leads are recorded. In the third phase, which usually presents after the second week of the course of the disease, and may last from a few days to several weeks, T wave inversion is recorded. Finally, in the fourth phase, the ECG becomes normal again. It must be stressed that the above mentioned sequence is not observed in all patients7. The patient of our case report depicted quite typical ECG changes similar to those that are usually seen in acute myocarditis. Indeed, on the first ECG (24 hours before his admission to the hospital) ST elevation was recorded (Figure 1 - A) which returned to normal 24 - 36 hours later (admission ECG). Moreover no Q waves were observed (Figure 1 - B). After seven days, small sized negative T waves in the same leads were seen (Figure 1 - C).
Myocarditis, as in our case, has been reported only occasionally as a manifestation of Coxiella burnetii infection (0.6 - 0.8% of cases of acute Q fever in large series). It is of interest, that Q fever myocarditis can affect people at all ages3.
The identification of the primary source of transmission is not always possible. In a recent study, the primary source was defined in only 4 of the 7 patients8. As already has been stated, infected ruminant animals (mostly newborn) such as sheep, cattle and goats are presumed to be the main sources for transmission to humans. Birds, cats, dogs and wild animals are rarely reported to be implicated in cases of the disease. In most but not all of the reported cases, there is a history of contact with an infected animal. In our case-study the most likely source of infection (though unusual) was the pigeons, since the patient mentioned no other exposure to pets or animals and moreover he was not consuming unpasteurized products.
The diagnosis of Q fever is mainly achieved by serologic techniques. Ideally two serum samples are obtained the first during the acute phase of illness and the second during recovery, 3 - 4 weeks later. It must be underscored that only the minority of the infected patients (30%) demonstrate leukocytosis in the acute form of the disease2. The isolation of Coxiella burnetii is not easy to perform as its culture is technically demanding and furthermore the bacterium is considered highly infectious. Thus serologic techniques are considered to be the diagnostic tests of choice when there is clinical suspicion of Q fever. Three techniques are available: complement fixation, indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA). Coxiella burnetii exists in two antigenic forms: phase I (predominant in the chronic form of the disease) and phase II (predominant in acute Q fever). In humans the antibody response against these two antigens of Coxiella burnetii is examined. In acute Q fever, high antibody titers (IgG ≥ 1:200, IgM ≥ 1:50 or both) against phase II antigens of the bacterium (as in our patient) are typically detected, while in chronic Q fever, a more intense antibody response (IgG ≥ 1:800) against phase I antigen is seen. Moreover, in acute Q fever it is usually possible to demonstrate a four fold rise in titer between acute and convalescent-phase samples4,8. The diagnosis can also be made by examination of biopsy specimens from affected organs using the polymerase chain reaction (PCR) technique or the immunohistochemical staining of the infected tissue technique (IHC staining). Interpretation of the results of serologic studies is far more difficult in animals compared to humans. It seems that the presence of antibodies against Coxiella burnetii is possibly a marker of prior infection but by no means can it predict reliably the risk of transmission of the bacterium to humans.
The pathogenesis of Q fever remains controversial. Some authors have proposed the hypothesis that acute and chronic forms of the disease are caused by specific strains9, whereas others have emphasized the role of host factors in the disease10 or the size of the infecting inoculum11. The role of specific strains in the different forms of Q fever seems unlikely, because some authors have demonstrated that a single strain can cause both acute and chronic infections12. Moreover, host factors seem to play a determinant role. Hence, an underlying heart abnormality seems to be a major risk factor of chronic Q fever: most patients who develop Q fever endocarditis or vascular infection have damaged valves or blood vessels10.
The role of the immune system has also been implicated in the occurrence of chronic Q fever but is not completely known. Several authors have described cases of chronic Q fever in immunocompromised hosts13, and patients who develop chronic Q fever have lymphocyte unresponsiveness to Coxiella burnetii, which results in a lack of macrophage activation and subsequent permanent rickettsemia. La Scola et al11 have demonstrated in animal models that the size of the infecting inoculum can play a major role in the type of acute Q fever manifestation, with the highest levels of inoculum being responsible for most cases of myocarditis.
Our patient developed no common manifestations of Q fever, other than fever. His clinical course until now is excellent, since no signs of dilated cardiomyopathy have been evident. However, the prognosis of myocarditis caused by Coxiella burnetii is not so good, with mortality rates reaching 16.7%, much higher than the usual mortality seen when there is no involvement of the myocardium. More specifically, patients who died from Q fever myocarditis were younger when compared to patients who died from Q fever but had no cardiac manifestations3. This feature is especially interesting, since myocarditis has been suspected to be a cause of sudden death in young patients. Postmortem examinations revealed evidence of previously unexpected myocarditis in 1 - 4% of unselected cases14 and a higher incidence in young people who died suddenly. Moreover, myocarditis has been found histologically in 10%-20% of cases of idiopathic dilated cardiomyopathy15. This feature suggests that cardiac involvement may be more prevalent in acute Q fever than previously expected and may be an important cause of death in young patients, even among those with no history of illness.
If left untreated, the disease continues for 2 to 14 days. In acute Q fever, treatment options are doxycycline 200 mg/day for 2 to 3 weeks or tetracycline 2 g/day for 2 weeks or chloramphenicol 50 mg/day every 6 hours (its main use is in children due to the fact that tetracyclines are contraindicated). In cases of pre-existing known valvular heart disease, hydroxychloroquine 600 mg/day is added on the above treatment for a total period of one year. Early initiation of treatment is essential since it can prevent the transition to the chronic form of the disease. Although our patient was asymptomatic when the diagnosis of Q fever myocarditis was made, we decided to add doxycycline in his therapy for 3 weeks, in order to enhance recovery.
It is important sanitary services to be informed when patients present with symptoms suggestive of Q fever such as prolonged fever, hepatitis, atypical pneumonia or endocarditis with negative blood cultures, especially when a history of exposure to sheep, goats or cattle is reported, so that the indicated serologic tests can be performed and preventive measures be taken to minimize the transmission of the bacterium.
As with other forms of Q fever, myocarditis has a non-specific clinical presentation, and its incidence may therefore be underestimated. Given the potential severity of Q fever myocarditis and given the existence of a reliable therapy, we suggest that C. burnetii should systematically be considered in the differential diagnosis of acute myocarditis.
References
- 1.Walker D, Raoult D, Brouqui P, Marrie T. Harrison's Principles of Internal Medicine. 14th edition. New York: Mc-Graw Hill Company; 1998. Rickettsial Diseases; pp. 1045–1052. [Google Scholar]
- 2.Raoult D, Marrie TJ. Q fever. Clin Infect Dis. 1995;20:489–496. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 3.Fournier PE, Etienne J, Harle JR, Habib G, Raoult D. Myocarditis, a rare but severe manifestation of Q Fever: Report of 8 cases and review of the literature. Clin Infect Dis. 2001;32:1440–1447. doi: 10.1086/320159. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Q fever--California, Georgia, Pennsylvania, and Tennessee, 2000-2001. MMWR Morb Mortal Wkly Rep. 2002;51:924–927. [PubMed] [Google Scholar]
- 5.Vogiatzis IA, Kambitsi H, Karamitsos TD, Prodromidis PS, Bougiouklis DG, Samanidis DK. Acute myocarditis imitating acute myocardial infarction. Galinos. 2004;46:37–46. [Google Scholar]
- 6.Chan TC, Brady WJ, Pollack M. Electrocardiographic manifestations: acute myopericarditis. Emerg Med. 1999;17:865–872. doi: 10.1016/s0736-4679(99)00097-9. [DOI] [PubMed] [Google Scholar]
- 7.Spodick DH. Electrocardiogram in acute pericarditis. Distributions of morphologic and axial changes by stages. Am J Cardiol. 1974;33:470–474. doi: 10.1016/0002-9149(74)90603-1. [DOI] [PubMed] [Google Scholar]
- 8.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q Fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel JE, Frazier ME, Mallavia LP. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985;49:775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein A, Raoult D. Q - Fever endocarditis. Eur Heart J. 1995;16:19–23. doi: 10.1093/eurheartj/16.suppl_b.19. [DOI] [PubMed] [Google Scholar]
- 11.La Scola B, Lepidi H, Raoult D. Pathologic changes during acute Q fever : influence of the route of infection and inoculum size in infected guinea pigs. Infect Immun. 1997;65:2443–2447. doi: 10.1128/iai.65.6.2443-2447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein A, Raoult D. Lack of pathotype specific gene in human Coxiella burnetii isolates. Microb Pathog. 1993;15:177–185. doi: 10.1006/mpat.1993.1068. [DOI] [PubMed] [Google Scholar]
- 13.Raoult D, Levy PY, Tissot-Dupont HT, et al. Q fever and HIV infection. AIDS. 1993;7:81–86. doi: 10.1097/00002030-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Gravanis MG, Sterby NH. Incidence of myocarditis. Arch Pathol Lab Med. 1991;115:390–392. [PubMed] [Google Scholar]
- 15.Sole MJ, Lui P. Viral myocarditis: a paradigm for understanding the pathogenesis and treatment of dilated cardiomyopathy. J Am Coll Cardiol. 1993;22:99A–105A. doi: 10.1016/0735-1097(93)90470-l. [DOI] [PubMed] [Google Scholar]