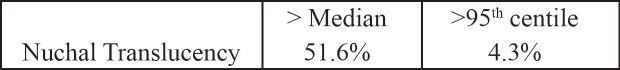Abstract
Introduction: Nowadays maternal age of pregnant women has increased in most developed countries. The rate of women above 35 years old constitutes about 15% of pregnancies.
Aim: The aim of our study is to prove that by first trimester screening, the number of women who have indication for invasive prenatal diagnostic procedure is significantly reduced.
Materials and methods: This prospective study lasted two years from 02/2005 to 02/2007. The participants to our study were 531 pregnant women with a mean maternal age of 30 years (19-42). We used the first trimester screening test for Down's syndrome. The biochemical blood test of free b-hCG (beta human chorionic gonadotropin) and PAPP-A (pregnancy associated plasma protein A) and the measurement of nuchal translucency were performed between 11-13 weeks +6 days (mean gestational age 12 weeks +2days).
Results: In our study group, 69 women (12%) were 35 years old or more. The risk estimate for Down syndrome was 1 in 300 or more in 14 (2%) cases. In all these 14 cases we offered CVS (chorionic villus sampling) or amniocentesis.
Conclusion: It is a fact that although the risk of any individual 36 years old is higher, most abnormalities (approximately 70%) occur in the low risk population. With the first trimester screening the sensitivity of detecting DOWN syndrome reaches 90%. Our study confirms that by first trimester screening, the number of women who have indication for invasive prenatal diagnostic procedure is significantly reduced. As a result the cost for prenatal diagnosis of the population and also the risk of iatrogenic missed miscarriages is also reduced. Finally, this screening method gives the advantage of early diagnosis.
Keywords: prenatal screening, nuchal translucency, amniocentesis
Screening for chromosomal abnormalities in an obstetric setting has traditionally meant screening for trisomy 211. It is the single most common cause of mental retardation of school-age children. The majority of chromosome abnormalities identified in prenatal samples are trisomy for chromosomes 13, 18, 21 and sex chromosome aneuploidies. These are associated with the newborn phenotypes, Patau, Edwards and Down syndromes (trisomy 13, 18 and 21 respectively), and the less severe Turner (monosomy X) and Klinefelter (XXY) syndromes. Down's syndrome, with an incidence rate of 1 in 800 pregnancies, is the predominant reason for women seeking prenatal diagnosis2, 3.
The distinction between screening and diagnostic tests is often spurious since most tests will be used in both ways in different patients. At present accurate prenatal diagnosis of chromosomal anomalies is only available by obtaining fetal cells through an invasive procedure, such as amniocentesis or chorionic villous sampling. Karyotype analysis of cells by culture is usually available in more than in one week. In order to reduce anxiety and improve pregnancy management, more rapid aneuploidy testing are used. The most widely established method is interphase-fluorescence in situ hybridization (FISH)4,5, in which a set of chromosome- specific fluorescence-labelled probes are hybridized to interphase nuclei of uncultured prenatal cells. The number of fluorescent signals in each nucleus represents chromosome copy number. Between 50 and 100 cells are usually analyzed to allow for low-level background and signal overlay that can occur during FISH procedures6. A quantitative fluorescence-PCR (QF-PCR) is a more recent addition to aneuploidy diagnosis7,8. The technique involves the relative quantification of microsatellite alleles to determine sequence copy number; amplification using fluorescence-labelled primers is followed by size separation and allele peak measurement on a semi-automated genetic analyzer.
Due to the fact that both amniocentesis and CVS are associated with a risk of miscarriage9 these procedures are currently applied only to small group of women who are in a higher risk of having an offspring with a chromosomal defect in comparison to the general population. The aim of the currently available screening tests is actually to identify, with the highest possible sensitivity and specificity, those women who should be offered the invasive procedure. The risk for many of the chromosomal defects increases with maternal age. Additionally, because fetuses with chromosomal defects are more likely to die in utero than normal fetuses, the risk decreases with gestational age. Maternal age of 35 years at delivery has been the medicoligal standard in USA and Europe for more than 20 years (Table 1). This high risk group constituted 5% of the pregnant population10. It was estimated that approximately 30% of trisomy 21 occurred to mothers >35 years old. It is a fact that although the risk of any individual 36 years old is higher than in a 26 years old woman for example, there are so many more pregnancies in the 26 years old group that from a population perspective, most abnormalities (approximately 70%) occur in the 'low risk' population11.
Table 1. Risk of Down's syndrome and maternal age at expected date of delivery (EDD) and at the time of amniocentesis(2).
Nowadays, such screening for prenatal diagnosis is provided by using the family history, the maternal serum screening and the ultrasonography. Every time a test is carried out the background risk is multiplied by the test factor to calculate a new risk, which then becomes the backround risk for the next test 12. This process is called sequential screening. Although screening tests are not diagnostic they can indeed alter the odds 13. The aim of our study is to prove that by first trimester screening, the number of women who have indication for invasive prenatal diagnostic procedure is significantly reduced.
Materials and methods
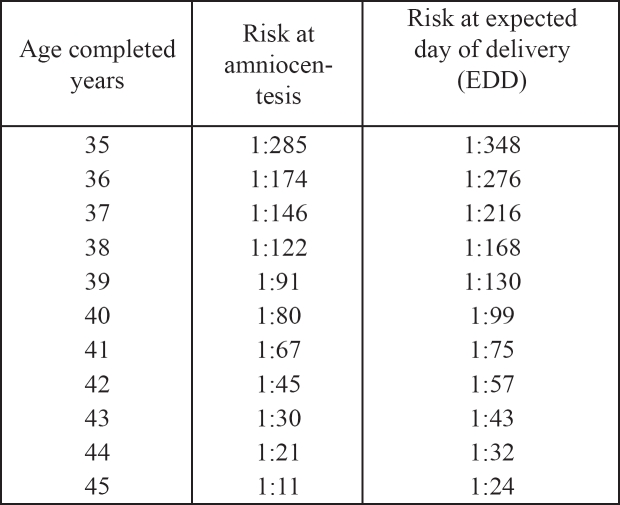
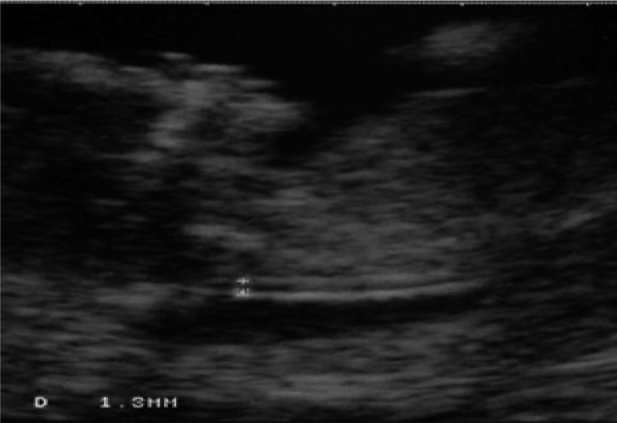
In our study screening started in February 2005 and was completed two years later (in February 2007). In this period 531 patients were examined with a mean maternal age of 30 years (19-42). The biochemical blood test of free b-hCG and PAPP-A and the measurement of nuchal translucency (NT) were performed between 11-13 weeks + 6 days (mean gestational age 12 weeks +2 days). All the measurements of nuchal translucency were performed by three obstetricians qualified in nuchal translucency ultrasound technique. A cut-off based on gestational age was used, since NT normally increases with gestation. The minimal crown rump length (CRL) was 45mm and the maximum 84mm, a good sagittal section of the fetus was obtained, with the fetus in a neutral position, and the magnification was such that the fetus occupied three-quarters of the image and fetal skin and amnion were distinguished (Figures 1,2). The biochemical measurements were performed with the use of Krypton (Brahms industry) and the statistical analysis of the data was based on Astraia programme.
Figures 1. Normal nuchal translucency.
Figures 2. Abnormal nuchal translucency.
Results
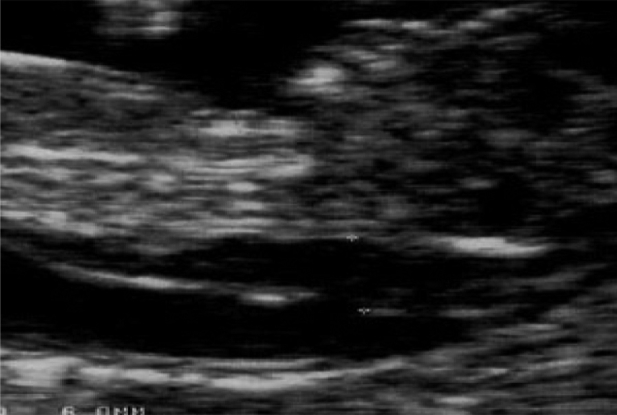
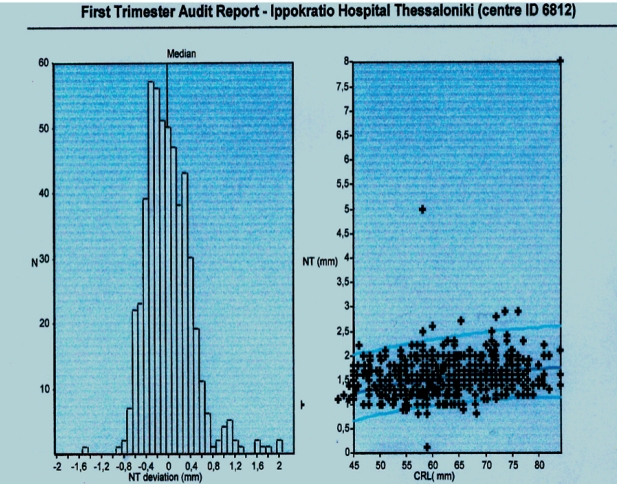
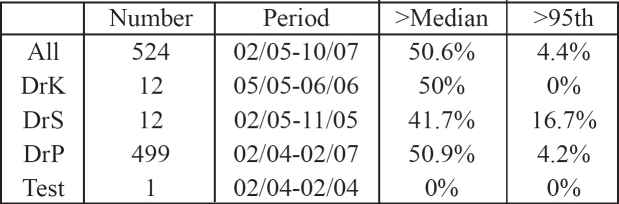
In our study group 69 women (12%) were 35 years old or more. On the basis of the maternal age distribution it is expected that the population contains 1.5 cases of trisomy 21 and approximately the same number of other chromosomal defects. The risk estimate for Down syndrome with the combination of maternal age, nuchal translucency, and biochemical markers was 1 in 300 or more in 14 (2%) cases. In all these 14 cases we offered CVS or amniocentesis. Two women (14%) of the high risk group declined to proceed to prenatal diagnosis, one due to religion believes and one due to valuable pregnancy after five trials with ICSI and maternal age (42years old). Five women out of fourteen were more than 35 years old (%). All twelve women had amniocentesis and in one of them the result was trisomy 21 (Figures 3, 4). The performance of operators and the results of NT measurements and biochemical markers are analyzed in tables 2, 3 and 4. All the infants from the rest 55 women aged more than 35 years were born with normal phenotype.
Figures 3. Distribution of NT and CRL measurements.
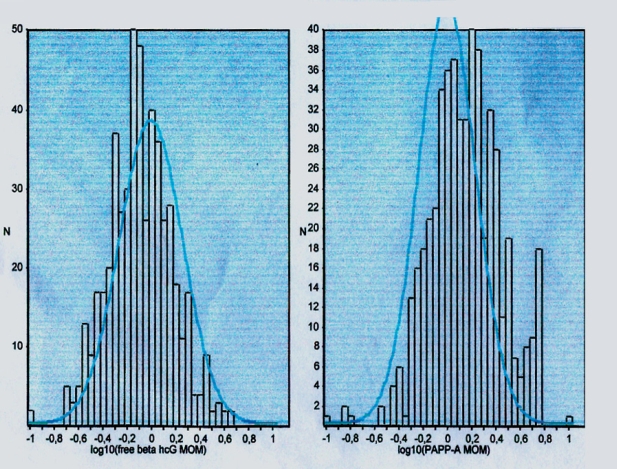
Figures 4. Distribution of biochemical measurements.
Table 2. The nuchal translucency distribution for all operators.
(K,S and P are the first letters of the surnames of the doctors who performed the examination)
Table 3. The nuchal translucency (NT) measurements are plotted on the attached reference range. The percentage of cases within each centile range are shown in the following table.
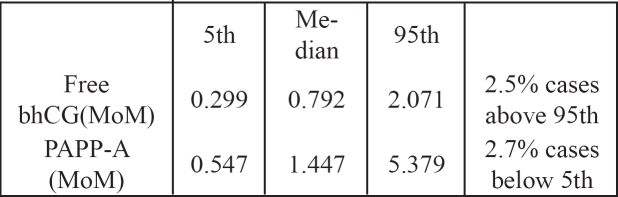
Table 4. Values for the maternal serum biochemical measurements are expressed as multiples of the expected median for gestational age. Median, 5th and 95th centiles, and the percentage of cases outside the expected 5th/95th centile.
(MoM=multiple of median)
Discussion
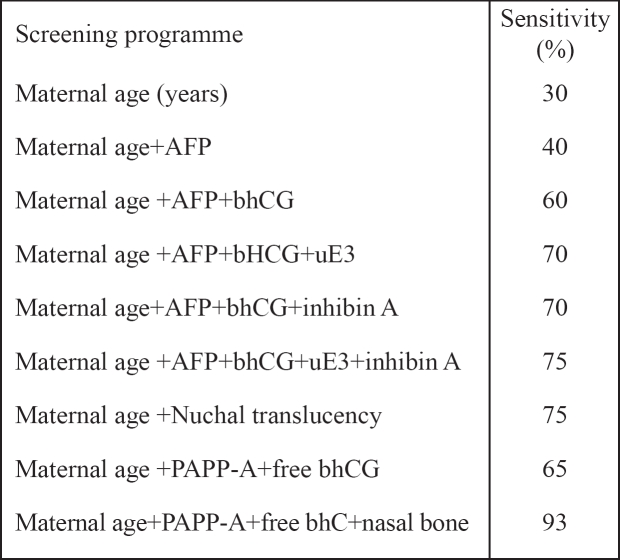
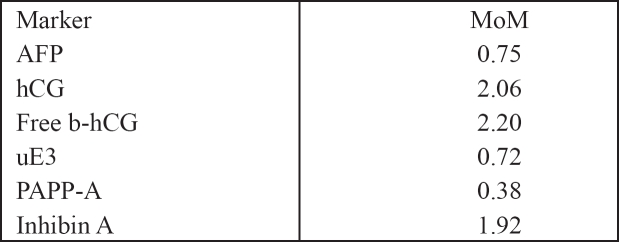
Nowadays prenatal screening and diagnosis between 11 and 14 weeks of gestation is becoming more and more available and efficient worldwide. Recent studies concluded that the sensitivity of first trimester screening is much more sufficient than second trimester tests (Table 5)14. Several maternal serum markers have been used and PAPP-A (pregnancy associated plasma protein) and b-HCG (free b-human chorionic gonadotropin) have been the most promising so far (Table 6)15,16. It is found that maternal serum concentration of b-HCG is increased during first (1.83 MoM) and second trimester in cases of Down's syndrome and that PAPP-A on the contrary is decreased (0.38). By using PAPP-A as a screening marker in combination with maternal age approximately 50% of cases of Down's can be detected17,18. By using maternal age combined with b-HCG about 45% of cases of Down syndrome are detected17,18 . The use of all three factors combined offers a detection rate of 65%17,18. In the last few years an ultrasonografic finding has been added as a quite important marker for the screening of Down's syndrome19 and other fetal anomalies such as trisomy 13, 18. Turner syndrome, triploidy and heart defects20. That is the nuchal translucency (NT), which is actually the measurement of the subcutaneous collection of fluid at the back of the fetal neck. Fetal nuchal translucency thickness increases with CRL and therefore it is essential to take gestation into account13. A lot of studies showed that the observed numbers of fetuses with trisomies 21, 18, 13 with NT measurements of 3mm, 4mm, 5mm, and >6mm at 10-14 weeks, were 3 times, 18 times, 28 times and 36 times higher than numbers observed on the basis of maternal age21 . A measurement of > 6mm identifies about 40% of cases with trisomy 21 in a high risk population.
Table 5. Sensitivity of first-trimester screening programmes, for a 5% false positive rate.
Table 6. Biochemical markers and median multiples of the median (MoM) in pregnancies affected by trisomy 21.
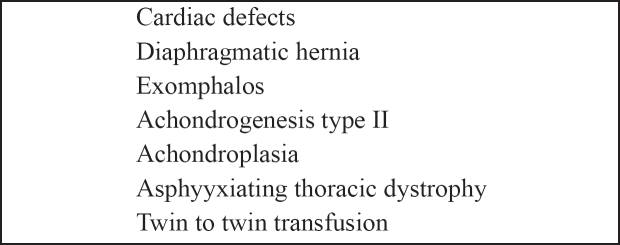
Fetal abnormalities other than Down's syndrome associated with increased NT are summarized in tables 7 and 8. Cardiac defects are found in about 1% of live fetuses. Evidence suggests that about 50% of all cardiac defects are in the subgroup with NT above the 95th centile22 . The combination of maternal age and NT leads to a detection rate of 75% for Down syndrome and false positive rate of 5%19,20, 22. By combining the first trimester biochemical screening with maternal age and the NT the detection rate for Down syndrome is estimated to be about 90% and the false positive rate 5%23–25. It should be mentioned though that it is not possible to perform screening for neural tube defects in this early stage of pregnancy.
Table 7. Nuchal translucency thickness above the 95th centile and chromosomal abnormalities other than trisomy 2142.
Table 8. Conditions associated with increased nuchal translucency.
Our prospective study comes in agreement with all these available until now data and confirms the advantages of first trimester screening. The high risk population that needs to proceed to invasive diagnostic test, calculated according to the measurement s of the first trimester test is much smaller than the high risk population according only to maternal age. So apart from the fact of higher sensitivity in comparison to other tests, the first trimester screening method is also cost effective since the number of invasive procedures is reduced. Of course one of the biggest advantages of the first trimester screening test is that counselling and invasive diagnosis of chromosomal defects can be accomplished early in pregnancy thus reducing significantly the anxiety. By providing the option of early termination of pregnancy the situation becomes less complicated for both the patient and the obstetrician.
Conclusion
Our study confirms that the first trimester screening, with the use of maternal age, nuchal translucency and the biochemical markers free bhCG & PAPP-A, dramatically reduces the number of women who will need CVS or amniocentesis for prenatal diagnosis. As a result the cost for prenatal diagnosis of the population and also the risk of iatrogenic missed miscarriages is also significantly lower. Finally, this screening method gives the advantage of early counselling and of diagnosis quite as early as in the first trimester of pregnancy.
References
- 1.Cameron A, Macara L, Brennand J, Milton P. Screening for chromosomal abnormalities. Fetal medicine for the MRCOG and Beyond. 2002;1:1–12. [Google Scholar]
- 2.Thornton FG, Onwude FL. Prenatal diagnosis. Progress in Obstetrics and Gynaecology. 1998;10:13–31. [Google Scholar]
- 3.Bubb JA, Matthews AL. Whats new in prenatal screening and diagnosis? Primary Care. 2004;31:561–582. doi: 10.1016/j.pop.2004.04.011. Clinics in Office Practice. [DOI] [PubMed] [Google Scholar]
- 4.Klinger K, Landes G, Shook D, et al. Rapid detection of chromosome aneuploidies in uncultured amniocytes by using fluorescence in situ hybridization (FISH) Am J Hum Genet. 1992;51:55–65. [PMC free article] [PubMed] [Google Scholar]
- 5.Tepperberg J, Pettenati MJ, Rao PN, et al. Prenatal diagnosis using interphase fluorescence in situ hybridization (FISH): 2-year multi-centre retrospective study and review of the literature. Prenat Diagn. 2001;21:293–301. doi: 10.1002/pd.57. [DOI] [PubMed] [Google Scholar]
- 6.Mann K, Donaghue C, Fox SP, Docherty Z, Ogilvie CM. Strategies for the rapid prenatal diagnosis of chromosome aneuploidy. Eur J Hum Genet. 2004;12:907–915. doi: 10.1038/sj.ejhg.5201224. [DOI] [PubMed] [Google Scholar]
- 7.Mansfield ES. Diagnosis of Down syndrome and other aneuploidies using quantitative polymerase chain reaction and small tandem repeat polymorphisms. Hum Mol Genet. 1993;2:43–50. doi: 10.1093/hmg/2.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Pertl B, Yau SC, Sherlock J, Davies AF, Mathew CG, Adinolfi M. Rapid molecular method for prenatal detection of Down's syndrome. Lancet. 1994;343:1197–1198. doi: 10.1016/s0140-6736(94)92404-x. [DOI] [PubMed] [Google Scholar]
- 9.Tabor A, Philip J, Madsen M, Bang J, Obel EB, Norgaard-Pedersen B. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986;1:1287–1293. doi: 10.1016/s0140-6736(86)91218-3. [DOI] [PubMed] [Google Scholar]
- 10.Crossley JA, Aitken DA, Cameron AD, McBride E, Connor JM. Combined ultrasound and biochemical screening for Down's syndrome in the first trimester: a Scottish multicentre study. BJOG. 2002;106:667–676. doi: 10.1111/j.1471-0528.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaides KH. Screening for chromosomal defects. Ultrasound Obstet Gynecol. 2003;21:313–321. doi: 10.1002/uog.128. [DOI] [PubMed] [Google Scholar]
- 12.Snijders RJM, Nicolaides KH. Ultrasound Markers for Fetal Chromosomal Defects. Canforth, UK: Parthenon Publishing; 1996. Assessment of risks; pp. 109–113. [Google Scholar]
- 13.Nikolaides KH, Sebire NJ, Snijders RJM. The 11-14 Weeks Scan. The diagnosis of fetal abnormalities. 1, Nuchal translucency and chromosomal defects. Parthenon publishing; 1999. pp. 3–65. [Google Scholar]
- 14.Lambert-Messerlian GM, Saller JDN, Tumber MB, French CA, Peterson CJ, Canick JA. Second trimester maternal serum inhibin A levels in fetal trisomy 18 and Turner syndrome with and without hydrops. Prenat Diagn. 1998;18:1061–1067. [PubMed] [Google Scholar]
- 15.Wald NJ, Watt HC, Hackshaw AK. Intergrated screening for Down's syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461–467. doi: 10.1056/NEJM199908123410701. [DOI] [PubMed] [Google Scholar]
- 16.Bromley B, Lieberman E, Shipp TD, Benacerraf B. The genetic sonogram: a method of risk assessment for Down syndrome in the second trimester. J Ultrasound Med. 2002;21:1087–1896. doi: 10.7863/jum.2002.21.10.1087. [DOI] [PubMed] [Google Scholar]
- 17.Benn PA. Advances in prenatal screening for Down syndrome: II First trimester testing intergraded testing, and future directions. Clin Chim Acta. 2002;324:1–11. doi: 10.1016/s0009-8981(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 18.Cuckle HS, van Lith JM. Appropriate biochemical parameters in first-trimester screening for Down syndrome. Prenat Diagn. 1999;19:505–512. doi: 10.1002/(sici)1097-0223(199906)19:6<505::aid-pd572>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosome dedefects in first trimester of pregnancy. BMJ. 1992;304:867–869. doi: 10.1136/bmj.304.6831.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snijders RJM, Noble, Sebire N, Souka A, Nicolaides KH. UK multicentre project of assessment of risk of trisomy 21 by maternal age and fetal nuchal translucency thickness at 10-14 weeks of gestation. Lancet. 1998;352:343–346. doi: 10.1016/s0140-6736(97)11280-6. [DOI] [PubMed] [Google Scholar]
- 21.Pandya PP, Kondylios A, Hilbert L, Snijders RJM, Nicolaides KH. Chromosomal defects and outcome in 1,015 fetuses with increased nuchal translucency. Ultrasound Obstet Gynecol. 1995;5:15–19. doi: 10.1046/j.1469-0705.1995.05010015.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyett JA, Perdu M, Sharland GK, Snijders RJM, Nikolaides KH. Using fetal translucency to screen for major cardiac defects at 10-14 weeks of gestation: population based cohort study. Br Med J. 1999;318:81–85. doi: 10.1136/bmj.318.7176.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlandi F, Damiani G, Hallahan W, Krantz DA, Macri JN. First-trimester screening for fetal aneuploidy: biochemistry and nuchal translucency. Ultrasound Obstet Gynecol. 1997;10:381–386. doi: 10.1046/j.1469-0705.1997.10060381.x. [DOI] [PubMed] [Google Scholar]
- 24.Bindra R, Heath V, Liao A, Spencer K, Nicolaides KH. One-stop clinic for assessment of risk for trisomy 21 at 11-14 weeks: a prospective study of 15030 pregnancies. Ultrasound Obstet Gynecol. 2002;20:219–225. doi: 10.1046/j.1469-0705.2002.00808.x. [DOI] [PubMed] [Google Scholar]
- 25.Spencer K, Spencer CE, Power M, Dawson C, Nikolaides KH. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years prospective experience. Br J Obstet Gynecol. 2003;110:281–286. [PubMed] [Google Scholar]