Nano- and micrometer-sized particles of organic polymers and inorganic materials play an important role in many applications, including catalysis, optics, biosensing, and data storage.1 As an alternative, our group and others recently have developed synthetic approaches for the preparation of amorphous particles from infinite metal-organic coordination polymers, which are based on the coordination chemistry of late transition metal ions and polydentate organic building blocks.2,3 The infinite coordination polymer (ICP) particles offer a high degree of tailorability through the choice of transition metal connectors and predefined functional organic precursors which have useful chemical and physical properties.2,3 However, the amorphous nature of ICP particles, in contrast to the well-defined crystalline metal-organic frameworks (MOFs),4 prohibit a detailed understanding of the structural connectivity and transition metal coordination environments of these systems due to their inability to diffract X-rays. Herein, we wish to report a new class of salen-based homochiral ICP particles and the discovery that judicious choice of solvent can be used to drive the amorphous spherical particles into rod-shaped crystalline structures that can be fully characterized by X-ray crystallography. These data provide key structural information about the likely molecular connectivity of the precursors that make up the amorphous ICP microparticles.
Acid-functionalized salen ligands were synthesized according to literature procedures and used for the preparation of ICP particles and crystalline rods.5 Spherical particles 2a and 2b were prepared by slow diffusion of diethyl ether into a precursor pyridine solution consisting of a 1:1 mixture of Ni(OAc)2·4H2O and metallo-salen precursors, H2-1a and H2-1b, respectively (Scheme 1).
Scheme 1.
Synthesis of Salen-Based Microparticles and Their Dynamic Solvent-Triggered Crystallization Process: (a) Pyridine/Ether, (b) Pyridine, (c) MeOH, (d) Pyridine/MeOH, (e) Pyridine
The particle formation reaction is completely reversible, as evidenced by the formation of a clear solution upon addition of excess pyridine to the particles in their dried form.2 1H NMR and UV-vis spectra confirm the re-formation of the metallo-salen precursors. The morphology of the microscale ICP particles was characterized by optical microscopy (OM) and field-emission scanning electron microscopy (FE SEM) (Figure 1). The images show spherical structures with an average diameter of 2.69 ± 0.13 and 5.70 ± 0.84 μm for 2a and 2b, respectively. The particle size was further studied by dynamic light scattering (DLS), and the DLS-determined mean particle size values of 2.46 μm for 2a and 5.52 μm for 2b are in good agreement with the SEM determined values. The chemical compositions of 2a and 2b were characterized by energy dispersive X-ray (EDX) spectroscopy and elemental analyses and are consistent with formation of the 1:1 structure derived from the metallo-salen and metal ion precursors.
Figure 1.
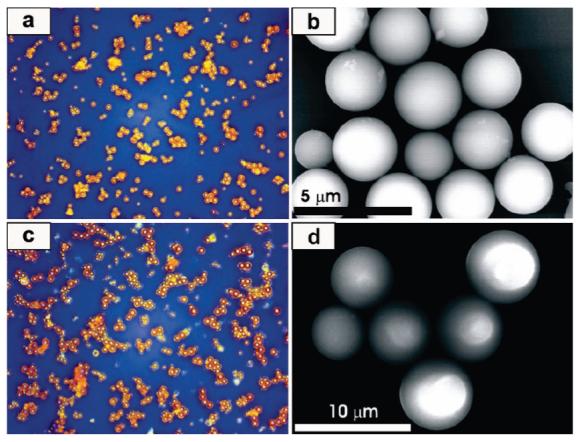
OM and SEM images of spherical ICP particles: (a) and (b) for 2a, (c) and (d) for 2b.
Infrared spectra taken of the microparticles show that the carboxylate groups are coordinated to metal ions, as evidenced by a shift of the carboxylate stretching frequency from 1683 cm-1 in the protonated metallo-salen precursor H2-1a to 1531 (νanti) and 1437 cm-1 (νsym) for 2a. These values compare very well with the stretching frequencies for Ni(OAc)2·4H2O at 1535 and 1422 cm-1, consistent with η1-coordination of the carboxylate groups to the Ni centers through their anionic O atoms. The IR spectra of ligand H2-1b and microparticle 2b show similar trends.
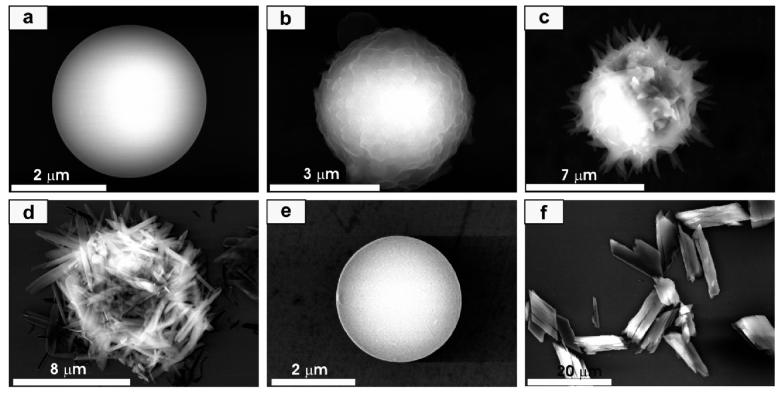
Interestingly, when microparticles 2a and 2b were completely dried under vacuum, they retained their spherical shapes, but when methanol was added to them, they transformed in the solid state into crystalline rod-shaped structures. Note the particles and the rod-shaped products are not soluble in methanol. This solid-state transformation could be followed by analyzing samples taken from the reaction mixture as a function of time over a 60 min time period (Figure 2). The initial microparticles 2a have a smooth surface (Figure 2a). However, in the presence of methanol, the surface morphology of the particle becomes ruffled as the solvent begins to penetrate the particle (Figure 2b). Then, sharp needles begin to grow from the surface (Figure 2c), and eventually the particles turn into crystalline rods 3a (Figure 2d).
Figure 2.
Representative SEM images monitoring the transformation of microparticles 2a to crystalline rods 3a. (a) Particle 2a grown from the precursor solution, (b) early intermediate (5 min), (c) intermediate at a later stage (30 min), (d) crystalline rods 3a, (e) particle grown by first dissolving the crystalline rods 3a with pyridine and then exposing to ether by vapor diffusion, and (f) crystalline products 3a grown from the pyridine precursor 1a solution, Ni(OAc)2·4H2O, and methanol.
Interestingly, the crystalline rods 3a can be transformed into the microparticles 2a again by first dissolving the rods in pyridine and then slowly diffusing ether into the solution (Figure 2e). The dissolution of the crystalline rods with pyridine results in the reformation of the salen and metal ion precursors as confirmed by 1H NMR and UV-vis spectroscopy. Taken together, these processes make the entire cycle between precursors, microparticles, and rodshaped crystalline structures pseudo-reversible (Scheme 1). In other words, the crystalline rods do not go back to the amorphous particles without redispersing the metallo-salen and metal ion precursors in solution.
The crystalline product 3a can be prepared directly by slow diffusion of methanol into the pyridine solution of precursors, as well (Figure 2f). However, the crystalline rods made from the amorphous microparticles by the methanol-triggered process are more uniform and have higher aspect ratios than those made from the direct crystallization in pyridine method. We also investigated the IR spectra of the crystalline products 3a and 3b. The carboxylate stretching frequencies of 3a at 1531 and 1439 cm-1 are comparable to those for the amorphous microparticle sample 2a (1531 and 1437 cm-1) and Ni(2,6-DMB)2(py)2(EtOH)2 (1555 and 1400 cm-),6 which suggest that they have similar metal carboxylate connectivities. Compound 3b shows similar trends (Supporting Information).
Significantly, a single crystal of 3a suitable for an X-ray diffraction study was grown by adding methanol to a pyridine solution of the salen and metal ion precursors. X-ray crystallography shows that the structure of 3a consists of a one-dimensional coordination polymer (Figure 3). The Ni ions exhibit a distorted octahedral coordination geometry with two trans-carboxylate (Ni-Oavg = 2.05 Å), two cis-pyridine (Ni-Navg = 2.10 Å), and two cis-methanol (Ni-Oavg = 2.13 Å) groups. The coordination geometry around the Ni is similar to that observed for the model monomeric Ni(py)2(OAc)2(H2O)2 complex.7 The IR and XRD spectra of the single crystal were superimposable with those for crystalline rods of 3a grown from the polymer particles, showing that crystalline materials grown the different ways are chemically identical from composition ratio and connectivity standpoints. Note that the major difference between the ICP spherical particles and crystalline rods is the coordination of methanol to the Ni atoms in the latter structure. This is likely the driving force for the amorphous to crystalline transformation.
Figure 3.
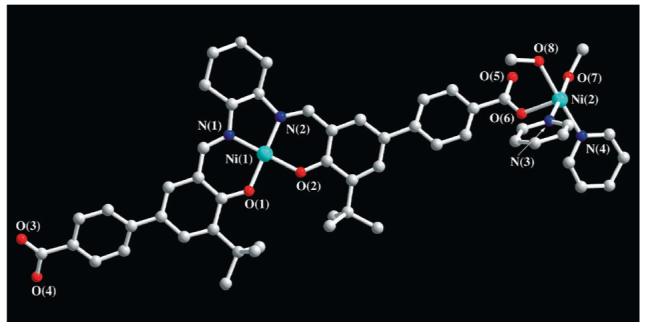
X-ray structure of crystalline 3a (one of the two repeating units: gray, carbon; blue, nitrogen; red, oxygen; sky blue, nickel). Hydrogen atoms and solvent molecules have been omitted for clarity.
In conclusion, this paper describes a new class of homochiral salen-based ICPs and the discovery of a novel and pseudo-reversible solvent-induced crystallization process. The solvent, methanol in this case, induces such a transformation through a change in the coordination environment around the metal bridging the metallosalen portion of the complex. This novel transformation has allowed us to use X-ray methods to structurally characterize the crystalline product and through spectroscopic comparisons better understand the connectivity and structure of the amorphous ICP particle precursor. To the best of our knowledge, this is the first single-crystal X-ray diffraction data for polymeric materials that derive from the amorphous ICPs.
Supplementary Material
Acknowledgment
C.A.M. acknowledges the ONR, NSF, and ARO for supporting this research. He is also grateful for a NIH Director’s Pioneer Award.
Footnotes
E-mail: chadnano@northwestern.edu
References
- (1)(a).Bell AT. Science. 2003;299:1688. doi: 10.1126/science.1083671. [DOI] [PubMed] [Google Scholar]; (b) Blanco A, Chomski E, Grabtchak S, Ibisate M, John S, Leonard SW, Lopez C, Meseguer F, Miguez H, Mondia JP, Ozin GA, Toader O, van Driel HM. Nature. 2000;405:437. doi: 10.1038/35013024. [DOI] [PubMed] [Google Scholar]; (c) Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]; (d) Cao YC, Jin R, Mirkin CA. Science. 2002;297:1536. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]; (e) Sun S, Murray CB, Weller D, Folks L, Moser A. Science. 2000;287:1989. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]; (f) Wang J, Gudiksen MS, Duan X, Cui Y, Lieber CM. Science. 2001;293:1455. doi: 10.1126/science.1062340. [DOI] [PubMed] [Google Scholar]
- (2)(a).Oh M, Mirkin CA. Nature. 2005;438:651. doi: 10.1038/nature04191. [DOI] [PubMed] [Google Scholar]; (b) Oh M, Mirkin CA. Angew. Chem., Int. Ed. 2006;45:5492. doi: 10.1002/anie.200601918. [DOI] [PubMed] [Google Scholar]
- (3)(a).Maeda H, Hasegawa M, Hashimoto T, Kakimoto T, Nishio S, Nakanishi T. J. Am. Chem. Soc. 2006;128:10024. doi: 10.1021/ja0637301. [DOI] [PubMed] [Google Scholar]; (b) Park KH, Jang K, Son SU, Sweigart DA. J. Am. Chem. Soc. 2006;128:8740. doi: 10.1021/ja062907o. [DOI] [PubMed] [Google Scholar]; (c) Sun X, Dong S, Wang E. J. Am. Chem. Soc. 2005;127:13102. doi: 10.1021/ja0534809. [DOI] [PubMed] [Google Scholar]
- (4)(a).Bradshaw D, Claridge JB, Cussen EJ, Prior TJ, Rosseinsky MJ. Acc. Chem. Res. 2005;38:273. doi: 10.1021/ar0401606. [DOI] [PubMed] [Google Scholar]; (b) Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]; (c) Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. doi: 10.1038/35010088. [DOI] [PubMed] [Google Scholar]
- (5).Jeon Y-M, Heo J, Mirkin CA. Tetrahedron Lett. 2007;48:2591. [Google Scholar]
- (6).Erre LS, Micera G, Gulinati B, Cariati F. Polyhedron. 1992;11:101. [Google Scholar]
- (7).Drew J, Hursthouse MB, Thornton P. J. Chem. Soc., Dalton Trans. 1972:1658. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.