Abstract
Infection by RNA viruses is detected by the host through Toll-like receptors or RIG-I-like receptors. Toll-like receptors and RIG-I-like receptors signal through the adaptors MyD88 and MAVS, respectively, to induce type I IFNs (IFN-I) and other antiviral molecules, which are thought to be essential for activating the adaptive immune system. We investigated the role of these adaptors in innate and adaptive immune responses against respiratory syncytial virus (RSV), a common human pathogen. Deletion of Mavs abolished the induction of IFN-I and other proinflammatory cytokines by RSV. Genome-wide expression profiling in the lung showed that the vast majority of RSV-induced genes depended on MAVS. Although Myd88 deficiency did not affect most RSV-induced genes, mice lacking both adaptors harbored a higher and more prolonged viral load and exhibited more severe pulmonary disease than those lacking either adaptor alone. Surprisingly, Myd88−/−Mavs−/− mice were able to activate a subset of pulmonary dendritic cells that traffic to the draining lymph node in response to RSV. These mice subsequently mounted a normal cytotoxic T-lymphocyte response and demonstrated delayed but effective viral clearance. These results provide an example of a normal and effective adaptive immune response in the absence of innate immunity mediated by MAVS and MyD88.
Keywords: adaptive immunity, RSV, IFN
Immune detection of pathogens is mediated by pathogen recognition receptors (PRRs), which, in the case of RNA viruses, involve a subset of Toll-like receptors (TLRs 3, 7, and 8) and the cytoplasmic RIG-I-like receptors (RLRs) (1–3). TLR 7/8 binds viral single-stranded RNA in endosomes and engages the cytosolic adaptor MyD88 to activate downstream signaling pathways leading to the activation of NF-κB and interferon regulatory factors (i.e., IRF3, IRF7). The RLRs, including RIG-I and MDA5, bind viral double-stranded RNA or 5′-triphosphorylated uncapped viral RNA in the cytosol (4, 5). Through their common adaptor, MAVS (also known as IPS-1, VISA, or CARDIF), RLRs also activate NF-κB and IRFs (6–9). Together with activated AP-1, NF-κB and IRFs subsequently induce transcription of antiviral genes, including type 1 IFNs (e.g., IFN-α and IFN-β, herein referred to as IFN-I). In addition to IFN-I, proinflammatory cytokines and chemokines are induced downstream of TLR and RLR signaling. It is thought that these innate responses serve to limit the extent of infection until the adaptive immune response is able to clear the virus.
Activation of adaptive B and T cell responses is driven by dendritic cells (DCs) (10). Upon detection of viral infection through PRRs, DCs become activated and traffic to lymph nodes, where they present viral antigens and provide additional signals to activate CD4+ and CD8+ T cells. Activated CD4+ T cells can, in turn, activate virus-specific B cells, resulting in antibody production.
Respiratory syncytial virus (RSV) belongs to the Paramyxovirus family of negative-sense single-stranded RNA viruses. RSV infection is currently recognized as the leading cause of respiratory disease in infants and young children, and it can also be life threatening in elderly and immune-compromised adults (11–13). There is currently no vaccine available against RSV. A previous failed vaccine attempt underscores the importance of a better understanding of the host response to the virus for the design of safe and effective treatment and prophylaxis (14, 15). In this report, we generated mice lacking Mavs, Myd88, or both (double knockout, DKO) to investigate the roles of RLR and TLR signaling in the innate and adaptive immune responses to RSV.
Results
Innate Cytokine Induction Depends on MAVS-Mediated Signaling.
In the first series of experiments, we studied the cell-specific IFN-I response to RSV. Lung fibroblasts, bone marrow-derived macrophages, and conventional DCs (cDCs) were isolated and infected with RSV. Measurement of IFN-α and -β production by ELISA, shown in Fig. 1 A–E, revealed that IFN-I induction by RSV in all these cell types completely depended on MAVS. Previous studies have suggested that plasmacytoid DCs (pDCs) rely on TLR7 and MyD88 to induce IFN-I in response to RNA viruses, whereas other cell types use the RLR-MAVS pathway (16, 17). Although Sendai virus (SeV) infection triggered IFN-I production in purified pDCs, we were unable to detect any IFN-α or -β in these cells after RSV infection [supporting information (SI) Fig. S1A]. Consistent with this result, the induction of IFN-α and -β by RSV depended on MAVS in bone marrow cells treated with the Flt3 ligand, which leads to the generation of pDCs and cDCs (Fig. S1 B and C). In contrast, infection of Flt3L-DCs with SeV triggered IFN-I production in a manner that was independent of MAVS. This is consistent with our previous finding that MAVS is dispensable for IFN-I induction by SeV in pDC, which is the major source of IFN-I in SeV-infected Flt3L-DCs (16).
Fig. 1.
In vitro and in vivo cytokine responses to RSV infection are lost in the absence of MAVS. Adult lung fibroblasts (A), bone marrow-derived macrophages (BMDM; B and C), and cDCs (D and E) from WT+/+ and Mavs−/− mice were infected with SeV or RSV for 24 h before culture supernatants were analyzed for IFN-β or -α by ELISA. Data are represented as mean ± SD. (F) Mice of the indicated genotypes were intranasally infected with RSV (107 pfu) for 24 h, and then BALF was harvested for cytokine measurements by ELISA (for IFN-α, IFN-β, and IL-1β) or Cytometric Bead Array (for IL-6, TNF-α, and MCP-1) (*, P < 0.001; **, P < 0.01; ***, P < 0.05; ANOVA, Tukey's test) (n = 4 per group). n.d., not detected. Data are represented as mean ± SEM.
To examine the role of MAVS and MyD88 in RSV infection in vivo, we infected Mavs−/−, Myd88−/−, and DKO mice with RSV via the intranasal route (Fig. 1F). Consistent with the in vitro results, 1 day after infection, WT mice secreted a large amount of IFN-I, which was detected in bronchoalveolar lavage fluid (BALF). This response was normal in MyD88-null mice but abolished in mice lacking MAVS. Consistently, MAVS-deficient mice failed to induce and activate STAT1 in the lung after RSV infection, whereas this IFN signaling response was normal when these mice were infected with vesicular stomatitis virus, an RNA virus known to induce IFN-I independent of MAVS (Fig. S2; ref. 16). Other proinflammatory cytokines, including IL-6, TNF-α, monocyte chemoattractant protein (MCP)-1, and IL-1β, also depended on MAVS for their expression. Interestingly, maximal production of TNF-α, MCP-1, and IL-1β also required MyD88. At the same time point, day 1, we were unable to detect significant levels of IFN-γ, IL-12, or IL-10 in the BALF (data not shown). Neither IFN-α nor -β was detectable in sera and mediastinal lymph nodes by ELISA or quantitative PCR in mice of any genotype (data not shown). Recently, it has been shown that in response to intranasal Newcastle disease virus infection, IFN-α–producing alveolar macrophages and cDCs could be detected after 24 h in WT mice (18). In MAVS-deficient animals, however, this response was abolished and a compensatory IFN-α response was detected in pDCs at 48 h. However, we found no evidence of IFN-I production 2, 5, 9, or 14 days after RSV infection in Mavs−/− mice (Fig. S3, data not shown), consistent with our in vitro observation that RSV does not induce IFN-I in pDCs.
To evaluate the roles of MyD88- and MAVS-mediated signaling in the global pulmonary innate immune response to RSV, we analyzed lung RNA by microarray 24 h after infection (Fig. 2). Of the 659 genes induced by ≥2-fold in WT mice, 440 (≈66.8%) depended on MAVS but not on MyD88, 12 (≈1.8%) depended on MyD88 but not on MAVS, and 90 (13.7%) depended on both (Fig. S4). The induction of most IFN-related genes was MAVS-dependent and MyD88-independent (Fig. 2A). A similar pattern of dependence was seen for most of the cytokine-, chemokine-, and PRR-related genes (Fig. 2 B–D, Fig. S5). In contrast, optimal expression of TNF-α and IL-1β depended on both adaptors (Fig. 2E). Interestingly, both IL-24 and Cxcl-16 were induced in all RSV-infected groups compared with mock-treated groups. With the exception of these chemokines, innate immune responses were largely absent in Mavs−/− and DKO mice.
Fig. 2.
Global gene expression analysis of lung RNA after infection with RSV reveals profound defects in mice lacking MAVS. For each genotype, two mice were mock treated and three were inoculated with RSV (107 pfu) for 24 hours before lungs were harvested for total RNA extraction, which was analyzed by microarray. (A–D) Mean relative expression of genes known to be involved in immune responses, including interferon-related (A), chemokine or cytokine receptors (B), cytokines (C), and chemokines (D). (E) Induction of selected genes was confirmed by qPCR. Data are represented as mean ± SEM.
Viral Clearance in the Absence of MAVS and MyD88.
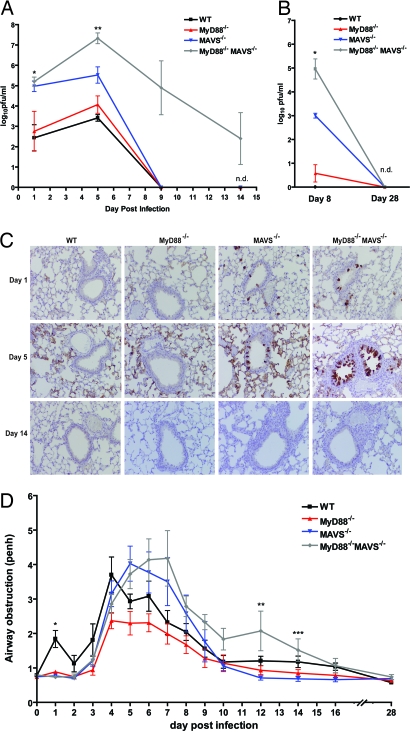
Next, we evaluated viral clearance in these mice by measuring viral loads in the BALF at various times after infection. In WT mice, viral loads peaked on day 5 after infection and reached undetectable levels by day 9 as previously observed in this model (Fig. 3A) (19). Consistent with a previous study, MyD88-deficient mice showed no significant differences in viral clearance compared with WT mice (20). In contrast, viral loads in Mavs−/− mice were ≈100 times higher than those in the WT mice on days 1 and 5 after infection. Surprisingly, despite the absence of IFN-I and other antiviral molecules, these mice were able to clear the virus to undetectable levels by day 9. The DKO mice showed no significant difference in viral loads compared with the Mavs−/− group 1 day after infection, suggesting the importance of MAVS- but not MyD88-mediated viral clearance in the early phase of infection. This is consistent with the lack of a pDC response, which would otherwise be the major source of early TLR-dependent antiviral interferons. As the infection progressed, however, DKO mice harbored significantly higher viral loads compared with all other genotypes. Remarkably, DKO mice were also able to clear the infection by day 28 (Fig. 3B).
Fig. 3.
Mavs−/− and DKO mice are able to clear RSV and resolve pulmonary disease. (A and B) WT, Myd88−/−, Mavs−/−, and Myd88−/−Mavs−/− (DKO) mice were infected with RSV (107 pfu) and BALF was harvested at the indicated times to measure viral loads by plaque assay. (A: *, mavs−/− vs. myd88−/−, P < 0.001; **, mavs−/− vs. DKO and mavs−/− vs. myd88−/−, P < 0.001; B: *, mavs−/− vs. DKO and mavs−/− vs. myd88−/−, P < 0.01; ANOVA) (n = 3–5). (C) Lungs from infected mice and mock controls were analyzed by immunohistochemistry using a polyclonal anti-RSV antibody. (D) Mice infected with RSV were assessed by whole-body plethysmography to measure airway obstruction (*, P < 0.0001; **, P < 0.03; ***, P < 0.04; ANOVA). n.d., not detected; Penh, enhanced pause. Data are represented as mean ± SEM.
Consistent with unrestricted viral replication in Mavs−/− and DKO mice in the early phase of RSV infection, immunohistochemical staining with RSV antibodies in the lungs of these mice showed evidence of infection of ciliated respiratory epithelium lining the bronchioles and bronchi on days 1 and 5 after infection (Fig. 3C). This staining was undetectable by day 14. Histological examination of lungs of infected mice revealed no striking differences on day 1 (Fig. S6A). On day 5, the inflammation seen in DKO mice appeared in patches compared with the diffuse inflammation in the other three genotypes. At this time point, there was no gross difference in the composition of the cellular infiltrate among all groups. However, few multinucleated syncytial cells were observed in the bronchioles of DKO mice (Fig. S6B). On day 14, inflammation had largely subsided in WT and Myd88−/− mice but remained in the other two groups. Although Mavs−/− mice had some chronic inflammation at this stage, DKO mice exhibited patchy acute pneumonia, numerous syncytial cells, and reactive bronchial epithelium (Fig. S6C). By day 28, inflammation was significantly reduced and comparable in all genotypes (Fig. S6D).
MAVS or MyD88 Deficiency Alleviates only the Initial Phase of Pulmonary Disease, and All Mice Eventually Recover.
Next, we evaluated the roles of MAVS and MyD88 signaling in the pulmonary function of mice by plethysmography (Fig. 3D). One day after infection, only WT mice exhibited pulmonary dysfunction, possibly attributable to inflammatory cytokine responses that cause airway obstruction (P < 0.0001). We also consistently noted that only WT mice had ruffled hair and were less active than other genotypes on day 1. This is consistent with cytokine measurements, which showed that mice lacking MAVS, MyD88, or both had markedly reduced levels of the proinflammatory cytokines TNF-α, IL-6, and IL-1β (Figs. 1 and 2). Between days 2 and 10, all groups developed increased airway obstruction compared with their baseline function. Whereas the WT, Mavs−/−, and Myd88−/− mice returned to normal function around day 10, the DKO mice experienced slightly prolonged pulmonary dysfunction but eventually also returned to normal lung function (Fig. 3D; day 12, P = 0.03; day 14, P = 0.04).
Antibody Production Is Defective in the Absence of MAVS or MyD88.
The surprising ability of Mavs−/− and DKO mice to clear RSV led us to examine anti-RSV adaptive immune responses in these mice. Serum anti-RSV antibody responses, including the production of both IgG1 and IgG2a, were significantly attenuated in mice lacking either MAVS or MyD88 and were reduced even further in the DKO mice (Fig. 4A). These results suggest that both MAVS and MyD88 play an important role in the generation of anti-RSV antibodies in the adaptive phase of the antiviral response. This is in contrast to antibody production in response to influenza, which is MyD88 dependent and MAVS independent (21). However, depletion of B cells, followed by RSV infection, has suggested that the antibody response is unlikely to be a major mediator of primary RSV clearance (22).
Fig. 4.
Adaptive immune responses in mice lacking Mavs and Myd88. (A) RSV-specific antibody subtypes, IgG, IgG1, and IgG2a, were measured by ELISA using sera taken on the indicated day after RSV infection (IgG; *, WT vs. Myd88−/−; P < 0.01, Myd88−/− vs. DKO, P < 0.05; ** and ***, WT vs. Myd88−/−, P < 0.001) (IgG1; *, Mavs−/− vs. DKO, P < 0.01; ** and ***, WT vs. Myd88−/− and Myd88−/− vs. DKO, P < 0.05) (IgG2a; *, WT vs. Myd88−/− and Mavs−/− vs. DKO, P < 0.01; **, WT vs. Mavs−/−, P < 0.001; ***, WT vs. Myd88−/−, P < 0.001) (ANOVA, Tukey's test) (n = 7–10 per group). (B) Mice were mock-infected or infected with RSV for 8 days before lung cells were harvested for stimulation with an RSV- or SeV (control)-derived peptide. Six hours after stimulation, intracellular IFN-γ levels in CD3+CD8+ cells were measured by FACS. The percentage of IFN-γ+CD3+CD8+ cells in RSV-peptide treated cultures minus that from SeV-peptide cultures is plotted for each genotype (P = 0.35; ANOVA) (n = 5 per group). (C) Bronchoalveolar lavage fluid IFN-γ was measured in WT and DKO mice at the indicated times after RSV infection (n = 3 per group). (D) Lung cells (effectors) from day 8 RSV- or mock-infected mice were incubated with EL4 target cells loaded with a peptide (RSV peptide or SeV control peptide). The target cells were differentially labeled with CFSE and incubated with the effector cells at the indicated effector:target (E:T) ratios for 4 hours. Cells were then analyzed by flow- cytometry and the specific killing of RSV peptide-loaded targets was calculated (n = 3 per group). (E) Mice were either depleted of CD8+ T cells (with antibody 2.43) or mock depleted (IgG) and infected with RSV. BALF was extracted on day 8 and IFN-γ was measured by ELISA (n = 3 per group). (F) BALF viral titers were measured for mice described in E. Data are represented as mean ± SEM.
Normal CD8+ T Cell Response in the Absence of MAVS and MyD88.
Next, we examined the activation of the CD8+ cytotoxic T-lymphocyte (CTL) response in the lungs of mice after intranasal infection of RSV. Eight days after infection, lung cells were stimulated in vitro with an RSV-derived peptide previously identified as a CD8+ T cell stimulating epitope or with a control SeV-derived peptide, both of which bind H2-Db (23). The percentage of IFN-γ– producing CD8+ pulmonary T cells was quantified by FACS (Fig. 4B and Fig. S7). Unexpectedly, RSV-specific CD8+ T cell activation was similar in Myd88−/− and Mavs−/− mice compared with WT counterparts. Even the DKO mice were clearly able to activate CD8+ T cells in response to RSV, although the responses were slightly but not significantly lower (P = 0.35). In fact, measurement of IFN-γ in the BALF of WT and DKO mice at various times after infection revealed that DKO mice produced this cytokine more robustly than WT counterparts (Fig. 4C). In WT mice, IFN-γ peaked on day 6 after RSV infection and was undetectable by day 10. In DKO mice, however, IFN-γ peaked on day 8 at a higher level than in WT mice, suggesting that the higher viral load in DKO mice led to a corresponding increase in IFN-γ production. Additionally, analysis of CD8+ T cell cytolytic activity showed that, compared with cells from mock-treated animals, lung cells from RSV-infected mice of all genotypes demonstrated increased ability to specifically kill target cells loaded with the MHC-I–binding RSV epitope (Fig. 4D). Furthermore, depletion of CD8+ T cells in conjunction with RSV infection firmly established a role for these cells. Analysis of BALF 8 days after infection revealed that production of IFN-γ was intact in all mice treated with control IgG but absent in mice depleted of CD8+ T cells, pointing to these cells as the likely source of the cytokine (Fig. 4E). Finally, measurement of viral titers in Mavs−/− and DKO mice showed much higher levels of virus when CD8+ T cells were depleted (Fig. 4F). In contrast, depletion of CD8 T cells did not increase the viral titers in WT and Myd88−/− mice, presumably because these mice still had an intact MAVS pathway to clear the virus. Together, these data clearly show that CTL activation was intact and effective in DKO mice.
Subset of Pulmonary DCs Is Activated in Mavs−/− and DKO Mice.
To investigate how Mavs−/− and DKO mice mount a largely normal CTL response in the absence of innate IFNs and cytokines known to be important for activating adaptive immunity, we examined the activation of DCs. We first measured up-regulation of CD86 in CD11c+ GM-CSF-derived bone marrow-derived DCs (BMDCs) in response to RSV and SeV infection in vitro. CD86 up-regulation was observed in WT and Myd88−/− DCs but not in Mavs−/− and DKO DCs (Fig. S8), suggesting a complete dependence on MAVS-mediated signaling. As a control, LPS-induced CD86 surface expression was observed in DCs of all genotypes.
Because the RSV-induced response in these cultured DCs did not explain intact CD8+ T cell activation in mice lacking MAVS, we examined in vivo activation of pulmonary cDCs, which were identified as CD11c+, CD2−, and F4/80− cells (18). Both WT and MyD88-deficient CD11c+ DCs up-regulated surface levels of CD86 (Fig. 5A) and CD80 (data not shown) after RSV infection compared with mock-infected mice. In both MAVS-deficient and DKO mice, up-regulation of these molecules was drastically reduced. However, a small percentage of the pulmonary DCs in these mice consistently up-regulated these molecules, indicating their activation. In contrast, CD86 up-regulation was completely MAVS-dependent in CD11c+CD2+F4/80+ alveolar macrophages of the same mice (Fig. 5A).
Fig. 5.
A subset of pulmonary DCs is activated by RSV in mice lacking Mavs. (A) Mice of the indicated genotypes were infected with RSV or mock treated for 24 hours before CD86 levels were assessed on pulmonary DCs and alveolar macrophages by flow cytometry. Shaded histograms represent isotype-control antibody staining, blue histograms represent DCs from mock-infected mice and red histograms represent RSV infected mice. (B) Mediastinal lymph nodes (MLNs) were taken from mice described in A and surface expression of CD86 was assessed on dendritic cells by flow cytometry. Representative mice are shown for each genotype (n = 3 per group).
After activation, antigen-loaded DCs traffic from their peripheral tissue location to draining lymph nodes, where they activate T cells. Therefore, we examined the phenotype of DCs in the mediastinal lymph nodes draining the lung (Fig. 5B). Upon RSV infection, the majority of lymph node DCs in WT and Myd88−/− mice were CD86high compared with DCs in mock-treated mice. Consistent with the notion that the subset of pulmonary DCs activated in the absence of MAVS may subsequently traffic to the lymph node, we found a significant percentage of CD86high DCs in the lymph nodes of Mavs−/− and DKO mice. This subset of DCs may be responsible for the effective activation of RSV-specific CTLs in the absence of innate immunity mediated by MAVS and MyD88.
Discussion
We have provided both in vivo and in vitro evidence that MAVS is essential for the production of IFN-I and inflammatory cytokines in response to RSV infection. Global gene expression profiling further demonstrated that MAVS is required for RSV-induced production of the vast majority of antiviral molecules. In contrast to MAVS, MyD88 is dispensable for the induction of IFN-I and the majority of antiviral cytokines, with a few exceptions such as TNF-α, IL-1β, and MCP-1. The defective production of these molecules in all of the mutant mice may reflect the complex regulation unique to these potent inflammatory mediators. Notably, Il-24 and cxcl-16 were induced independently of MAVS and MyD88. Whether these cytokines play any role in the immune response to RSV requires further study.
It has recently been shown that systemic infection with the RNA virus, LCMV, results in IFN-I, inflammatory cytokine and chemokine production in a MyD88-dependent but largely MAVS-independent manner (24). Using an intranasal mode of infection, the same group found that influenza virus-induced IFN-I is defective only in mice lacking both MAVS and MyD88 (21). Further, pulmonary NDV infection indicated that mice lacking either MAVS or MyD88 are still capable of producing IFN-α (18). These results indicate that the requirement of MAVS and MyD88 for innate cytokine responses depends on the pathogen and on the route of infection. Our data suggest that RSV solely relies on the RLR-MAVS pathway for IFN-I induction in vivo. Further, we find no evidence of compensatory MyD88-mediated IFN-I production, which is consistent with recent data on the lack of IFN production by pDCs in response to RSV infection (25) but inconsistent with other reports suggesting IFN-I induction in these cells by RSV (26, 27). Our observation that RSV-infected Myd88−/− mice induce normal levels of IFN-I suggests that in this model, pDCs do not produce these cytokines because they are expected to use TLR7, which requires MyD88 for signaling. The reason for these seemingly conflicting results is unclear but may be partially explained by differences in virus strain or cell depletion methods.
Despite such a drastically defective cytokine response and the fact that Mavs−/− mice harbored higher viral loads shortly after RSV infection, these mice still cleared the virus effectively by activating a normal CTL response. Antibody-mediated depletion of CD8+ T cells significantly prolonged RSV replication, confirming their appropriate activation and demonstrating the important role of these cells in controlling the virus. Although deletion of Myd88 alone had no effect on RSV loads, mice lacking both adaptors had higher and more prolonged viral loads than those lacking either one alone, suggesting that in the absence of MAVS, MyD88 signaling contributed to antiviral immunity through a mechanism independent of IFN-I induction. Remarkably, even the DKO mice were able to activate CD8+ T cells and clear the virus effectively.
It is surprising that CD8+ T cell activation is normal in the absence of MAVS, because it is widely believed that IFNs and cytokines produced during innate antiviral responses are required for activating adaptive immunity, including cross-priming (28, 29). A possible answer to this conundrum is our finding that a subset of pulmonary DCs is activated by RSV in the lung and then migrates to the mediastinal lymph nodes in the absence of MAVS and MyD88. It is possible that this subset of DCs may be responsible for the cross-priming of CD8+ T cells in a manner that depends on TLR3 and Trif (30). We have assessed the expression of several surface markers, including CD8α, CD4, CD11b, B220, mPDCA1, and GR-1, on the pulmonary DCs and found that none are specifically enriched or lower in the activated subset in the WT mice or in those lacking MAVS or MyD88. Therefore, it is still not clear what makes this subset of DCs unique in getting activated by RSV in the absence of MAVS and MyD88. Further characterization of this DC subset should provide important insights into the regulation of T cell responses to RSV and possibly other pathogens.
Our results highlight the sophistication of the host's antiviral defense mechanisms and provide an example of an effective adaptive immune response in the absence of known innate immune mediators. Our results also have important implications for the development of RSV therapeutics and vaccines, as a significant contributor of RSV disease is an inappropriate CD8+ T cell response. Indeed, antibody-mediated depletion of CD8+ T cells alleviates disease progression in RSV-infected mice (22, 31). Because MAVS is required for optimal anti-RSV antibody production but not for a CD8+ T cell response, agents that activate the RLR-MAVS pathway may serve as important adjuvants in future RSV vaccines and therapeutics.
Materials and Methods
Mice.
The generation of Mavs−/− mice has been described in ref. 16. Briefly, Mavs−/− mice were made by homologous recombination with a targeting vector in 129/Sv ES cells, which were then injected into C57BL/6 blastocysts to create chimeric mice. Myd88+/− mice, which had been back-crossed to the C57BL/6 background, were kindly provided by Shizuo Akira (Osaka University). To obtain mice lacking mavs, myd88, or both (DKO), mavs+/− and myd88+/− mice were bred with each other, and the resulting progeny with appropriate genotypes were used in the experiments. All mice described in this report were engineered and housed in animal facilities at the University of Texas Southwestern Medical Center, and the experimental protocols were approved by the Institutional Animal Care and Use Committee.
In Vivo Infection.
Mice were anesthetized with inhaled isoflurane before intranasal inoculation with 107 pfu of live RSV in 100 μl of Eagle Minimal Essential Medium supplemented with l-glutamine, Hepes, penicillin, streptomycin, and 10% FBS. Control animals were sham-inoculated with 100 μl of cell-culture media (19).
CD8+ T Cell Analyses.
For IFN-γ production, lung cells were incubated in complete media with either an SeV-derived peptide (FAPGNYPAL) or an RSV-derived peptide (NAITNAKII) (23) (10 μg/ml) and brefeldin A (10 μg/ml; Sigma) for 6 h. Cells were then incubated with antibodies against CD3 and CD8, followed by fixation, permeabilization, and staining for IFN-γ using the Becton Dickinson fix/perm reagent according to the manufacturer's instructions. Samples were run on a Becton Dickinson FACSCalibur and analyzed using FlowJo 8.3 (Tree Star, Inc.). Cytotoxicity assays were performed as described (23). EL4 targets were incubated with either RSV- or SeV-dervived peptides (10 μg/ml) overnight. Respiratory syncytial virus peptide-loaded targets were then labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) and control targets with 0.5 μM CFSE before mixing them at a 1:1 ratio. Mixed targets were then incubated at the indicated effector/target (E/T) ratios with lung cells from mice infected with RSV for 8 days. Cells were incubated at 37°C for 4 h and then were analyzed by flow cytometry. Percent specific lysis was calculated as 100 − (100 × Percent CFSEhigh [at E/T of 2 or 20)/Percent CFSEhigh [at 0:1]).
More information on materials and methods, including statistical analyses, can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Nitin Karandikar, James Forman, and John Schatzle for much invaluable advice. We thank Dr. Nitin Karandikar (University of Texas Southwestern, Dallas) for the 2.43 hybridoma and Dr. Lijun Sun (University of Texas Southwestern, Dallas) for help with antibody purification. This work was supported by grants from the National Institutes of Health (Grant AI-09919) and the Welch Foundation. V.G.B. was supported in part by a National Institute of Allergy and Infectious Diseases training grant (Grant 5T32 AI005284–30). Z.J.C. is a Burroughs Wellcome Fund Investigator of Pathogenesis of Infectious Diseases and an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804717105/DCSupplemental.
References
- 1.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 4.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 5.Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 7.Meylan E, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 8.Xu LG, et al. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Simoes EA, Carbonell-Estrany X. Impact of severe disease caused by respiratory syncytial virus in children living in developed countries. Pediatr Infect Dis J. 2003;22:S13–S18. doi: 10.1097/01.inf.0000053881.47279.d9. discussion S18–S20. [DOI] [PubMed] [Google Scholar]
- 12.Murata Y, Falsey AR. Respiratory syncytial virus infection in adults. Antivir Ther. 2007;12:659–670. [PubMed] [Google Scholar]
- 13.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 14.Openshaw PJ. Antiviral immune responses and lung inflammation after respiratory syncytial virus infection. Proc Am Thorac Soc. 2005;2:121–125. doi: 10.1513/pats.200504-032AW. [DOI] [PubMed] [Google Scholar]
- 15.Collins PL, Murphy BR. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc. 2005;2:166–173. doi: 10.1513/pats.200501-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai Y, et al. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Jafri HS, et al. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189:1856–1865. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 20.Rudd BD, et al. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 21.Koyama S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 22.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutigliano JA, Rock MT, Johnson AK, Crowe JE, Jr, Graham BS. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology. 2005;337:335–343. doi: 10.1016/j.virol.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Jung A, et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jewell NA, et al. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J Virol. 2007;81:9790–9800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R, Janeway CA., Jr Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol. 1999;64:429–435. doi: 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 29.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 30.Schulz O, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze J, et al. CD8 T cells are essential in the development of respiratory syncytial virus-induced lung eosinophilia and airway hyperresponsiveness. J Immunol. 1999;162:4207–4211. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.