Abstract
The interaction between cronic heart failure, cronic kidney insufficiency and anemia, form a vicious cycle, termed as the cardio-renal anemia syndrome. The interaction between these three conditions causes deterioration of the cardiac and renal function and increases anemia. Each of the three can cause or be caused by the others.
We herein analyze and speculate the mechanisms involved in the pathophysiology of this new syndrome highlighting the main points of interest that seem to expand upon more than one specialty. The cardio-renal anemia syndrome is emerging in the area of clinical investigation with progressively elevated significance.
Additionaly we report the data related to anemia treatment as part of therapeutic perspective concerning the management of patients manifesting the profile of this syndrome.
Keywords: anemia, renal failure, heart failure, kidney insufficiency, erythropoetin
Congestive heart failure (CHF) remains a major challenge for medical community. The current prevalence of CHF is about 2% (≈ 5 million people in USA). However among people older than 65 this prevalence is elevated more than 5% and becomes 10% among people older than 80 years of age1.
Despite remarkable advances in diagnosis and therapy over the past decade, the prognosis of patients with heart failure remains poor. During the decade 1950-1959 according to data extracted from the Framingham Study the 1st year mortality for CHF in men was 30%. In the period from 1990 to 1999, even when adjusted for many confounding factors, this mortality rate was almost unchanged at 28%2. This unacceptable rate of improvement rendered the investigation to search for novel mechanisms by which to address the unchanged morbidity and mortality associated with CHF.
The first approach has been done by Silverberg et al, evaluated the records of 142 patients seen in outpatient CHF clinic3. They found that, despite the fact that these patients were all being treated with ACE inhibitors and b-blockers in the highest recommended doses, in addition to digoxin, nitrates, aldospirone and furosemide, a remarkable percentage among them did not respond to therapy, resulting in poor outcome. Severe fluid retention, extreme fatigue, shortness of breath on no or minimal exertion (New York Heart Association (NYHA) functional class IV) were still present. The great majority of these patients were anemic. Anemia (Hb < 12 g/dl) was present in 55.6% of all patients, increasing in prevalence from 9.1% in mild CHF (NYHA I) to 79.1% in severe CHF (NYHA IV). The mean Hb fell from group I to IV, from 13.73±0.83 g/dl to 10.9±1.70 g/dl, concluding that anemia is more prevalent and severe along with the severity of the CHF.
In patients with Chronic Kidney Disease (CKD) the severity of anemia is an independent predictor of death4,5 whereas the risk for cardiovascular events, including stroke, is increased significantly when anemia is present6,7.
Other source of information suggesting that anemia contribute to the CHF is the literature on correcting anemia with erythropoietin (EPO) in patients with CKD and patients with End Stage Renal Disease (ESRD), who are under renal replacement therapy8,9. In both groups, anemia was associated with left ventricular dilation and hypertrophy, an increased prevalence and incidence of CHF and an increased mortality and hospitalization.10 Anemia may also increase the rate of deterioration of renal function in patients with CKD and therefore the rate of progression of renal failure to dialysis11. On the other hand, correction of anemia in CKD patients reduced hospitalization for CHF12. Similarly in ESRD patients, correction of anemia prevented, improved or even corrected LV dilation and LV hypertrophy13, reduced hospitalization and mortality7 and improved quality of life14.
The importance of the interaction between CHF, Chronic Kidney Insufficiency (CKI) and anemia was suggested by a study including approximately 1 million elderly patients, who represented a 5% sample of the Medicare population in the USA.5 The 2-year risk for dying or starting dialysis is shown in table 1. Individually, each of these three conditions increases the risk of death or ESRD by 50-100% and the three together increase the probability up to 300%.
Table 1. Two-year mortality and incidence of ESRD in a 5% sample of Medicare patients from the USA.
CHF: Congestive Heart Failure, CKI: Chronic Kidney Insufficiency,
ESRD: End Stage Renal Disease
Therefore, Silverberg et al, proposed a new mechanism involved in the interaction between CHF, CKI and anemia, which form a vicious cycle, termed as the cardio- renal anemia syndrome (Figure 1). The interaction between these three conditions causes deterioration of the cardiac and renal function and increases anemia. Each of the three can cause or be caused by the others.15
Figure 1. The interaction between anemia, congestive heart failure (CHF) and chronic kidney insufficiency (CKI).
Pathophysiology
The causative role of anemia in CHF and CKI
Since the beginning of 1990s, the mechanism by which anemia can induce cardiac and renal damage, have been gradually elucidated.16 Indeed it has been shown that severe anemia, could be causative factor for cardiac and renal disease in patients without previous basic heart disease.17
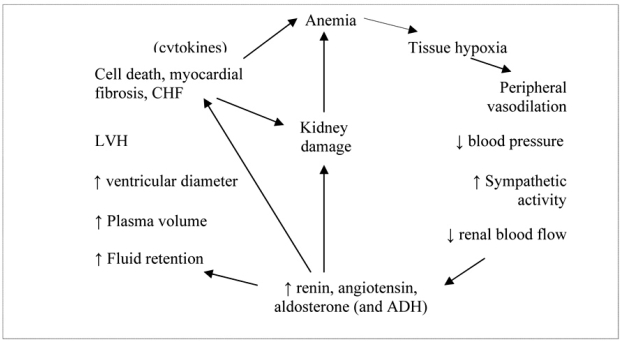
Tissue hypoxia due to anemia, leads to peripheral vasodilatation and decreased vascular resistance, which in turn reduces blood pressure. The sympathetic system is activated and this causes tachycardia, increased stroke volume and peripheral vasoconstriction in order to maimaintain adequate blood pressure. However, increased sympathetic activity also causes renal vasoconstriction, resulting in renal blood flow and glomerular filtration rate (GFR) reduction, leading consequently to renal ischemia. The reduced renal blood flow activates the renin-angiotensinaldosterone system (RAAS) and antidiuretic hormone, causing further renal vasoconstriction, as well as salt and water retention. The renal insufficiency thus produced may also cause anemia through reduced eryptropoietin production and bone marrow activity. The fluid retention mentioned above, causes plasma volume expantion which leads to LV dilatation and stress on an already stressed myocardium. The consequent LV hypertrophy leads to necrosis and apoptosis of myocardial cells, myocardial fibrosis and cardiomyopathy resulting in CHF. Additionally, elevated levels of renin, angiotensin and aldosterone cause damage of cardiac cells directly, exacerbating the damage already done.18,19
The levels of tumor–necrosis factor a (TNFa) are increased in CHF and there is evidence that cardiac cells produce this cytokine in response to injury, which damage the heart further.20 This increased production of cytokines has also been implicated in the development of chronic disease anemia21 and may also worsen anemia in CKD and CHF patients, thus forming a vicious cycle of disease progression (Figure 2).
Figure 2. The mechanism by which anemia can cause heart failure and renal failure.
The causative role of CKD in anemia and heart failure
In patients with advanced renal disease, progressive renal deterioration leads to decrease in circulating levels of erythropoietin, with a subsequent decrease in bone marrow erythrocyte production and Hb levels.8 It is well-established that anemia in patients with ESRD is associated with a variety of adverse cardiac consequences, including the development of left ventricular dilation, hypertrophy and clinical HF.22,23
Despite the high prevalence of renal dysfunction, circulating levels of EPO are generally normal-to-elevated in HF, with a correlation between the degree of EPO elevation and worsening functional class.17 The worsening cardiac performance leads to renal hypoperfusion and hypoxia, which are powerful stimulus for EPO production. In light of this elevation of EPO levels in advanced HF, the high prevalence of anemia in this group of patients is notable. It seems that anemia in HF may be a condition of relative resistance to the EPO action, with persistent anemia despite the usually high levels of EPO.
The role of CKI as a causative factor of HF, apart from the anemia related consequences is well known. As CKI progresses inevitably to the end stage, volume expansion due to water and sodium retention causes overload hypertension, which is the rule in ESRD patients. Among other factors, accelerated untreated hypertension leads to LVH and HF. Additionally, atherosclerotic events due to accelerated atherogenesis attributed to traditional and uremia-related risk factors in CKD patients, are involved significantly in myocardial damage causing HF in a wide proportion of these patients.
The role of HF in causing CKI and anemia
Renal failure is quite common in patients with heart failure, with a prevalence from 30% to 50% depending on the definition used and the population studied.17 Hypoperfusion is considered as the leading direct mechanism involved in CKI related to HF, whereas cytokines and growth factors contribute to chronic kidney insufficiency progression.
Even though anemia is common in patients with HF, it is not well-documented whether this evidence is the result of direct relationship between HF and anemia or there are multiple potential mechanisms by which HF could contribute to the development of anemia. Heart failure is a disease of the elderly, a population where the prevalence of anemia is high irrespective of cardiac status. 24 Multiple comorbid conditions are common in HF patients and a major one is the renal insufficiency, which is associated with the development of anemia.25,26 On the other hand, there are many anaemic patients with HF that have normal levels of serum creatinine, and thus it is unlikely that renal insufficiency is the unique causative factor of anemia in patients with HF.
Other possible comorbid factors causing anemia in HF patients are haemodilution, proinflammatory cytokines, malnutrition due to right-sided HF, iron deficiency, decreased bone marrow perfusion and drug therapy (ACE inhibitors, aspirin). It is likely that several of these mechanisms act simultaneously, and that anemia in HF is the result of a complex interaction between them.
A characteristic of HF syndrome is plasma volume expansion, so that anemia may be due to hemodilution rather than to true decrease in red blood cell mass.27 In a recent study of 37 HF patients, true anemia (as expressed by decreased red blood cell mass) was present in 54%, and hemodilution was present in 46%. The interesting is that both these situations were associated with adverse survival, with the worst survival seen in patients with hemodilution. 28
Chronic HF is known to be a state of persistent inflammatory activation, and higher levels of circulating proinflammatory cytokines are known to be associated with greater disease severity and worsened clinical outcomes.29,30 The two major proinflammatory cytokines, tumor necrosis factor (TNF)-alpha and interleukin-6, have been found to have direct effect on the bone marrow, and are implicated to the mechanism of anemia of chronic disease.31 Elevated levels of these cytokines lead to decreased EPO production and resistance to the effects EPO on the bone marrow production of red blood cells. Furthermore, proinflammatory cytokines inhibit the release of iron from the reticuloendothelial system so that it cannot reach the bone marrow to be utilized in hemoglobulin production, and lastly they inhibit iron absorption through the gut. This is done through cytokine production of the protein hepcidin in the liver which inhibits iron absorption in the small bowel.32,33 It has been shown that the higher the TNF-a level in patients with CHF, the lower the Hb level.34
Patients with severe CHF have often reduced appetite and, consequently, show signs of malnutrition such as weight loss, anemia and hypoalbuminemia.35 The converse may also be true, namely that anemia itself could cause loss of appetite and malnutrition. Additionally, iron deficiency, which is common in anemic HF patients, may be due to malnutrition. Aspirin, which often is used in HF patients, may cause blood loss in the gut. Also, these patients often have proteinuria which causes the loss of significant amounts of EPO, iron and transferrin in the urine contributing to the anemia.36 CHF alone may cause malabsorption, probably because of edema in the gut and through hepcidin production in the liver.
Finally, treatment with ACE inhibitors is a mainstay of HF therapy, with clearly documented improvements in long-term survival.37,38 Some data have suggested, however, that ACE inhibitor therapy may reduce Hb concentration in patients with HF. Therapeutic doses of ACE inhibitors have been shown to decrease renal secretion of EPO in patients with hypertension,39 renal insufficiency,40 polycythemia41 and chronic HF.42
Treatment Conciderations
EPO is the gold standard for treatment of anemia among end stage renal disease (ESRD) patients. Patients under EPO therapy experienced significant improvement in terms of quality of life and overall survival as well as a decrease of left ventricular hypertrophy, increase of cardiac output and left ventricular ejection fraction.43,44,45 In this group of patients there was a benefit concerning their exercise capacity, depression symptoms, social relationships, sexual function, sleep, appetite and nutritional status.46,47,48 According to the same studies,46,47,48 correction of anemia by EPO, is responsible for an increase in aerobic metabolism and less anaerobic metabolism and lactate production, improved peak oxygen utilization, improved skeletal muscle function, an improvement in angina pectoris, improved cerebral blood flow, improved amino acid and glucose metabolism, improved endothelial cell function and blood rheology. Ofsthun et al. reported that between levels of 9 and 13 g/dL a 1 g elevation in hemoglobin was associated with improved 6-month survival among dialysis patients.49 Li and Collins reported an inverse association between lower hematocrit at the start of dialysis and increased risk of death and hospitalization from cardiovascular disease among 50,579 hemodialysis patients.50
However it is not yet precisely known which is the ideal timing in a patient with CKD to start EPO treatment and which should be the ideal hematocrit target in order to reduce cardiovascular morbidity and mortality. The Canada-Europe Normalization of Hemoglobin Study 51, a randomized double-blind comparison of lower (9.511.5 g/dL) versus higher (13.514.5 g/dL) hemoglobin treatment targets of 596 hemodialysis patients, failed to find significant differences between the two groups in changes in left ventricular volume index. These findings were supported by a small (n=155) randomized open-label trial which found that maintenance hemoglobin levels above 12.0 g/dL compared with levels between 9.0 and 10.0 g/dL in patients with stage 3 or 4 CKD were not associated with reduced left ventricular mass index or risk of progressive left ventricular hypertrophy.52 In an another large, randomized trial, the Normal Hematocrit Treatment trial,53 there were a non significant increase in mortality among dialysis patients with clinical heart disease (congestive heart failure or coronary heart disease) under EPO with a hematocrit treatment target of 42% vs. 30%. Relative risk of death or non fatal myocardial infarction was 1,3 (CI 95%: 0,9-1,9) in the group of higher hematoctit. Despite the fact that the elevation in mortality was statistically none significant the safety monitoring panel decided to discontinue the study. It is noteworthy that when each group was examined separately, higher hematocrit values correlated with reduced mortality rates. Maybe the excess of deaths observed in higher hematocrit treatment arm was not due to the hematocrit itself but due to other covariates not taken into consideration by the investigators.
Levin et al. performed a randomized clinical trial with 172 CKD patients in order to find out whether prevention or early correction of anemia using EPO is superior to late onset of therapy concerning a retain in the of left ventricular mass increase. Participants were divided into two groups with a Hb target of 12.0-14.0 g/dL and 9.0-10.5 g/dL respectively. After 24 months of followup, there was no significant difference in respect of left ventricular mass growth between the two groups. The authors conclude that the relationship between Hb levels and left ventricular mass index, as it was documented from observational studies, is not being verified by clinical trials. Perhaps these two variables are not causally related. 54 CHOIR study aimed to define the best level of anemia correction in CKD patients. One thousand forty hundred thirty two CKD patients participated and they were divided into two groups. Both groups were treated with EPO and a Hb target of 13.5 and 11.3 g/dL respectively. After a mean follow-up of 16 months incidence of deaths, myocardial infarctions, hospitalizations due to congestive heart failure and cerebrovascular events were assessed. According to the investigators tight correction of hematocrit vs usual does not confer grater improvement of quality of life in this group of patients while a significant raise in cardiovascular morbidity and mortality is being observed.55
At the moment two studies are being at progress, TREAT (the Trial to Reduce Cardiovascular Events with Aranesp Therapy) and CREATE (Cardiovascular risk Reduction by Early Anemia Treatment with Epoetin Beta). The first one is a multi-center, double-blind, randomized controlled trial with 4000 patients. Primary end-point is the assessment of EPO therapy on mortality and non-fatal cardiovascular events (myocardial infarction, myocardial ischemia, congestive heart failure and cerebrovascular events) among patients suffering from CKD and diabetes mellitus. Participants will be randomized in a 1:1 ratio either to EPO, with a Hb target of 13.0 g/dL, or to placebo, as long as they have a Hb level of ≥9g/dL, or to active treatment, if they have Hb<9 g/dL, with a Hb target of ≥9g/dL.56 The latter is a multi-center randomized trial with 600 CKD patients, not yet under dialysis, dived into two treatment arms. Half of the patients will be administered EPO at an earlier stage of anemia, Hb levels of 11.0-12.5 g/dL, while the rest of them will not get any treatment unless their Hb levels drop below 10.5 g/dL. The Hb target is set to 13.0-15.0 g/dL and 10.5-11.5 g/dL respectively. The annual change of left ventricular mass index and time until the first cardiovascular event are going to be assessed.57 According to preliminary results early onset of EPO therapy aiming to a fully correction of anemia is not accompanied with a reduction in cardiovascular morbidity.58
Silverberg et al. were the pioneers in the area of clinical trials on patients suffering from CHF and anemia. They started with a small, uncontrolled trial with 26 patients suffering from CHF with a functional class IV and anemia (Hb<12 g/dL). All patients, who were already under maximum tolerated therapy, were assigned to EPO subcutaneously and iron (Venofer-iron sucrose) intravenously for about 7 months. The subsequent rise of Hb correlated with a significant increase of ejection fraction and a decrease of the mean NYHA functional class. In addition, while before EPO treatment glomelural filtration rate (GFR) was falling, it started rising at the end of the study. Compared with the pre-treatment period, the rate of rehospitalization fell markedly and the dose of oral and i.v. diuretics needed was greatly reduced.3 These findings were supported from another series of 32 patients.59
The same group of investigators recruited 126 CHF patients, resistant to all CHF medications, who were suffering from anemia and mild renal insufficiency as well. The same treatment strategy was followed. After 12.4 months anemia, renal function and ejection fraction were improved. In addition patients were asked on their first visit and at the completion of the study to assess their fatigue and shortness of breath on a visual analogue scale. On this scale, 10 is the worst they could possibly feel and 0 is the best. The score fell from a mean of 8.8 at onset to a mean of 2.8 on completion.60,61 Similar results were observed after EPO and iron administration in 179 CHF and anemic patients including a subset of diabetics.62 The fact that renal function improved in both diabetics and non diabetics at the same extent led the authors to assume that the deterioration of renal function observed in diabetics is in part due to the underlying anemia and the poorly treated CHF.63
The above findings were supported by a randomized, single-blind, placebo controlled trial conducted by Mancini et al. They evaluated the effect of 3 months of erythropoietin treatment on exercise capacity in 26 patients with anemia and NYHA functional class III to IV CHF. This study demonstrated significant improvement in peak oxygen consumption with erythropoietin treatment vs. no significant change in the control patients. A significant correlation was observed between elevations in Hb with EPO treatment and increased peak oxygen consumption. Notably, the improvement in exercise performance with EPO treatment was observed whether the anemia was found to be from decreased red blood cell mass or from hemodilution.64
New erythropoietin analogs, such as darbepoetin alfa, with a greater half period of life, have now been developed which makes them more appealing for the treatment of anemia in CHF patients. At the moment guidelines concerning the use of EPO are referring exclusively to CKD patients. All patients suffering from CKD and anemia should be considered as candidates for EPO therapy, irrespective of renal functional status or the need for dialysis. Patients with CKD should maintain Hb levels above 11 g/dL (Hct>33%) or achieve this target in a 4 month period after the treatment onset, irrespective of age, gender or race.
References
- 1.Heart disease and stroke statistics; Dallas. American Heart Association; 2005. pp. 28–29. [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, et al. Long term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Djurdjev O, Duncan J, Werb R, Taylor P. Hemoglobin levels prior to therapy predict survival in chronic kidney disease (CKD) patients. Nephrol Dial Transplant. 2003;18(T 358) suppl 4:393–394. [Google Scholar]
- 5.Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease and anemia on survival in the medicare population. J Card Fail. 2004;10:467–472. doi: 10.1016/j.cardfail.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Jurcovitz CT, Abramson JL, Vaccarino V, Weintraub WS, Weintraub WS, Mc-clellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919–2925. doi: 10.1097/01.asn.0000092138.65211.71. [DOI] [PubMed] [Google Scholar]
- 7.Abramson JL, Jurcovitz CT, Vaccarino V, Weintraub WS, Mc-Clellan W. Chronic kidney disease, anemia and incident stroke in a middle-aged community based population: the ARIC study. Kidney Int. 2003;64:610–615. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation. K/DOQI clinical practice guidelines 2000 update. Am J Kidney Dis. 2001;37(suppl 1):S1–S238. [Google Scholar]
- 9.Revised European Best Practice Guidelines for the management of anemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19(suppl 2):1–47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 10.Collins AJ. Influence of target haemoglobin in dialysis patients on morbidity and mortality. Kidney Int. 2002;61(suppl 80):44–48. doi: 10.1046/j.1523-1755.61.s80.9.x. [DOI] [PubMed] [Google Scholar]
- 11.Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL Study. Kidney Int. 2003;63:1499–1507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 12.St Peter WL, Xue J, Ebben J, Collins A. Pre-end stage renal disease erythropoietin use predicts hospitalisation in the periods before and after end-stage renal disease prognosis. J Am Soc Nephrol. 2001;12:247A. [Google Scholar]
- 13.Hampl H, Sternberg C, Berweck S, et al. Regression of left ventricular hypertrophy in haemodialysis patients is possible. Clin Nephrol. 2002;58(suppl 1):S73–S96. [PubMed] [Google Scholar]
- 14.Moreno F, Sanz-Guajardo D, Lopez-Gomez JM, Jofre R, Valderrabano F. Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. J Am Soc Nephrol. 2000;11:335–542. doi: 10.1681/ASN.V112335. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg DS, Wexler D, Blum M, Wollman Y, Iaina A. The cardio-renal anemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(suppl 8):7–12. doi: 10.1093/ndt/gfg1084. [DOI] [PubMed] [Google Scholar]
- 16.Anand IS, Chandrashekhar Y, Ferrari R, Ferrari R, Poole-Wilson PA, Harris PC. Pathogenesis of edema in chronic anemia: studies of body water and sodium, renal function, haemodynamics and plasma hormones. Br Heart J. 1993;70:357–362. doi: 10.1136/hrt.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felker GM, Kirkwood FA, Gattis WA, Christopher MO. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–966. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 18.Katz AM. The cardiomyopathy of overload: an unnatural growth response in the hypertrophied heart. Ann Int Med. 1994;121:363–371. doi: 10.7326/0003-4819-121-5-199409010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DB, Dell'Italia LJ. Cardiac hypertrophy and failure in hypertension. Curr Opin Nephrol Hypertens. 1996;5:186–191. doi: 10.1097/00041552-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Torre-Amione G, Bozkurt B, Deswal A, Mann DL. An overview of tumor necrosis factor alpha and the failing human heart. Curr Opin Cardiol. 1999;14:206–210. doi: 10.1097/00001573-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Means RT., Jr Advances in the anemia of chronic disease. Int J Hematol. 1999;70:7–12. [PubMed] [Google Scholar]
- 22.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61. doi: 10.1016/s0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 23.Harnett JD, Kent GM, Foley RN, Parfrey PS. Cardiac function and hematocrit level. Am J Kidney Dis. 1995;25:S3–S7. doi: 10.1016/0272-6386(95)90673-8. [DOI] [PubMed] [Google Scholar]
- 24.Joosten E, Pelemans W, Hiele M, Noyen J, Verhaeghe R, Boogaerts MA. Prevalence and causes of anemia in a geriatric hospitalized population. Gerontology. 1992;38:111–117. doi: 10.1159/000213315. [DOI] [PubMed] [Google Scholar]
- 25.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 26.Hillege HL. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 27.Volpe M, Tritto C, Testa U, et al. Blood levels of erythropoietin in congestive heart failure and correlation with clinical, hemodynamic, and hormonal profiles. Am J Cardiol. 1994;74:468–473. doi: 10.1016/0002-9149(94)90905-9. [DOI] [PubMed] [Google Scholar]
- 28.Androne AS, Katz SD, Lund L, et al. Haemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–229. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 29.Deswal A, Peterson MJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 30.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 31.Voulgari PV, Kolios G, Papadopoulos GK, Katsaraki A, Seferiadis K, Drosos AA. Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 1999;92:153–160. doi: 10.1006/clim.1999.4736. [DOI] [PubMed] [Google Scholar]
- 32.Iverson PO, Woldbaek PR, Tonnessen T, Christensen G. Decreased hematopoiesis in bone marrow of mice with congestive heart failure. Am J Physiol. 2002;282:166–172. doi: 10.1152/ajpregu.2002.282.1.R166. [DOI] [PubMed] [Google Scholar]
- 33.Means RT. Hepcidin and anemia. Blood Rev. 2004;18:219–225. doi: 10.1016/S0268-960X(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 34.Bolger AP, Doehner W, Sharma R, Coate JS, Anker S. Anemia in chronic heart failure: the relationship to inflammatory cytokine production and prognostic importance. Circulation. 2002;106 [Google Scholar]
- 35.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 36.Vaziri ND. Erythropoietin and transferrin metabolism in nephrotic syndrome. Am J Kidney Dis. 2001;38:1–8. doi: 10.1053/ajkd.2001.25174. [DOI] [PubMed] [Google Scholar]
- 37.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 38.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 39.Griffing GT, Melby JC. Enalapril (MK-420) and the white cell count and haematocrit. Lancet. 1982;319:1361. doi: 10.1016/s0140-6736(82)92430-8. [DOI] [PubMed] [Google Scholar]
- 40.Kamper AL, Nielsen OJ. Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scan J Clin Lab Invest. 1990;50:611–618. doi: 10.3109/00365519009089178. [DOI] [PubMed] [Google Scholar]
- 41.Plata R, Cornejo A, Arratia C, et al. Angiotensin-converting-enzyme inhibition therapy in altitude polycythaemia: a prospective randomised trial. Lancet. 2002;359:663–666. doi: 10.1016/s0140-6736(02)07812-1. [DOI] [PubMed] [Google Scholar]
- 42.Fyhrquist F, Karppinen K, Honkanen T, Saijonmaa O, Rosenlof K. High serum erythropoietin levels are normalized during treatment of congestive heart failure with enalapril. J Intern Med. 1989;226:257–260. doi: 10.1111/j.1365-2796.1989.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 43.Low-Friedrich I, Grutzmacher P, Marz W, Bergmann M, Schoeppe W. Therapy with recombinant human erythropoietin reduces cardiac size and improves heart function in chronic hemodialysis patients. Am J Nephrol. 1991;11:54–60. doi: 10.1159/000168273. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg N, Lundin AP, Delano B, Friedman EA, Stein RA. Changes in left ventricular size, wall thickness, and function in anaemic patients treated with recombinant human erythropoietin. Am Heart J. 1992;124:424–427. doi: 10.1016/0002-8703(92)90608-x. [DOI] [PubMed] [Google Scholar]
- 45.Linde T, Wikstrom B, Andersson LG, Danielson BG. Renal anemia treatment with recombinant human erythropoietin increases cardiac output in patients with ischemic heart disease. Scand J Urol Nephrol. 1996;30:115–120. doi: 10.3109/00365599609180900. [DOI] [PubMed] [Google Scholar]
- 46.Tong EM, Nissenson AR. Erythropoietin and anemia. Semin Nephrol. 2001;21:190–203. doi: 10.1053/snep.2001.20939. [DOI] [PubMed] [Google Scholar]
- 47.Silverberg DS, Iaina A, Wexler D, Blum M. The pathological consequences of anemia. Clin Lab Haematol. 2001;23:1–6. doi: 10.1046/j.1365-2257.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- 48.Mann JF. What are the short-term and long-term consequences of anemia in CRF patients. Nephrol Dial Transplant. 1999;14(Suppl 2):29–36. doi: 10.1093/ndt/14.suppl_2.29. [DOI] [PubMed] [Google Scholar]
- 49.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher haemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63:1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Collins AJ. Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodialysis patients. Kidney Int. 2004;65:626–633. doi: 10.1111/j.1523-1755.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 51.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 52.Roger SD, McMahon LP, Clarkson A, et al. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. J Am Soc Nephrol. 2004;15:148–156. doi: 10.1097/01.asn.0000102471.89084.8b. [DOI] [PubMed] [Google Scholar]
- 53.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 54.Levin A, Djurdjev O, Thompson C, et al. Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. Am J Kidney Dis. 2005;46:799–811. doi: 10.1053/j.ajkd.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Singh AK, Szczech L, Tang KL, et al. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 56.Mix CH, Brenner RM, Cooper ME, et al. Rationale-Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. Am Heart J. 2005;149:408–413. doi: 10.1016/j.ahj.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 57.Eckardt KU. The CREATE trial-building evidence. Nephrol Dial Transplant. 2001;16(suppl 2):16–18. doi: 10.1093/ndt/16.suppl_2.16. [DOI] [PubMed] [Google Scholar]
- 58.Locatelli F, Del Vecchio L, Pozzoni P. Anemia and Cardiovascular Risk: The lesson of the CREATE trial. J Am Soc Nephrol. 2006;17:S262–S266. doi: 10.1681/ASN.2006080924. [DOI] [PubMed] [Google Scholar]
- 59.Silverberg DS, Wexler WD, Sheps Detal. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 60.Silverberg DS, Wexler D, Blum M, et al. Aggressive therapy of congestive heart failure and associated chronic renal failure with medications, and correction of anemia stops the progression of both diseases. Peritoneal Dialysis Int. 2001;21:S236–S240. [PubMed] [Google Scholar]
- 61.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail. 2002;4:681–686. doi: 10.1016/s1388-9842(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 62.Silverberg DS, Wexler D, Blum M, et al. The effect of correction of anemia in diabetic and non diabetics with severe resistant congestive heart failure and chronic renal failure by subcutanous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18:141–146. doi: 10.1093/ndt/18.1.141. [DOI] [PubMed] [Google Scholar]
- 63.Wexler D, Silverberg D, Blum M, et al. Anemia as a contributor to morbidity and mortality in congestive heart failure. Nephrol Dial Transplant. 2005;20(Suppl 7):vii11–vii15. doi: 10.1093/ndt/gfh1101. [DOI] [PubMed] [Google Scholar]
- 64.Mancini DM, Katz SD, Lamanca J, Lamanca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]