Abstract
In acute inflammation, infiltrating polymorphonuclear leukocytes (also known as PMNs) release preformed granule proteins having multitudinous effects on the surrounding environment. Here we present what we believe to be a novel role for PMN-derived proteins in bacterial phagocytosis by both human and murine macrophages. Exposure of macrophages to PMN secretion markedly enhanced phagocytosis of IgG-opsonized Staphylococcus aureus both in vitro and in murine models in vivo. PMN secretion activated macrophages, resulting in upregulation of the Fcγ receptors CD32 and CD64, which then mediated the enhanced phagocytosis of IgG-opsonized bacteria. The phagocytosis-stimulating activity within the PMN secretion was found to be due to proteins released from PMN primary granules; thorough investigation revealed heparin-binding protein (HBP) and human neutrophil peptides 1–3 (HNP1–3) as the mediators of the macrophage response to PMN secretion. The use of blocking antibodies and knockout mice revealed that HBP acts via β2 integrins, but the receptor for HNP1–3 remained unclear. Mechanistically, HBP and HNP1–3 triggered macrophage release of TNF-α and IFN-γ, which acted in an autocrine loop to enhance expression of CD32 and CD64 and thereby enhance phagocytosis. Thus, we attribute what may be a novel role for PMN granule proteins in regulating the immune response to bacterial infections.
Introduction
Acute inflammatory processes are characterized by an early appearance of polymorphonuclear cells (PMNs) followed by a second wave of monocytes (1), which differentiate into macrophages. During the journey from blood to tissue, PMNs release their granules via which they communicate with their close environment (2, 3). Recent research provides evidence for the importance of PMN granule proteins in the interaction with other immune cells, in particular monocytes and macrophages. For instance, neutrophil-specific granule deficiency exhibits obvious changes in macrophages maturation, migratory capacity, cytokine gene expression, and phagocytosis in humans (4) and mice (5). Moreover, different models of neutropenia have provided evidence that monocyte extravasation depends on PMNs (6). Direct proof illustrating the importance of PMN secretion (PMN-sec) products for monocyte and macrophage function is also accumulating. We could recently demonstrate that the PMN-derived heparin-binding protein (HBP), a multifunctional modulator of inflammation (7), promotes monocyte adhesion (8) and extravasation (9). In addition, certain proteins in PMN granules are known to induce monocyte/macrophages chemotaxis (10), cytokine and chemoattractant production and release (11), and antigen presentation (12).
Phagocytosis of bacteria by macrophages is an essential mechanism of host defence (13). To make phagocytosis more efficient, pathogens are opsonized by complement-derived factors (14) or immunoglobulins (15). Recognition after opsonization occurs by either complement receptors or Fc receptors. Among the FcRs are those recognizing IgG: Fcγ receptor I (FcγRI) (CD64), FcγRII (CD32), and FcγRIII (CD16). Complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) on macrophages are involved in the binding and ingestion of opsonized particles.
In this report, we demonstrate what we believe to be a novel role for PMN-sec products in phagocytosis of bacteria by macrophages. We hypothesized that the well-established PMN-monocyte/macrophage axis in inflammation may be of importance in the regulation of bacterial phagocytosis by macrophages. Our results show that secretion products derived from PMNs trigger an active response in macrophages, resulting in enhanced bacterial phagocytosis. This mechanism contributes to the capability of activated PMNs to modulate macrophage function as well as the effectiveness of the immune response in host defense.
Results
PMN-sec enhances phagocytosis of bacteria in macrophages.
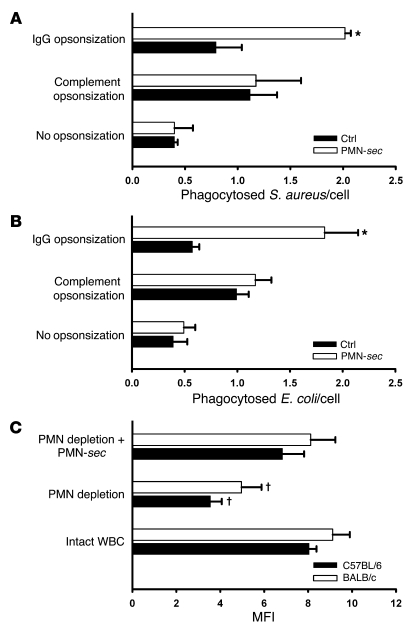
PMN activation via β2 integrin cross-linking caused release of secretory vesicles and tertiary, secondary, and primary granules as shown by Western blot analysis for marker proteins in the PMN-sec (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI35740DS1). Human macrophages derived from monocytes were treated with PMN-sec for 24 hours followed by a 1-hour incubation period with Staphylococcus aureus or Escherichia coli that were IgG opsonized, complement opsonized, or nonopsonized. Treatment with PMN-sec caused a strong enhancement of phagocytosis of IgG-opsonized S. aureus or E. coli but not of complement-opsonized or nonopsonized bacteria (Figure 1, A and B). Treatment with PMN-sec also resulted in a comparable enhancement of phagocytosis of IgG-opsonized S. aureus by murine RAW264.7 cells and WEHI-3B cells (data not shown). Interestingly, treatment of human macrophages with PMN-sec not only increased the uptake of bacteria but also their capacity to intracellularly kill S. aureus and E. coli (Supplemental Figure 2). In further experiments, only IgG-opsonized bacteria were used in the phagocytosis assay.
Figure 1. PMN-sec products enhance phagocytosis in macrophages.
(A and B) Human macrophages were treated with PMN-sec or medium alone (ctrl) for 24 hours. After stimulation, fluorescent S. aureus (A) or E. coli (B) were injected into the medium. Bacteria were either opsonized with IgG or with complement or left nonopsonized. The number of incorporated bacteria per cell was quantified by fluorescence microscopy. For each analysis, 3–6 independent experiments were performed. *P < 0.05 versus respective control. (C) Comparison of phagocytic activity of peritoneal macrophages obtained from BALB/c or C57BL/6 mice. Macrophages were isolated from mice with intact WBC, neutropenic mice, and from neutropenic mice treated with PMN-sec. MFI as a measure of phagocytosed IgG-opsonized bacteria was read in a plate reader. For each analysis, 6 independent experiments were performed. †P < 0.05 versus intact WBC and PMN depletion plus PMN-sec of the respective strain.
PMN granule proteins stimulate bacterial phagocytosis in peritoneal macrophages in vivo.
To further investigate the PMN-macrophage cross-talk in vivo a thioglycollate-induced peritonitis model, in which macrophages are exposed to PMN-sec products released into the peritoneum, was used. Subsequent incubation with bacteria and analysis of phagocytic capacity were done ex vivo. In BALB/c and C57BL/6 mice, we found that peritoneal macrophages obtained from neutropenic mice showed markedly reduced ability to phagocytose bacteria compared with mice with normal white blood cell count (WBC). The i.p. injection of human PMN-sec to neutropenic animals enhanced the phagocytic capacity of peritoneal macrophages (Figure 1C). To compare the quantity of PMN granule proteins in the PMN-sec with the conditions found in the peritoneal cavity in vivo, we analyzed the PMN-derived granule proteins myeloperoxidase (MPO) and MMP-9 in the PMN-sec as well as in the peritoneal lavage fluid. The activity of MPO and MMP-9 assessed in both specimens was found to be in a similar range (Supplemental Table 1). To exclude a direct effect of the PMN-depleting antibody on the phagocytic capacity, we treated peritoneal macrophages from mice with intact WBC with RB6-8C5 or control IgG. However, no differences regarding the phagocytic capacity could be detected (data not shown).
PMN-sec activates macrophages and enhances surface expression of FcγRs.
In further experiments human macrophages were treated with PMN-sec over different times. Treatment for 24 hours was more effective in stimulating phagocytosis than treatment for 2 hours (Figure 2A). Moreover, we found that absence of PMN-sec during incubation with bacteria had no influence on the phagocytic capacity (Figure 2A), suggesting that PMN-sec acts rather on the macrophages than on the bacteria and that PMN-sec causes a long-standing activation of macrophages.
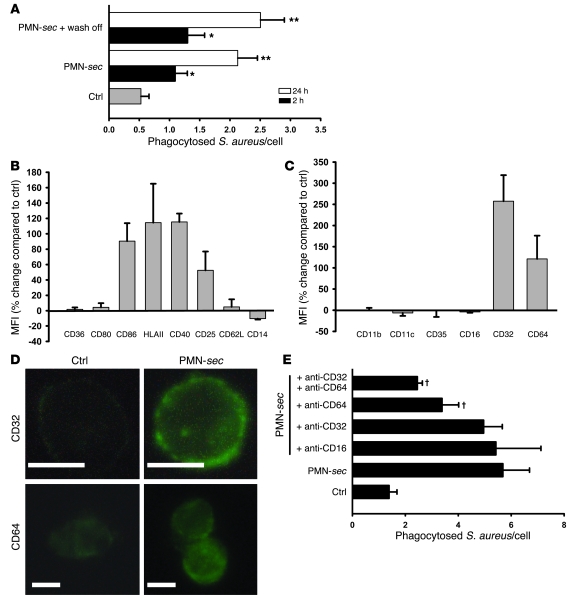
Figure 2. PMN-sec enhances activation of macrophages and expression of FcγRs.
(A) Human macrophages were incubated with PMN-sec for either 2 or 24 hours or with medium. In some wells, the PMN-sec was washed off after the 2- or 24-hour incubation period and replaced by medium. Subsequently the phagocytic activity was quantified. For each analysis, 4 independent experiments were performed. *P < 0.05 versus control; **P < 0.05 versus control and the respective 2-hour treatment group. (B and C) Expression of activation markers (B) and phagocytic receptors (C) in macrophages in response to treatment with PMN-sec for 24 hours. Expression is given as percentage change compared with basal expression. All values are isotype corrected. For each analysis, 6–8 independent experiments were performed. (D) Representative images of antibody staining for CD32 and CD64 in macrophages after treatment with PMN-sec or medium. Scale bar: 10 μm. (E) Human macrophages were incubated with PMN-sec or medium for 24 hours. Blocking antibodies toward CD64, CD32, or CD16 were added 30 minutes before incubation with IgG-opsonized S. aureus. The number of phagocytosed bacteria after 1 hour was quantified. For each analysis, 6 independent experiments were performed. †P < 0.05 versus group treated with PMN-sec.
To investigate whether this activation may involve altered activity of specific cell surface molecules, we measured the expression of activation markers (Figure 2B) and phagocytic receptors (Figure 2, C and D) on macrophages. While expression of CD36, CD80, and CD62L was not altered by treatment with PMN-sec, we found a clear increase in CD86, HLA class II, CD40, and CD25 expression, reflecting phenotypic changes seen in classically activated macrophages (16). Analysis of complement and FcγRs revealed a marked upregulation of CD32 and CD64 but not of CD16 or complement receptors (CD11b, CD11c, CD35). In control experiments the cell number was controlled for by counterstaining with the DNA-binding dye DAPI. In these experiments, we obtained identical results (Supplemental Figure 3). To investigate the role of the different FcγRs in enhanced phagocytosis, neutralizing antibodies to these molecules were applied in the phagocytosis assay. While antibodies to CD16 were without effects, blockade of CD64 or CD64 plus CD32 attenuated the enhanced phagocytosis significantly (Figure 2E).
The enhanced macrophage phagocytosis originates from proteins of PMN primary granules.
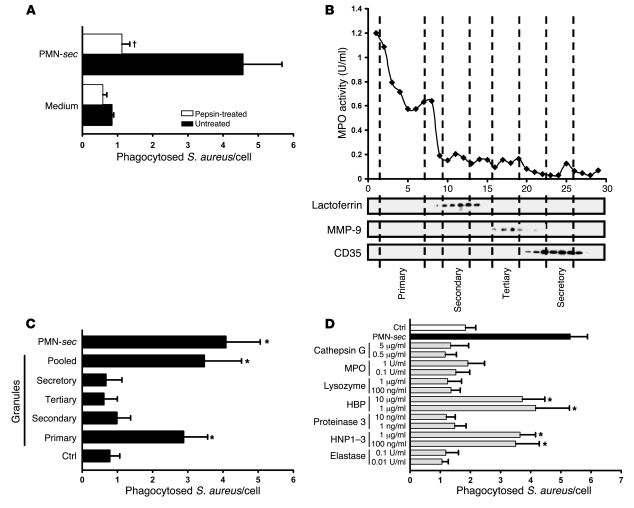
To investigate the chemical nature of the secreted compounds that stimulate phagocytosis, the activity of the PMN-sec was tested after heat inactivation or pepsin treatment. Heat inactivation (data not shown) and treatment with pepsin completely abrogated the induction of phagocytosis in macrophages (Figure 3A), indicating that the PMNs release one or several proteins that are responsible for enhancing phagocytosis. To identify these proteins, we isolated the individual subsets of PMN granules using subcellular fractionation (17) and assayed the activity of the respective granule subset. To determine which fraction corresponded to which granule compartment, each individual fraction was analyzed for the presence of specific granule markers (Figure 3B). Fractions that contained only one type of granule were pooled and used to stimulate human macrophages. Using this approach, we found that the phagocytosis-enhancing activity resided predominantly in primary granules (Figure 3C).
Figure 3. Identification of active PMN granule components.
(A) PMN-sec was digested with pepsin and the remaining activity was tested. For each analysis, 4 independent experiments were performed. †P < 0.05 versus group treated with active PMN-sec. (B) Localization of neutrophil granules in fractions (x axis) obtained by subcellular fractionation of PMNs is shown by marker analysis. MPO activity was measured as a marker for primary granules. Western blots of the fractions probed with antibodies to lactoferrin, MMP-9, and CD35 indicate the localization of secondary and tertiary granules and of secretory vesicles, respectively. (C) Human macrophages were treated with PMN-sec, fractions of the indicated PMN granules, a mixture of the 4 different granule fractions (pooled), or medium and phagocytosis assay was performed. For each analysis, 6 independent experiments were performed. *P < 0.05 versus control. (D) Human macrophages were treated with proteins and peptides stored in human primary granules. Phagocytosis was compared with that obtained from treatment with PMN-sec or medium. For each analysis, 6 independent experiments were performed. *P < 0.05 versus control.
Hence, we used purified or recombinant forms of proteins and peptides that are stored in primary granules and analyzed their activity in the phagocytosis assay. The selection was based upon their abundance in primary granules and their reported capability to activate macrophages. The concentration at which each individual protein was used was adjusted to what has earlier been found to activate monocytes or macrophages (8, 18–20). The panel of proteins consisted of elastase, human neutrophil peptides 1–3 (HNP1–3, α-defensins), proteinase-3, HBP (CAP37/azurocidin), lysozyme, MPO, and cathepsin G. Notably, the concentrations of the proteins used in our assay were similar to the amounts we found in the PMN-sec (HBP, 3.4 μg/ml; HNP1–3, 2.5 μg/ml; cathepsin G, 2.1 μg/ml; MPO, 1.3 U/ml). Among the proteins tested, only recombinant HBP and HNP1–3 purified from human PMNs were found to enhance the phagocytosis of S. aureus by human macrophages (Figure 3D).
Chemical identification of HBP and HNP1–3 as enhancers of bacterial uptake in macrophages.
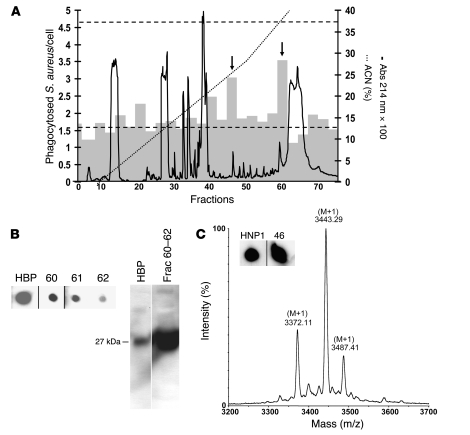
Complementary to the previous approach, the PMN-sec was separated by reversed-phase HPLC, and the fractions were tested for their biological activity. Only pooled fractions 45–47 and 60–62 caused a marked enhancement of phagocytic activity (Figure 4A). Screening of HPLC fractions for the presence of HBP and HNP1–3 revealed positive signals for HBP in fractions 60–62 (Figure 4B). For HNP1–3, strong immunoreactivity was found in fraction 46 (Figure 4C) and weak staining in fraction 47 (data not shown). Western blot analysis of pooled fractions 60–62 revealed a strong immunoreactive band with a molecular weight corresponding to that of HBP (Figure 4B). After Coomassie staining of the remaining protein in the gel, a band of 27 kDa was visualized. The protein in this gel plug was trypsinized, and the masses of the generated fragments were determined with MALDI–mass spectrometry (MALDI-MS). Further MS-Fit search on these mass values indicated 33.8% coverage and 6 matched fragments corresponding to HBP, supporting the presence of HBP in fractions 60–62.
Figure 4. Identification of HBP and HNP1–3 as enhancers of bacterial phagocytosis in macrophages.
(A) Fractionation of PMN-sec by reversed-phase HPLC. PMN-sec was loaded onto a C18 column. Proteins (right y axis, solid curve) were eluted with a gradient of ACN with 0.1% TFA (right y axis, dotted line). Gray bars indicate the phagocytic activity resulting from stimulation with material of 3 consecutive fractions (left y axis). Basal phagocytosis and phagocytosis in response to PMN-sec are indicated by the lower and upper dashed line, respectively. Arrows indicate active fractions (45–47 and 60–62). For each analysis, 4 independent experiments were performed. (B) Immunological detection of HBP in fraction 60–62 using dot blot (left panel). These fractions were pooled and further analyzed with Western blot analysis (right panel). As positive control for both Western and dot blot, 40 ng recombinant HBP was used. (C) HPLC fractions were screened for the presence of HNP1–3 by dot blot analysis. Positive staining was detected in fractions 46 (insert). Inserts of dot blots in B and C were run on the same gel but were noncontiguous. As positive control 20 ng HNP1 was used. Determination of the mass values of material in fraction 46 with MALDI-MS gave molecular weights close to the theoretical values of HNP1–3.
To verify the presence of HNP1–3 in fraction 46, the material was analyzed with mass spectrometry (Figure 4C) and N-terminal sequence analysis. The obtained mass values were 3,442.30, 3,371.1, and 3,486.4 Da, corresponding to HNP1 (3,442.0 Da), HNP2 (3,371.0 Da), and HNP3 (3,486.1 Da). N-terminal sequence analysis of the material in fraction 46 yielded 3 sequences comprising 20 amino acids residues, which are identical to the N-terminal residues of HNP1–3 (ACYCRIPACIAGERRYGTCI, CYCRIPACIAGERRYGTCIY, and DCYCRIPACIAGERRYGTCI).
Enhanced phagocytosis in response to PMN granule proteins is mediated by HBP and HNP1–3.
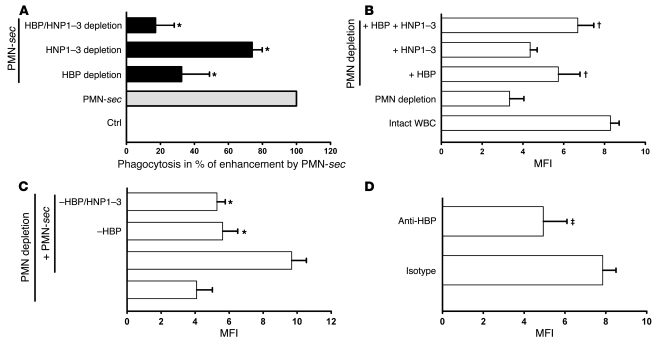
To investigate the contribution of HBP and HNP1–3 to enhanced phagocytosis, we immunodepleted the PMN-sec of these polypeptides. HBP removal caused a reduction of the enhanced phagocytosis by almost 70%, while HNP1–3–depletion damped the effect by approximately 25% (Figure 5A). Combined depletion of HBP and HNP1–3 resulted in almost complete elimination (83%) of the enhanced phagocytosis, attributing a virtually exclusive role to HBP and HNP1–3.
Figure 5. Contribution of HBP and HNP1–3 to enhanced phagocytosis.
(A) Human macrophages were treated with medium, PMN-sec, or secretion depleted of HBP, HNP1–3, or both. Phagocytic activity is expressed in percentage of enhanced phagocytosis above control in response to PMN-sec, which was set to 100%. For each analysis, 4 independent experiments were performed. *P < 0.05 versus group treated with PMN-sec. (B) Comparison of phagocytic activity of peritoneal macrophages from mice with intact WBC or neutropenic mice. Neutropenic mice were also injected i.p. with HBP (10 μg/mouse), HNP1–3 (2 μg/mouse), or both. MFI, as a measure of phagocytosed IgG-opsonized bacteria, was read in a plate reader. For each analysis, 5 independent experiments were performed. †P < 0.05 versus fluorescence intensity in macrophages obtained from PMN-depleted mice. (C) Thioglycollate-treated neutropenic mice received PMN-sec, PMN-sec depleted of HBP, or PMN-sec depleted of both HBP and HNP1–3. *P < 0.05 versus group treated with PMN-sec. For each analysis, 5 independent experiments were performed. (D) A polyclonal antibody to HBP or control IgG were injected i.p. into thioglycollate-treated mice. The phagocytic capacity of peritoneal macrophages was tested ex vivo 4 days after initiating peritonitis. ‡P < 0.05 versus isotype control. For each analysis, 4 independent experiments were performed.
In our peritonitis model, we tested the effect of HBP and HNP1–3 on the phagocytic capacity of peritoneal macrophages from neutropenic BALB/c mice. Injection of HNP1–3 tended to increase the ability to phagocytose, whereas HBP significantly enhanced the phagocytic capacity in peritoneal macrophages (Figure 5B). Interestingly, combination of HBP and HNP1–3 almost completely restored the phagocytic competence of peritoneal macrophages. In further experiments, we injected PMN-sec, PMN-sec depleted of HBP, and PMN-sec depleted of both polypeptides. Depletion of HBP from the PMN-sec almost completely abolished its phagocytosis-enhancing effect (Figure 5C). In yet another approach, we injected 50 μg polyclonal anti-HBP i.p. together with the thioglycollate broth at day 0 and every 24 hours after until the macrophages were harvested. Application of the anti-HBP antibody resulted in a significant reduction of the phagocytic capacity of macrophages harvested from the peritoneum as compared with mice treated with irrelevant IgG (Figure 5D). To investigate if the recruitment of PMNs to the peritoneal cavity was compromised by this treatment, we analyzed the number of PMNs by FACS. Importantly, we found no difference in the recruitment of PMNs (CD45+Gr1+F4/80–) to the peritoneal cavity (data not shown). Also the number of macrophages (CD45+Gr1–F4/80+) was not affected by the HBP antibody (data not shown).
Although murine macrophages respond to human HNP1–3 in this study and elsewhere (21), it is well established that murine PMNs are devoid of HNPs. Therefore, we wanted to confirm our findings in a different species known to have HNPs (22). In a rat peritonitis model, we could demonstrate that PMN depletion reduced the phagocytic function in peritoneal macrophages (Supplemental Figure 4). Injection of HBP or HNP1–3 partly restored phagocytic activity in these animals with predominance for HBP.
HBP and HNP1–3 stimulate enhanced phagocytosis via distinct signaling pathways.
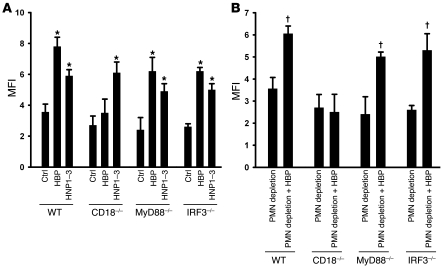
Above we show that the enhanced phagocytosis stimulated by PMN granule proteins critically depends on FcγRs and on the presence of HBP and HNP1–3. In further experiments, we attempted to tie these observations together. Treatment of macrophages with HBP or HNP1–3 caused a strong increase in expression of CD32 and CD64 (Figure 6, A and C). HBP has previously been shown to function as a soluble ligand to β2 integrins (23) and to activate monocytes through binding this receptor (24). In our experiments, incubation of macrophages with HBP caused an immediate Ca2+ mobilization, which was inhibited in the presence of the β2 integrin blocking mAb IB4 (Figure 6B). Blockade of β2 integrins not only prevented activation of macrophages but also attenuated expression of CD32 and CD64 by treatment with HBP (Supplemental Table 2). Similar observations were made in a murine system. There, enhanced phagocytosis of bacteria by murine RAW264.7 macrophages in response to HBP was abolished in the presence of the CD18 blocking antibody GAME46 (data not shown). In peritoneal macrophages from neutropenic CD18-deficient mice, stimulation with HBP did not induce a significant increase in bacterial uptake, which is in contrast to macrophages from neutropenic wild-type mice (Figure 7A). In a further step, we injected HBP into the peritoneum of neutropenic wild-type and CD18-deficient mice. While testing the phagocytic capacity ex vivo we found that HBP significantly enhanced phagocytosis in wild-type mice but not in CD18-deficient mice (Figure 7B). Moreover, we used isolated peritoneal macrophages from neutropenic MyD88- and IRF3-deficient mice to investigate the potential involvement of TLR signaling. In contrast to macrophages from CD18-deficient mice, HBP induced a significant increase in bacterial phagocytosis in macrophages obtained from neutropenic MyD88- and IRF3-deficient mice (Figure 7A), indicating that (a) enhanced phagocytosis in response to HBP does not involve TLR signaling and (b) that the effect of HBP is not due to LPS contamination. The same relationships were found when HBP was injected i.p. into neutropenic MyD88- and IRF3-deficient mice (Figure 7B). To gain insight into which intracellular pathway HBP activates in macrophages after binding to β2 integrins, we used inhibitors of various signaling molecules. Of the substances tested, inhibitors of tyrosine kinases and of MAP kinases greatly reduced the HBP-induced expression of CD32 and CD64, while inhibition of phospholipase C, protein kinase C, and phosphatidylinositol-3-kinase were without effects (Supplemental Table 2).
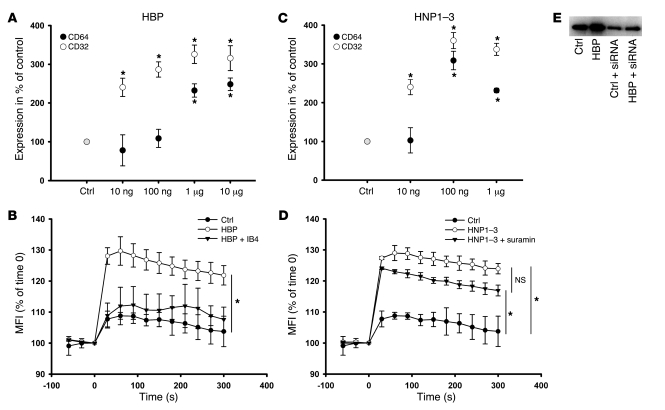
Figure 6. Characterization of the enhanced phagocytosis by HBP and HNP1–3.
(A and C) Human macrophages were treated with increasing concentrations of (A) HBP or (C) HNP1–3 for 24 hours, and the expression of CD32 and CD64 was assessed by immunofluorescence. Data are expressed as a percentage of basal expression, which was set to 100%. For each analysis, 5 independent experiments were performed. *P < 0.05 versus control. (B and D) Intracellular Ca2+-mobilization in macrophages labeled with the Ca2+-sensitive dye fluo4/AM in response to stimulation with (B) HBP (1 μg/ml) and (D) HNP1–3 (1 μg/ml). After labeling and washing, fluorescence was read in a plate reader every 30 seconds, from 60 seconds before stimulation until 300 seconds after stimulation. Data are expressed as percent of fluorescence intensity at time 0. IB4 (10 μg/ml) or suramin (100 μM) were added to analyze the involvement of β2 integrins and P2Y6 receptors in the respective activation. For each analysis, 4 independent experiments were performed. *P < 0.05 versus control treatment. (E) Western blot for CD64 of whole cell lysate of human macrophages treated with HBP (1 μg/ml, 24 hours) in the presence or absence of siRNA to CD64.
Figure 7. Enhanced phagocytosis mediated by HBP depends on CD18 but not TLR signaling, while enhanced phagocytosis induced by HNP1–3 is independent of CD18 and TLR signaling.
(A) Peritoneal macrophages were isolated from neutropenic mice of various strains and treated ex vivo with medium, HBP (1 μg/ml), or HNP1–3 (1 μg/ml) for 24 hours. Thereafter, the phagocytic capacity was assessed using IgG-opsonized S. aureus. For each analysis, 4 independent experiments were performed. *P < 0.05 versus respective control. (B) Comparison of phagocytic capacity of peritoneal macrophages from different strains. Mice were rendered neutropenic and then injected i.p. with either PBS or HBP. For each analysis, 3–5 independent experiments were performed. †P < 0.05 versus neutropenic mice of the respective strain.
In contrast to what was found for stimulation with HBP, IB4 treatment did not prevent HNP1–3–induced macrophage activation (Supplemental Table 3). In addition, none of the inhibitors used to block intracellular signaling pathways reduced the HNP1–3–induced expression of CD32 and CD64 (Supplemental Table 3). Recently, Kinee et al. demonstrated that PMN-derived HNPs mediate their effect on epithelial cells via binding to P2Y6 receptors (25). Therefore, it was of interest to investigate the influence of the P2Y-specific inhibitor suramin on the activation of macrophages by HNP1–3. HNP1–3 clearly activated macrophages as indicated by intracellular Ca2+ mobilization (Figure 6D). However, suramin failed to block HNP1–3–mediated activation and FcγR expression (data not shown). Similarly, treatment with Pertussis toxin (PTx) did not have any influence on the HNP1–3–induced macrophages activation (Supplemental Table 3). The efficiency of HNP1–3 treatment was also not blocked in macrophages deficient in CD18, MyD88, or IRF3 (Figure 7A). Taken together, these data indicate that HNP1–3 act via a pathway independent of TLR, CD18, and adenosine receptor signaling.
To reveal if the enhanced expression of CD32 and CD64 induced by HBP/HNP1–3 was due to de novo expression, we used emetine and anisomycin. Both compounds abrogated the HBP- and HNP1–3–mediated expression of CD32 and CD64 (Supplemental Tables 2 and 3). Moreover, whole-cell lysates of HBP-treated macrophages exhibited higher amounts of CD64 compared with control cells (Figure 6E). siRNA to CD64 resulted in a decreased expression of this receptor in control cells and a markedly reduced responsiveness to HBP (Figure 6E), suggesting that HBP stimulates de novo synthesis of CD32 and CD64.
The effects of HBP and HNP1–3 on the macrophage phenotype depend on autocrine stimulation via TNF-α and IFN-γ.
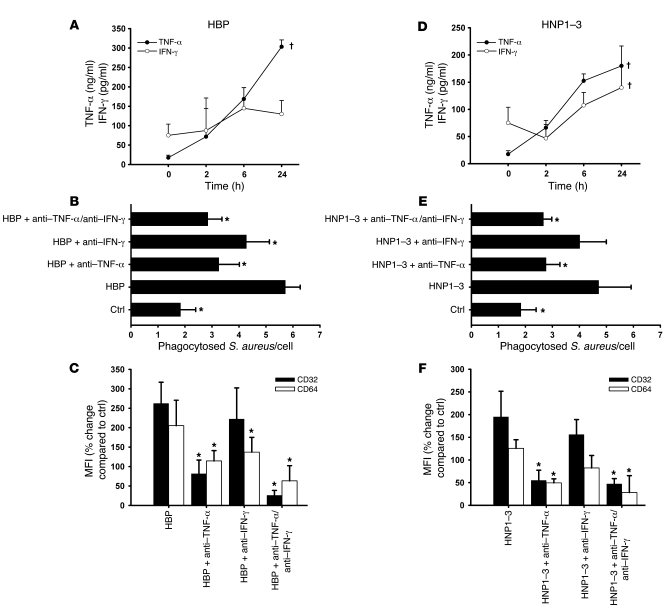
The classic activation of macrophages is initiated by TNF-α and IFN-γ (16). We therefore investigated whether treatment with HBP or HNP1–3 induces release of these cytokines from macrophages. Both HBP and HNP1–3 stimulated release of TNF-α and, to a smaller extent, IFN-γ (Figure 8, A and D). Blocking antibodies to TNF-α significantly reduced the effect of HBP and HNPs in the phagocytosis assay, whereas antibodies to IFN-γ had minor effects (Figure 8, B and E). Reduced phagocytosis in response to anti–TNF-α was related to a reduced CD32 and CD64 expression, while anti–IFN-γ exerted effects on the CD64 expression only (Figure 8, C and F). In control experiments, we counterstained the cells with the DNA-binding dye DAPI to adjust for the cell number. Here we reached identical results (Supplemental Figure 5). Antibodies to TNF-α also reduced the HBP-mediated enhancement of HLA class II, CD40, and CD86 expression (Supplemental Figure 6, A and B). As for the expression of CD32 and CD64, we investigated the involvement of specific signaling pathways in the release of TNF-α and IFN-γ upon stimulation with HBP or HNP1–3. The HBP-induced cytokine release was clearly prevented in the presence of the CD18 antibody IB4 and inhibitors to tyrosine kinases and MAP kinases, while none of these treatments reduced the HNP1–3–mediated release of TNF-α or IFN-γ (data not shown). Furthermore, treatment with siRNA to CD64 blocked the enhanced expression of this receptor in response to HBP, while levels of TNF-α and IFN-γ remained elevated (data not shown).
Figure 8. Involvement of TNF-α and IFN-γ in the HBP- and HNP1–3–mediated enhanced phagocytosis.
(A and D) Human macrophages were treated with (A) HBP or (D) HNP1–3 and the concentration of TNF-α and IFN-γ was determined by ELISA at different time points. For each analysis, 4 independent experiments were performed. †P < 0.05 indicates significant effect of treatment. (B and E) The contribution of TNF-α and IFN-γ was assessed in the phagocytosis assay after stimulation of human macrophages with (B) HBP or (E) HNP1–3 by addition of neutralizing antibodies to TNF-α (5 μg/ml) or IFN-γ (10 μg/ml). For each analysis, 4 independent experiments were performed. *P < 0.05 versus HBP or HNP1–3 treatment groups. (C and F) Importance of TNF-α and IFN-γ for the upregulation of CD32 and CD64 on human macrophages in response to (C) HBP or (F) HNP1–3 evaluated through use of neutralizing antibodies. Receptor expression is assessed by immunofluorescence staining and displayed as percent change compared with control. For each analysis, 5 independent experiments were performed. *P < 0.05 versus HBP or HNP1–3 treatment groups.
Discussion
Monocytes and macrophages are important players in the immune response and constitute together with PMNs the first line of defense in bacterial infections. PMNs contribute to antimicrobial activity through a variety of mechanisms, including release of antimicrobial polypeptides (26). Here we identify what we believe to be a novel antimicrobial mechanism of PMN-derived granule proteins enhancing bacterial phagocytosis by macrophages. When reaching the site of infection, PMNs release HBP and HNP1–3 from their primary granules. These accumulate in the tissue and activate adjacent macrophages in a paracrine manner. HBP was found to exert its effect by activation via β2 integrins, whereas HNP1–3 act via a yet undefined receptor. Macrophage activation leads to phenotypic changes, representing the pattern of classical macrophage activation, including increased expression of CD32 and CD64. The induction of the 2 FcγRs relates to enhanced phagocytosis of IgG-opsonized bacteria. This functional response is essentially mediated through autocrine stimulation via TNF-α and IFN-γ triggered by HBP and HNP1–3.
HNP1–3 were originally isolated as endogenous peptide antibiotics from the primary granules of human PMNs (27). Besides their antimicrobial activities, a number of innate and adaptive immune responses have been attributed to HNPs released from PMNs (2). HNPs were shown to opsonize several different bacterial strains, which may lead to enhanced phagocytosis (28). Since the interval between treatment of macrophages with HNP1–3 and addition of bacteria in our study was 24 hours and enhanced phagocytosis was observed even in the absence of PMN-sec, we concluded that the activity of HNPs in our model, as well as that of HBP, can be attributed to an effect on the macrophages rather than on the bacteria. Our findings indicate that HNPs directly activate macrophages, and rapid mobilization of intracellular Ca2+ suggests binding of HNPs to a receptor. In our study, macrophage activation was not inhibited by treatment with the P2Y-selective inhibitor suramin, which was reported to block HNP-mediated Il-8 release (25), or by pretreatment with PTx, indicating that there is an additional receptor for HNPs on macrophages.
Like HNPs, HBP has primarily been identified as an antimicrobial PMN-derived protein with potent activity against Gram-negative bacteria (29). HBP is known to be localized in both secretory vesicles and primary granules (30), and therefore it may seem surprising that no activity was detected in the secretory vesicle fraction. However, this granule subset is easily mobilized even after mechanical irritations such as the PMN isolation procedure. Therefore it is not unexpected that ELISA measurements showed much lower HBP concentrations in the secretory vesicle fraction compared with the primary granule fraction. By increasing the amount of secretory vesicle fraction added to the macrophages we could detect an enhanced phagocytic activity also in this fraction. Besides its direct antimicrobial activity, HBP acts as an opsonin, which enhances the uptake of S. aureus by monocytes (31). Like for HNPs, this mechanism is unlikely to play a role in our study since macrophages are exposed to recombinant HBP or PMN-sec well in advance of the exposure to bacteria, at which time HBP is likely internalized (32). Furthermore, the enhancing effect on phagocytosis persists even after the protein has been washed away prior to incubation with the bacteria. Thus, HBP has to act directly on the macrophages as indicated by a rapid Ca2+ mobilization. In line with the mechanism proposed by our data, an enhanced phagocytosis of zymosan by microglial cells in response to HBP was recently shown (33). This response was associated with enhanced expression of MHC class II (33), which is in agreement with the upregulation of HLA class II in our study. The significance of our finding is further highlighted by observations in a murine disease model, in which it is reported that i.p. application of HBP before induction of fecal peritonitis significantly increased the survival of the mice as well as phagocytosis of bacteria by peritoneal macrophages (34).
A critical finding of our study is the identification of β2 integrins as the functional receptor for HBP on monocytes. Cai et al. first described the binding of HBP to β2 integrins (23). We later demonstrated that monocyte activation in response to HBP is blocked by an antibody to CD18 (24). However, we now provide data that go beyond the activation of monocytes and macrophages and show that a physiological effect, the upregulation of CD32 and CD64 on macrophages, is blocked by pretreatment with a mAb to CD18, suggesting β2 integrins to be the HBP receptor. This was also confirmed in experiments where CD18-deficient macrophages were used. Similar to HBP, elastase and proteinase-3 interact with β2 integrins, enhancing adhesion (23, 35). However, it is not known if this interaction merely mediates changes in the cytoskeleton or if signaling events also induce gene transcription. In our study, however, elastase and proteinase-3 did not enhance phagocytosis.
The prototypic soluble activator of macrophages function is IFN-γ. IFN-γ is best known for induction of antimicrobial effector pathways and in conjunction with TNF-α as a second signal (either from exogenous sources or, more physiologically, as a result of TLR ligation) for a classical activation of macrophages, resulting in enhanced expression of MHC class II antigens, FcγRs, CD86, and CD40 and the release of proinflammatory cytokines (16). The CD40 and CD86 molecules on the macrophages surface function as costimulatory receptors during the MHC class II/T cell receptor–based interaction with T helper lymphocytes. Here we show an activation pattern that resembles that of the classical activation of macrophages. However, HBP and HNP1–3 are not known to activate macrophages via TLRs, which is in agreement with the conserved activity of HBP and HNP1–3 in MyD88- and IRF3-deficient mice. Also, PMN granules do not contain TNF-α or IFN-γ and are therefore not readily released (36). Nevertheless, both TNF-α and IFN-γ can be produced and released by PMNs during microbial stimulation, but it is unlikely that freshly isolated PMNs will release sufficient amounts of TNF-α and IFN-γ to induce a classical activation of macrophages. Instead, we show that HBP and HNP1–3 activate macrophages in a paracrine manner to produce TNF-α and IFN-γ themselves. In this context, both HBP and HNP1–3 were shown to induce TNF-α production by monocytic cells (32, 37). Interestingly, the activation pattern of macrophages by PMN-sec demonstrated here is similar to what has earlier been described for dendritic cells (38), which was in part due to the release of TNF-α.
PMN-derived antimicrobial proteins contribute to bacterial clearance by several mechanisms. They exert direct antimicrobial activity (26) or they opsonize bacteria, allowing a more efficient phagocytosis (28, 31). A third antimicrobial mechanism of PMN granule proteins was recently identified by Tan et al., who showed that the transfer of PMN granule proteins from apoptotic PMNs to macrophages contributes to enhanced intracellular killing of M. tuberculosis (39). Here we identify what we believe to be a novel mechanism of bacterial clearance involving HBP and HNP1–3. These 2 PMN-derived granule components directly activate the macrophages and strongly enhance its phagocytic activity, which we believe is of potential value in designing new antimicrobial strategies.
Methods
Cell culture.
Human PMNs and monocytes were isolated as described previously (8). Whole blood for isolation of PMNs and buffy coats (Blood center, Karolinska Hospital) for isolation of monocytes were obtained with informed consent from healthy blood donors. Approval for experiments on human subjects was given by the Regional Ethical Committee, Stockholm. Differentiation of monocytes to macrophages was achieved by plating monocytes on cell culture dishes and subsequent culture over 8 days in M199 (Invitrogen), containing 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). siRNA SMARTpool for FcγR1 was purchased from Dharmacon and transfection was performed as described by the manufacturer. GenBank accession numbers and sequences are listed in Supplemental Table 4. CD32 and CD64 expression was analyzed in the presence of various signaling inhibitors: emetine (100 μM; Sigma-Aldrich), anisomycin (1 μM; Sigma-Aldrich), IB4 (10 μg/ml; a gift from S.D. Wright, Rockefeller University, New York, New York, USA), PTx (100 ng/ml; Gibco), genistein (100 μM; Calbiochem), wortmannin (200 μM; Calbiochem), GF109203 (5 μM; Calbiochem), PD98059 (20 μM; Calbiochem), U73122 (5 μM; Calbiochem), herbimycin (1 μM; Calbiochem).
Preparation of PMN-sec.
PMNs were activated by antibody cross-linking of CD18 as described before (8). PMNs were sedimented and the supernatant containing PMN-sec was collected, filtered, and stored at –70°C until use. Granule release in response to cross-linking of β2 integrins was monitored by Western blot and MPO analyses. In some experiments, PMN-sec was either heat inactivated for 15 minutes at 60°C or digested with pepsin.
Phagocytosis assay.
Fluorescent S. aureus and E. coli as well as opsonizing reagent (Molecular Probes) were reconstituted as indicated by the manufacturer. IgG opsonization was achieved according to the manufacturer’s instructions. Complement opsonization was attained by incubation of bacteria with fresh human serum at 37°C for 1 hour. Opsonized particles were washed and seeded onto macrophages cultured in a 96-well plate at a ratio of 10 bacteria per cell for 1 hour at 37°C. Thereafter, trypan blue was added and cells were fixed. The amount of fluorescent particles per cell was quantified by a blinded observer using fluorescence microscopy (Nikon TE300), counting 2 random microscopic fields per well.
Anti-CD16 (MEM-154; BioVendor), anti-CD32 (AT10; GeneTex), and anti-CD64 (10.1; ImmunoTools) were added to the macrophages at 10 μg/ml 30 minutes before addition of IgG-opsonized bacteria. Anti–TNF-α (5 μg/ml; Mab1; BioLegend) and anti–IFN-γ (10 μg/ml; NIB42; BioLegend) were added 30 minutes before stimulation.
The following proteins were used at the indicated concentrations to stimulate human macrophages for 24 hours before accomplishment of the phagocytosis assay: elastase (Costa Mesa), proteinase-3 (Wieslab), HNP1–3 (purified from human PMN; ref. 40), lysozyme (Sigma-Aldrich), cathepsin G (Sigma-Aldrich), MPO (Green Corporation), and HBP (produced as described in ref. 41; gift from H. Flodgaard, Leukotech A/S, Copenhagen, Denmark).
Bacterial killing assay.
Macrophages were incubated with S. aureus (Newman) or E. coli (D21). After 1 hour, extracellular bacteria were washed off by extensive rinsing. Macrophages were disrupted by hypotonic lysis at 0, 1, 4, and 24 hours, releasing ingested bacteria. Viable bacteria were then grown on Luria Bertani agar over night and the colonies enumerated.
Peritonitis model.
Wild-type BALB/c or C57BL/6 mice as well as gene-targeted CD18–/–, MyD88–/–, and IRF3–/– mice (a gift from P. Liljeström, Karolinska Institute, Stockholm, Sweden) were used for in vivo experimentation. All genetically modified animals were on C57BL/6 background. Mice were of either sex, weighed 20–25 g, and were between 4 and 8 weeks old. Thioglycollate broth was injected i.p. and peritoneal macrophages were harvested and isolated after 4 days as described (42). PMN depletion was performed by daily i.p. injections of 250 μg RB6-8C5 mAb (a gift from A.G. Rothfuchs, NIH, Bethesda, Maryland, USA) starting 1 day before thioglycollate injection. Such treatment induces a rapid and selective depletion of PMNs (9, 36, 43). Controls were injected with isotype-matching antibody. To monitor neutropenia (<500 PMN/μl and <20% of basal value) peripheral blood cells were counted manually and differentiated using FACS. PMN-sec (500 μl) was injected i.p. daily. HNP1–3 (2 μg/mouse) or HBP (10 μg/mouse) were injected i.p. 48 and 24 hours before peritoneal macrophages were harvested. Peritoneal macrophages were seeded onto a 96-well plate (105 cells/well) and the phagocytosis assay was performed. The fluorescence intensity reflecting the number of phagocytosed bacteria was quantified in a plate reader (Fluoroskan Ascent; Labsystems). The number of macrophages and PMNs in the peritoneal lavage fluid was analyzed by FACS based on staining with anti-Gr1 (BioLegend), anti-CD45 (Serotec), and anti-F4/80 (Serotec). The activity of MPO and MMP-9 in the lavage fluid was determined using specific substrates (9, 36).
Peritonitis experiments with male Sprague-Dawley rats (500–550 g) were performed as described for mice. PMN depletion was achieved by injection of anti-PMN serum (1 ml/kg; Cedarlane). Injections with HNP1–3 (20 μg/rat) and HBP (100 μg/rat) and subsequent analysis of the phagocytic capacity of peritoneal macrophages was conducted as described for mice. All animal experiments were approved by Stockholm North Animal Ethics Committee.
Ca2+-mobilization.
Macrophages were cultured in a 96-well plate and labeled (37°C, 30 minutes) with the Ca2+-sensitive fluorophore fluo-4/AM (Molecular Probes) as described (8). Cells were then subjected to HBP, HNP1–3, or sham treatment. The fluorescence intensity was recorded in a plate reader before and every 30 seconds until 300 seconds after stimulation.
Protein enrichment and HPLC.
Proteins in the PMN-sec were enriched with an OASIS column (Waters). After addition of trifluoroacetic acid (TFA) to the PMN-sec to a final concentration of 0.1% TFA, the solution was centrifuged. The supernatant was then loaded onto an OASIS 1-cc column (Waters), activated by acetonitrile (ACN), and equilibrated in 0.1% aqueous TFA. The column was washed with 0.1% TFA and proteins were eluted with 80% ACN in 0.1% TFA followed by lyophilization. The lyophilized material was redissolved in 0.1% TFA and protein content was quantified.
Proteins and peptides were separated by HPLC using an ÄKTA purifier system (Amersham Pharmacia Biotech). Reversed-phase chromatography was performed on a Vydac C18 column (Separations Group) equilibrated in 0.1% TFA at a flow rate of 1 ml/min. A gradient of 0%–30%, 30%–60%, and 60%–80% in 37 minutes, 30 minutes, and 12 minutes, respectively, was employed, and the effluent was monitored at 214 nm. The collected fractions were lyophilized and used for further analyses.
Amino acid sequence analysis.
To confirm the identity of HNP1–3, N-terminal sequence analysis was employed. Peptides were first reduced with 1 mM DTT in 500 mM ammonium bicarbonate (Ambic) at 37°C for 2 hours. Thereafter, they were alkylated with 10 mM iodoacetamide in 500 mM Ambic for 15 minutes and applied onto Applied Biosystems Prosice cLC sequencer (PE Applied Biosystems).
Trypsin digestion and peptide mass fingerprinting.
The SDS-PAGE of fraction 60–62 used for Western blot was Coomassie stained after the transfer step. A visualized band of 27 kDa was manually excised and proteins were in-gel digested with trypsin, using the MassPREP robotic protein handling system (Waters). The gel plug was destained twice with 100 μl of 50 mM Ambic in 50% (volume/volume) ACN at 40°C for 10 minutes. Thereafter protein(s) were reduced with 10 mM DTT in 100 mM Ambic for 30 minutes, alkylated with 55 mM iodoacetamide in 100 mM Ambic for 20 minutes, and dehydrated in ACN. A solution of 25 μl trypsin (12 ng/μl, Promega) in 50 mM Ambic was added and digestion was carried out for 5 hours at 40°C. Peptides were extracted with 30 μl 1% formic acid/2% ACN, followed by extraction with 2 × 15 μl 50% ACN. The molecular weight of the obtained fragments was determined with MALDI-MS and polypeptides were identified by database searches using the ProteinProspector MS-Fit program ( http://prospector.ucsf.edu).
MALDI-MS.
Samples from the HPLC fraction 46 and the digested protein extracted from the gel piece were mixed (1:1) with matrix (saturated α-cyano-4-hydroxy-cinnamic-acid in ACN containing 0.1% TFA) on a target plate and left to dry. The peptides were then analyzed for their mass values with MALDI-MS using an Applied Biosystems Voyager DE-PRO instrument (Foster City).
Immunocytochemistry.
Human macrophages were cultured in 96-well plates and stimulated with PMN-sec for 24 hours. Thereafter, the cells were washed and incubated with 0.5% BSA in PBS for 15 minutes. Cell surface staining was carried out applying fluorescently labeled mAbs for 30 minutes at room temperature directed against the following antigens: CD36, CD80, CD86, HLA class II, CD40, CD25, CD14, CD11c, CD35, CD32, and CD64 (all FITC-labeled, ImmunoTools); and FITC-CD62L, PE-CD11b, and PE-CD16 (all Becton Dickinson). After staining, macrophages were washed and the fluorescence intensity was analyzed in a fluorescence plate reader. In selected experiments, macrophages were counterstained with DAPI (300 nM, 2 min, in PBS) to adjust for the number of cells in the 96-well dish.
Subcellular fractionation of PMNs.
Subcellular fractionation of PMN was performed by density centrifugation on Percoll gradients as described before (17). Using a peristaltic pump, 1-ml fractions were collected by aspiration from the bottom of the tube. To remove Percoll, fractions were centrifuged for 90 minutes at 100,000 g in an ultracentrifuge (L-60; Beckman Instruments Inc.) at 4°C.
Western blot and dot blot analyses.
Localization of subcellular organelles in the fractions obtained by subcellular fractionation was determined by analysis for markers specific for certain granules. MPO was quantified as previously described (36). Relative amounts of CD35, MMP-9, and lactoferrin in the fractions were analyzed by SDS-PAGE and Western blotting as described before (44). Western blot analysis was also used to investigate the presence of HBP in fraction 60–62 and to monitor release of PMN granules after β2 integrin cross-linking. The following antibodies were used: anti–MMP-9 (1:500; Sigma-Aldrich), anti-lactoferrin (1:500; Sigma-Aldrich), anti-CR1 (1:200; ImmunoTools), anti-HBP (1:200; a gift from Hans Flodgaard), and anti-albumin (1:500; DAKO).
All chromatographic fractions were screened for the presence of HNP1–3 and HBP with dot blot analyses. The 1-μl samples were spotted onto a membrane (Amersham Biosciences) and left to dry. For the HNP1–3–dot blot, the membrane was fixed with 0.05% glutaraldehyde for 30 minutes, followed by washing for 3 × 10 minutes. Thereafter, the membranes were treated in the same manner as for the PVDF membranes described above.
Immunoabsorption of HNP1–3 and HBP.
Monoclonal HNP1–3 antibody or monoclonal HBP antibody were coupled to Protein A Sepharose (CL-4B; Amersham Pharmacia Biotech). After PMN-sec was precleared with Protein A Sepharose the antibody/Protein A Sepharose complexes were incubated with the PMN-sec under gentle rotation over night at 4°C. The beads were spun down, and the efficacy of immunoadsorption was verified with Western blot for HBP and dot blot for HNP1–3.
ELISA.
Concentrations of TNF-α and IFN-γ (eBioscience) were analyzed in the poststimulatory cell-free supernatant using a commercial ELISA kit following the manufacturer’s instruction. HBP concentrations were determined by ELISA as described before (30).
Statistics.
The statistical calculations were done using Statistica 7.1 (Statsoft Inc.). Data were tested for normality and are expressed as mean ± SD. Data in Figure 6, B and F, were analyzed with 2-way repeated-measures ANOVA (treatment × time) followed by planned comparisons. Data in Figure 5B, Figure 6, A and C, and Figure 8, A and D, were analyzed using 1-way ANOVA followed by Tukey’s HSD test. All other data were analyzed with t test for independent samples followed by Bonferroni correction for multiple comparisons when appropriate. A P value of < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This study was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, the AFA Health Fund, the Torsten and Ragnar Söderbergs Foundations, and the Lars Hierta Memorial Fund. O. Söhnlein is a recipient of a post-doc grant from the Deutsche Forschungsgemeinschaft (SO 876/1-1). The authors would like to thank Megan Osler for critical reading and Ella Cederlund, Marie Ståhlberg, and Monica Heidenholm for excellent technical assistance.
Footnotes
Nonstandard abbreviations used: ACN, acetonitrile; Ambic, ammonium bicarbonate; FcγRI, Fcγ receptor I; HBP, heparin-binding protein; HNP1–3, human neutrophil peptides 1–3; MALDI-MS, MALDI–mass spectrometry; MPO, myeloperoxidase; PMN, polymorphonuclear cell; PMN-sec, PMN secretion; TFA, trifluoroacetic acid.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3491–3502 (2008). doi:10.1172/JCI35740
References
- 1.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. . 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 2.Chertov O., Yang D., Howard O.M., Oppenheim J.J. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol. Rev. . 2000;177:68–78. doi: 10.1034/j.1600-065X.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N., Cowland J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 4.Shiohara M., et al. Phenotypic and functional alterations of peripheral blood monocytes in neutrophil-specific granule deficiency. J. Leukoc. Biol. 2004;75:190–197. doi: 10.1189/jlb.0203063. [DOI] [PubMed] [Google Scholar]
- 5.Tavor S., et al. Macrophage functional maturation and cytokine production are impaired in C/EBP epsilon-deficient mice. Blood. 2002;99:1794–1801. doi: 10.1182/blood.V99.5.1794. [DOI] [PubMed] [Google Scholar]
- 6.Doherty D.E., Downey G.P., Worthen G.S., Haslett C., Henson P.M. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab. Invest. 1998;59:200–213. [PubMed] [Google Scholar]
- 7.Pereira H.A. CAP37, a neutrophil-derived multifunctional inflammatory mediator. J. Leukoc. Biol. 1995;57:805–812. doi: 10.1002/jlb.57.6.805. [DOI] [PubMed] [Google Scholar]
- 8.Soehnlein O., et al. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J. Immunol. . 2005;174:6399–6405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 9.Soehnlein O., et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertov O., et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J. Exp. Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer L.T., et al. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L514–L520. doi: 10.1152/ajplung.00099.2003. [DOI] [PubMed] [Google Scholar]
- 12.Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. . 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. . 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 14.Barrington R., Zhang M., Fischer M., Carroll M.C. The role of complement in inflammation and adaptive immunity. Immunol. Rev. . 2001;180:5–15. doi: 10.1034/j.1600-065X.2001.1800101.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderson C.L., Shen L., Eicher D.M., Wewers M.D., Gill J.K. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J. Exp. Med. . 1990;171:1333–1345. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bogdan, C. 2006. Macrophages. InEncyclopedia of life sciences. John Wiley & Sons, Ltd. Chichester, United Kingdom. http://www.els.net. doi:10.1038/npg.els.0004007 . [Google Scholar]
- 17.Kjeldsen L., Sengelov H., Borregaard N. Subcellular fractionation of human neutrophils on Percoll density gradients. J. Immunol. Methods. . 1999;232:131–143. doi: 10.1016/S0022-1759(99)00171-4. [DOI] [PubMed] [Google Scholar]
- 18.Sun R., et al. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J. Immunol. . 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 19.Territo M.C., Ganz T., Selsted M.E., Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro-Gomes F.L., et al. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 2007;179:3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 21.Grigat J., Soruri A., Forssmann U., Riggert J., Zwirner J. Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human {alpha}-defensin Family. J. Immunol. . 2007;179:3958–3965. doi: 10.4049/jimmunol.179.6.3958. [DOI] [PubMed] [Google Scholar]
- 22.Yount N.Y., et al. Rat neutrophil defensins. Precursor structures and expression during neutrophilic myelopoiesis. J. Immunol. 1995;155:4476–4484. [PubMed] [Google Scholar]
- 23.Cai T.Q., Wright S.D. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. J. Exp. Med. . 1996;184:1213–1223. doi: 10.1084/jem.184.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Påhlman L.I., et al. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J. Immunol. . 2006;177:1221–1228. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- 25.Khine A.A., et al. Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood. . 2006;107:2936–2942. doi: 10.1182/blood-2005-06-2314. [DOI] [PubMed] [Google Scholar]
- 26.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 2004;76:909–925. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 27.Ganz T., et al. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischmann J., Selsted M.E., Lehrer R.I. Opsonic activity of MCP-1 and MCP-2, cationic peptides from rabbit alveolar macrophages. Diagn. Microbiol. Infect. Dis. . 1985;3:233–242. doi: 10.1016/0732-8893(85)90035-5. [DOI] [PubMed] [Google Scholar]
- 29.Shafer W.M., Martin L.E., Spitznagel J.K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect. Immun. . 1984;45:29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapper H., Karlsson A., Morgelin M., Flodgaard H., Herwald H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood. 2002;99:1785–1793. doi: 10.1182/blood.V99.5.1785. [DOI] [PubMed] [Google Scholar]
- 31.Heinzelmann M., Platz A., Flodgaard H., Miller F.N. Heparin binding protein (CAP37) is an opsonin for Staphylococcus aureus and increases phagocytosis in monocytes. . Inflammation. 1998;22:493–507. doi: 10.1023/A:1022398027143. [DOI] [PubMed] [Google Scholar]
- 32.Heinzelmann M., Mercer-Jones M.A., Flodgaard H., Miller F.N. Heparin-binding protein (CAP37) is internalized in monocytes and increases LPS-induced monocyte activation. J. Immunol. 1998;160:5530–5536. [PubMed] [Google Scholar]
- 33.Pereira H.A., Ruan X., Kumar P. Activation of microglia: a neuroinflammatory role for CAP37. Glia. 2003;41:64–72. doi: 10.1002/glia.10167. [DOI] [PubMed] [Google Scholar]
- 34.Heinzelmann M., Mercer-Jones M.A., Peyton J., Flodgaard H., Cheadle W.G. Heparin binding protein increases survival in murine fecal peritonitis. Crit. Care Med. 2000;28:2926–2931. doi: 10.1097/00003246-200008000-00040. [DOI] [PubMed] [Google Scholar]
- 35.David A., Kacher Y., Specks U., Aviram I. Interaction of proteinase 3 with CD11b/CD18 (beta2 integrin) on the cell membrane of human neutrophils. J. Leukoc. Biol. 2003;74:551–557. doi: 10.1189/jlb.1202624. [DOI] [PubMed] [Google Scholar]
- 36.Soehnlein O., et al. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur. Respir. J. 2008;32:405–412. doi: 10.1183/09031936.00173207. [DOI] [PubMed] [Google Scholar]
- 37.Chaly Y.V., et al. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. . 2000;11:257–266. [PubMed] [Google Scholar]
- 38.Megiovanni A.M., et al. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J. Leukoc. Biol. 2006;79:977–988. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 39.Tan B.H., et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 40.Faurschou M., Sorensen O.E., Johnsen A.H., Askaa J., Borregaard N. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim. Biophys. Acta. 2002;1591:29–35. doi: 10.1016/S0167-4889(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen P.B., et al. Characterization of recombinant human HBP/CAP37/azurocidin, a pleiotropic mediator of inflammation-enhancing LPS-induced cytokine release from monocytes. FEBS Lett. 1996;390:109–112. doi: 10.1016/0014-5793(96)00639-4. [DOI] [PubMed] [Google Scholar]
- 42.Davies J.Q., Gordon S. Isolation and culture of human macrophages. Methods Mol. Biol. 2005;290:105–116. doi: 10.1385/1-59259-838-2:105. [DOI] [PubMed] [Google Scholar]
- 43.Zernecke A., et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 44.Soehnlein O., et al. ACE inhibition lowers angiotensin-II-induced monocyte adhesion to HUVEC by reduction of p65 translocation and AT 1 expression. J. Vasc. Res. . 2005;42:399–407. doi: 10.1159/000087340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.