Abstract
The interplay between dopamine and glutamate in the basal ganglia regulates critical aspects of motor learning and behavior. Metabotropic glutamate receptors (mGluR) are increasingly regarded as key modulators of neuroadaptation in these circuits, in normal and disease conditions. Using PET, we demonstrate a significant upregulation of mGluR type 5 in the striatum of MPTP-lesioned, parkinsonian primates, providing the basis for therapeutic exploration of mGluR5 antagonists in Parkinson disease.
Keywords: PET, dopamine, glutamate, mGluR5, Parkinson disease, MPTP
Increasing understanding of receptor function is revealing complex and dynamic interactions between neurotransmitter systems (Zhang and Sulzer, 2003), including heterosynaptic transmission, alternative second messengers and integration of intracellular cascades at different levels. In the basal ganglia, dopamine (DA) regulation of glutamate (Glu) neurotransmission is complex and the loss of DA-mediated inhibition in the striatum in Parkinson disease (PD) results in an imbalance in other neurotransmitters, mostly excitatory. Accordingly, Glu antagonists at ionotropic glutamate receptors (iGluR) (as well as anticholinergic drugs) have demonstrated antiparkinsonian effects, although their side effects are intolerable (Gubellini et al., 2004). In contrast, metabotropic (m)GluRs are activated when there is excess Glu in the synaptic cleft, that spills over to activate perisynaptic receptors –and therefore act as sensors and modulators when or where Glu transmission is enhanced (Gubellini et al., 2004; Konradi et al., 2004). This functional specificity makes them attractive pharmacological targets, although it cannot be excluded that drugs will also interact with mGluRs in other regions, masking or altering the effects (Gubellini et al., 2004). In contrast to iGluR antagonists, such side effects may be subtle and not discernible in animal models (Gubellini et al., 2004). The prevalent distribution of mGluR5 in the striatum and limbic system supports their role modulating DA and Glu-dependent signaling and synaptic plasticity within the basal ganglia cortico-subcortical loops (Gubellini et al., 2004). While the role of mGluR5 in cocaine addiction has been firmly established (Chiamulera et al., 2001) using mGluR5 knockout mice, data from Parkinson models are equivocal (Armentero et al., 2006; Breysse et al., 2003; Mela et al., 2007; Oueslati et al., 2005; Samadi et al., 2007) except for a likely involvement in the pathophysiology of dyskinesias (Mela et al., 2007), a frequent complication of long-term L-DOPA replacement therapy. Taking advantage of novel PET tracers (Pellegrino et al., 2007; Wang et al., 2007) we examined the distribution of mGluR5 in the primate brain and the effect of DA denervation in MPTP-lesioned parkinsonian primates to determine whether mGluR5 drugs may be therapeutically relevant for PD.
Methods
Eight young adult male primates (macaca fascicularis) were included in the study. Animals were individually housed at the New England Regional Primate Center. Studies were conducted following NIH guidelines for animal use and care and were approved by the Internal Animal Care and use Committee at Harvard Medical Area and the Massachusetts General Hospital.
PET imaging studies (MicroPET P4, Concord Microsystems) were conducted in 4 control (naïve) and 4 MPTP-lesioned, parkinsonian primates. Systemic administration of MPTP to induce a stable parkinsonism and rating of parkinsonian severity was performed as previously reported by our group in detail (Jenkins et al., 2004). Imaging studies were performed under propofol anesthesia (0.3mg/kg/min iv). In six animals mGluR5 and DA transporter binding were investigated in the same imaging session, using first a carbon-11 labeled pyridine analog, 2-(2-(5-[11C]methoxypyridin-3-yl)ethynyl)pyridine ([11C]MPEPy), which has fast binding kinetics. Starting from the administration of [11C]MPEPy (10–13 mCi i.v., specific activity 900 mCi/µmol) dynamic volumetric data were acquired for 90 min. One hour after the data acquisition was completed (i.e.150 min after injection) the carbon-11 labeled cocaine analog, 2ß-[11C]carbomethoxy-3ß-(4-fluoropheny) tropane ([11C]CFT) was injected (8–10 mCi i.v., specific activity 1400 mCi/µmol) and imaging data were acquired for 90 min. Transmission imaging was done using a cobalt-57 source for processing maps for attenuation correction. Image processing was done using filtered backprojection and software provided by the manufacturer (Asipro 6.0, Concord Microsystems/Siemens). In two animals imaging studies of mGluR5 and DAT were done in different session to avoid background activity, since [18F]FPEB has long retention time. After administration of [18F]FPEB (3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile) (0.8–1.2 mCi i.v., specific activity 1900 mCi/µmol) dynamic volumetric data were acquired for 120min followed by transmission imaging and data reconstruction as above.
The synthesis and labeling of these tracers has been described by us (Brownell et al., 2003; Pellegrino et al., 2007; Wang et al., 2007). Regions of interest (ROIs) were delineated based on MRI high resolution anatomical images acquired on a Siemens 3T Trio system, as previously reported by our group (Jenkins et al., 2004; Sanchez-Pernaute et al., 2007) and anatomical atlas (Fig. 1c). In vivo binding potential (BPND) (Innis et al., 2007) of these ligands was calculated using the cerebellum as reference tissue (Zhu et al., 2007).
Figure 1.
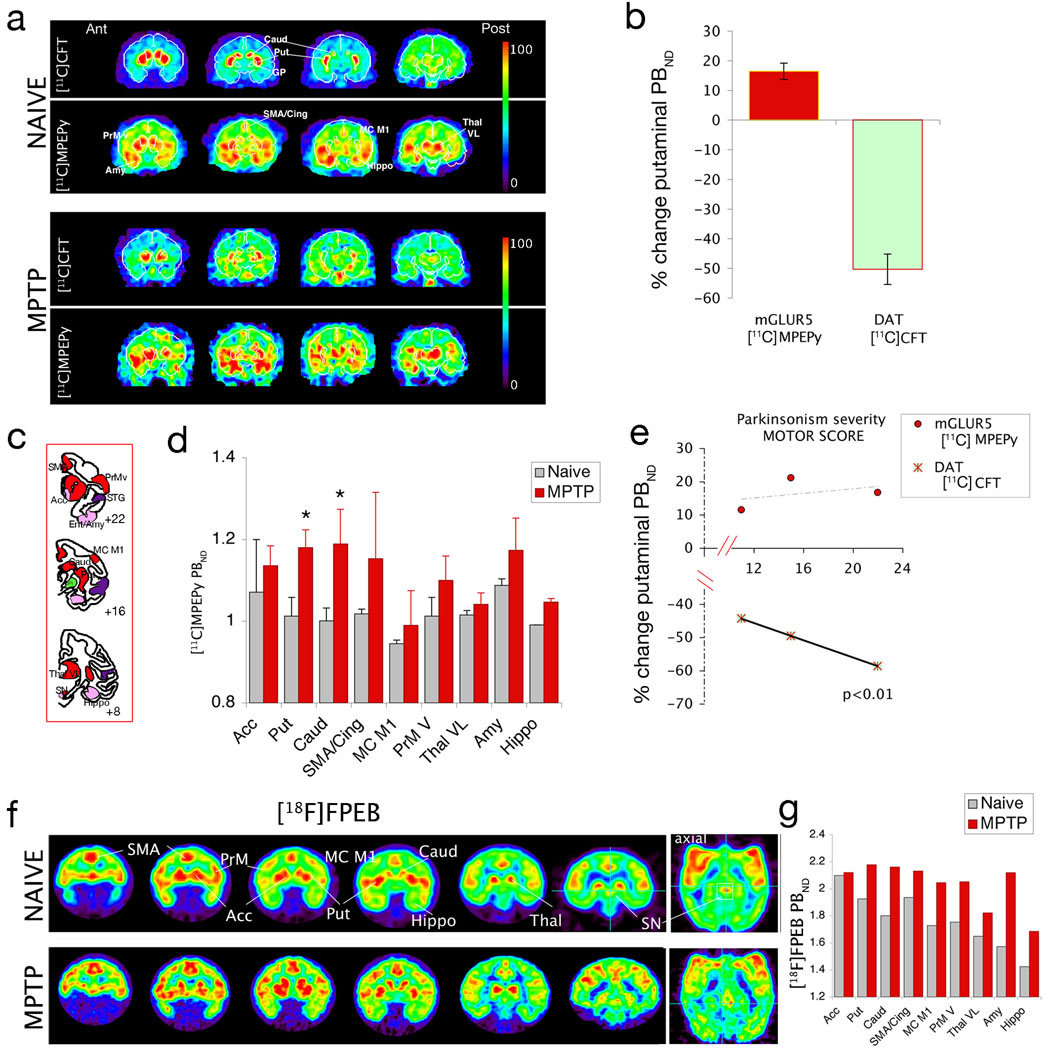
a) Representative images of the distribution of the dopamine transporter (DAT) and mGluR5 in a naïve (top panel) and a parkinsonian primate. Identical coronal slices shown at 4 anteroposterior levels were acquired consecutively in the same imaging session, to facilitate delineation of the basal ganglia. Color scale is adjusted to maximal activity for each tracer. Distribution of [11C]CFT accumulation is illustrated at 40–45 min after administration of radioactivity (8–10 mCi i.v., 1400mCi/µmol). [11C]MPEPy accumulation is illustrated at 10–25 min after administration of radioligand (10–13 mCi, iv., specific activity 900 mCi/µmol). b) Average change from naïve baseline in the putaminal binding in MPTP lesioned animals. c) Schematic representation of anatomical regions integrated in the mesolimbic (pink) mesostriatal (red) and temporal (purple) loops that are affected in diseases in which the DA/Glu interaction appears to play a crucial pathogenic role, i.e. addiction, Parkinson’ disease and schizophrenia, respectively. Globus pallidus pars interna is filled in green, to represent that no significant binding was observed in this region. d) ROI analysis of [11C]MPEPy binding demonstrated a significant increase in caudate and putamen. e) Putaminal change in [11C]MPEPy binding was not significantly correlated with the severity of parkinsonian signs (global score 0–24), unlike the change in [11C]CFT binding. f) Expression of mGluR5 in the brain of a naïve (top) and a parkinsonian primate, using the highly selective tracer [18F]FPEB delineated primary and downstream DA regions. SN/VTA are shown in coronal and axial reconstruction. Distribution of [18F]FPEB accumulation is illustrated at 60–70 min after administration of radioligand (0.8–1.2 mCi i.v., specific activity 1900 mCi/µmol); g) Regional values in binding potential follow the pattern described above for [11C]MPEPy. Acc=Accumbens, Amy=Amygdala, Caud=Caudate, Cing=cingulate Cortex, Ent= Entorhinalis cortex, GP=Globus Pallidus, Hippo=Hippocampus, MC M1=Primary Motor Cortex, PBND=in vivo binding potential, PrM= Premotor Cortex, Put=Putamen, SMA=supplementary motor area, SN=substantia nigra, Thal=Thalamus, V= ventral, VL=ventrolateral
Statistical analysis
Results are shown as mean ± SD. Two-tailed unpaired t test was used for comparison between conditions and simple regression analysis to assess the correlation with motor signs.
Results and Discussion
The distribution of [11C]MPEPy in the brain of naïve (n=3) and MPTP-lesioned, parkinsonian primates (n=3) was compared to that of [11C]CFT, a cocaine analog that binds to the DA transporter (DAT) as described (Brownell et al., 2003) (Fig. 1a). In naïve animals [11C]MPEPy rapidly accumulated in discrete cortical and subcortical regions encompassing the premotor and cingulate cortices, superior temporal gyrus and limbic (paraentorhinal/amygdala/hippocampal) cortex, the nucleus accumbens, caudate and putamen (predominantly at rostral levels), the ventral thalamus and the midbrain. This distribution corresponds to areas that have been shown to display high mGluR5 mRNA expression in the rodent brain (Messenger et al., 2002). Of interest is the lack of binding in the globus pallidus, which agrees with mRNA data in rodent (but not with published immunohistochemistry (Smith et al., 2000)).
MPTP-lesioned animals had a significant loss of [11C]CFT binding in the putamen (t1,3=8.27; p<0.05) with typical preservation of DA innervation of the nucleus accumbens (Fig 1b, (Jenkins et al., 2004)). Regional analysis of [11C]MPEPy was performed in cortical and subcortical areas to examine the motor and limbic DA loops (color coded in Fig.1c, at 3 coronal levels of the macaque brain). We found a significant enhancement of binding in the motor regions of the striatum (putamen t1,4 = 4.56; p = 0.01; caudate t1,4 = 3.57; p = 0.02) (Fig. 1d). The average increase in the motor striatum, 18.6 ±8.1% was moderate (16% in the putamen, Fig. 1b) and not significantly correlated with the loss of [11C]CFT binding, (Fig. 1e) or with the severity of the parkinsonian score –although the slope of the regression was positive (0.34). We acknowledge that the small volumes of ROIs are vulnerable for partial volume effects and the recorded activity might be less than the “real” activity. However, in this case it means that enhancement of mGluR5 accumulation is even more than in the presented data. The loss of [11C]CFT binding was directly correlated with the severity of the parkinsonian signs (p < 0.005) measured by the global motor score in a rating scale based on the motor subscale of the UPDRS (Fig. 1e) as we have previously described in this model (Jenkins et al., 2004). To confirm that the change in [11C]MPEPy binding reflected changes in mGluR5, we examined in 2 other primates, the distribution of the novel compound [18F]FPEB (Hamill et al., 2005; Wang et al., 2007), which has exceptionally high affinity to mGluR5. The reported in vitro Bmax/Kd value based on the saturation binding studies in rhesus caudate-putamen tissue is 210 (Patel et al., 2007) and in vivo studies have shown that it is subgroup specific (Wang et al., 2007). These imaging studies confirmed mGluR5 binding to DA target regions (Fig. 1f) matching cortical and subcortical areas with identical distribution to that we described previously using fMRI and amphetamine (Jenkins et al., 2004) (i.e. the areas in which DA release induces an increase in regional cerebral blood volume). Interestingly, there was no significant binding in the pallidal complex, either in the naïve or DA denervated condition. Given the high affinity of this tracer, it was feasible to resolve the VTA/SN region (Fig. 1f, see axial reconstruction in the far right panel) although more studies are necessary for quantification of MPTP-induced changes using this tracer (Fig. 1g).
In conclusion, here we identified a significant enhancement in [11C]MPEPy mGluR5 binding in the striatum of MPTP-lesioned parkinsonian primates. Because, in principle, DA denervation in PD enhances Glu transmission, further amplification through upregulation of striatal mGluR5 appears to be a possible pathogenic mechanism involved in aspects of disease progression, as well as in the development of long-term complications. Thus, these novel selective mGluR5 PET tracers are valuable tools for in vivo mechanistic studies in patients and in the development of novel therapeutic approaches for PD.
Acknowledgements
Supported by NIH-1R01 EB001850 and NIH-1P50 NS39793. We are grateful to Jack McDowell for technical support and Dr BG Jenkins for MRI studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson's disease. Neurobiol Dis. 2006;22:1–9. doi: 10.1016/j.nbd.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell AL, Canales K, Chen YI, Jenkins BG, Owen C, Livni E, Yu M, Cicchetti F, Sanchez-Pernaute R, Isacson O. Mapping of brain function after MPTP-induced neurotoxicity in a primate Parkinson's disease model. Neuroimage. 2003;20:1064–1075. doi: 10.1016/S1053-8119(03)00348-3. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 2004;74:271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford ND, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O'Malley SS, Hargreaves R, Burns HD. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong D, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell AL, Chen YC, Isacson O. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24:9553–9560. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis. 2004;17:219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson's disease. J Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- Messenger MJ, Dawson LG, Duty S. Changes in metabotropic glutamate receptor 1–8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology. 2002;43:261–271. doi: 10.1016/s0028-3908(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Oueslati A, Breysse N, Amalric M, Kerkerian-Le Goff L, Salin P. Dysfunction of the cortico-basal ganglia-cortical loop in a rat model of early parkinsonism is reversed by metabotropic glutamate receptor 5 antagonism. Eur J Neurosci. 2005;22:2765–2774. doi: 10.1111/j.1460-9568.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Hamill TG, Connolly B, Jagoda E, Li W, Gibson RE. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [(18)F]F-PEB. Nucl Med Biol. 2007;34:1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Pellegrino D, Cicchetti F, Wang X, Zhu A, Yu M, Saint-Pierre M, Brownell AL. Modulation of dopaminergic and glutamatergic brain function: PET studies on parkinsonian rats. J Nucl Med. 2007;48:1147–1153. doi: 10.2967/jnumed.106.037796. [DOI] [PubMed] [Google Scholar]
- Samadi P, Gregoire L, Morissette M, Calon F, Hadj Tahar A, Dridi M, Belanger N, Meltzer LT, Bedard PJ, Di Paolo T. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O. In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiol Dis. 2007;27:220–227. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Charara A, Hanson JE, Paquet M, Levey AI. GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates. J Anat. 2000;196(Pt 4):555–576. doi: 10.1046/j.1469-7580.2000.19640555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Tueckmantel W, Zhu A, Pellegrino D, Brownell AL. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61:951–961. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J Neurosci. 2003;23:10585–10592. doi: 10.1523/JNEUROSCI.23-33-10585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Wang X, Yu M, Wang JQ, Brownell AL. Evaluation of four pyridine analogs to characterize 6-OHDA-induced modulation of mGluR5 function in rat brain using microPET studies. J Cereb Blood Flow Metab. 2007;27:1623–1631. doi: 10.1038/sj.jcbfm.9600461. [DOI] [PubMed] [Google Scholar]