Abstract
When proliferating fission yeast cells are exposed to nitrogen starvation, they initiate conjugation and differentiate into ascospores. Cell cycle arrest in the G1-phase is one of the prerequisites for cell differentiation, because conjugation occurs only in the pre-Start G1-phase. The role of ste9+ in the cell cycle progression was investigated. Ste9 is a WD-repeat protein that is highly homologous to Hct1/Cdh1 and Fizzy-related. The ste9 mutants were sterile because they were defective in cell cycle arrest in the G1-phase upon starvation. Sterility was partially suppressed by the mutation in cig2 that encoded the major G1/S cyclin. Although cells lacking Ste9 function grow normally, the ste9 mutation was synthetically lethal with the wee1 mutation. In the double mutants of ste9 cdc10ts, cells arrested in G1-phase at the restrictive temperature, but the level of mitotic cyclin (Cdc13) did not decrease. In these cells, abortive mitosis occurred from the pre-Start G1-phase. Overexpression of Ste9 decreased the Cdc13 protein level and the H1-histone kinase activity. In these cells, mitosis was inhibited and an extra round of DNA replication occurred. Ste9 regulates G1 progression possibly by controlling the amount of the mitotic cyclin in the G1-phase.
INTRODUCTION
Each cell must duplicate its cellular constituents for proliferation. Two major events in this process are precise replication of the whole genome and accurate transmission of the duplicated genomes to two daughter cells. A typical eukaryotic cell cycle consists of four distinct phases, i.e., G1, S, G2, and M. Among these phases, G1 is most important for strict regulation of cell proliferation in many types of cells. Before the specific duration in the G1-phase called the ’restriction point’ in mammalian cells or the ’Start’ in yeast, a cell determines whether it enters the next cell cycle or ceases proliferation (Hartwell et al., 1974; Nurse and Bissett, 1981; Pardee, 1989). To make this decision, cells in the G1-phase monitor many types of environmental and intracellular information such as the presence or absence of nutrients, completion of the events of the previous cycle, and cell size. When all requirements are fulfilled, the cells override the restraint for the restriction point/Start control and are committed to progression through G1 to the S-phase. In contrast, when environment conditions, such as the absence of essential nutrients or the presence of negative growth factors, are unfavorable for cell proliferation, cells exit the cycling phase and enter into a quiescent G0-phase.
Tumor cells have many characteristics that differ from those of normal cells. The most noticeable one is their uncontrolled proliferation, suggesting that cell cycle regulation is ruined in tumor cells (Sherr, 1996). Tumor cells override the restraint for the “restriction point” even under unfavorable conditions for growth. Indeed, many oncogenic proteins strongly induce cell proliferation. Conversely, most of the tumor suppressor genes encode proteins that restrain progression to the S-phase from G1. Inactivation of these tumor suppressor genes leads to uncontrolled cell multiplication due to lack of the inhibition of G1/S progression. Therefore, cell cycle regulation in the G1-phase is very important to guarantee normal cell proliferation.
Fission yeast, Schizosaccharomyces pombe, has provided a useful model system with which to study these cell cycle controls (Nurse, 1990; Forsburg and Nurse, 1991; MacNeill and Fantes, 1995). There are three alternative fates for the proliferating fission yeast cells in response to environmental situation; they continue to proliferate, enter into a dormant stationary phase, or differentiate into ascospores (Egel, 1989; Su et al., 1996). Upon nitrogen starvation, the cells arrest in the pre-Start G1 period and withdraw from the cycling phase (Costello et al., 1986). In homothallic (h90) strains, haploid vegetative cells of opposite mating types conjugate to form a diploid zygote that subsequently undergoes meiosis and sporulation to differentiate into dormant ascospores. Alternatively, heterothallic cells (h+ or h−) exit from a proliferating phase and maintain quiescence (Costello et al., 1986; Su et al., 1996). Importantly, cells can initiate the conjugation process only in the pre-Start G1-phase (Nurse and Bissett, 1981). Once the cells pass Start, they are committed to a new round of the mitotic cycle and never enter the developmental cycle until they complete mitosis and again arrest in the pre-Start G1 period. Thus, in fission yeast as in other eukaryotes, the regulatory mechanism operates to block cell cycle progression in the G1-phase in response to unfavorable environmental conditions.
The Rum1 protein encodes a critical regulator of the G1-phase in fission yeast (Labib and Moreno, 1995). First, it acts as an inhibitor of the S-phase onset, delaying Start until a cell has attained a critical minimal mass. Second, Rum1 influences the dependence of Start and the S-phase upon completion of the mitosis of the previous cycle. Third, the Rum1 protein defines the cell as being in the pre-Start G1 period and prevents such a cell from undergoing mitosis. As an inhibitor of cdk, Rum1 inhibits cdk activity of the Cdc2/Cdc13 complex strongly and the Cdc2/Cig2 complex weakly (Correa-Bordes and Nurse, 1995). The rum1 gene is not essential for viability, and the rum1 mutant grows normally. However, the rum1 mutant cannot arrest its cell cycle in the pre-Start G1-phase upon nitrogen starvation and is sterile (i.e., mating-deficient) (Moreno and Nurse, 1994).
Although Rum1 is an important regulator of G1-phase progression, our knowledge about the cellular mechanism of G1 arrest upon unfavorable environmental conditions is not enough. To identify other genes involved in this control, we have searched for mutants whose phenotype mimicked the rum1 mutant. In this paper, we provide evidence that the ste9 mutant exhibits defects indistinguishable from those of the rum1 mutant. The Ste9 regulates the G1-phase progression possibly by down-regulating the amount of mitotic cyclin (Cdc13) during G1-phase.
MATERIALS AND METHODS
General Techniques for Yeast
The S. pombe strains used in this study are listed in Table 1. Yeast cells were grown in YE (complete medium), EMM2, or SD (minimal medium). For mating and sporulation, MEA and SSA were used. For liquid culture, EMM2 and its nitrogen-free version (EMM2−N) were used. Standard methods for S. pombe were as described (Gutz et al., 1974; Moreno et al., 1991). Sterile mutants were crossed using protoplast fusion (Kitamura et al., 1990). Transformation of S. pombe cells was done by the lithium acetate method (Okazaki et al., 1990). Viability was monitored by plating and counting the number of colonies after 3–4 d of incubation. To discriminate haploid cells from diploid cells, the cells were plated onto YE medium containing phloxine B (Moreno et al., 1991).
Table 1.
S. pombe strains
| Strain | Genotype |
|---|---|
| L972 | h−s |
| C766-1A | h90 leu1 |
| KJ100-8B | h90 ste9::ura4+ ura4-D18 |
| KJ32-11B | h90 ste9-B36 leu1 |
| KJ153-1A | h90 rum1::ura4+ leu1 ura4-D18 |
| KJ218-5C | h90 ste9::ura4+ cig2::ura4+ ade6-M210 leu1 ura4-D18 |
| KJ57-4C | h90 ste9-B36 cig2-S18 leu1 ura4 |
| KJ192-3A | h90 ste9-59 cig2::ura4+ leu1 ura4-D18 |
| KJ109-5A | h90 wee1-50 cig2::ura4+ ade6-M216 leu1 ura4-D18 |
| KJ111-1A | h90 ste9::ura4+ wee1-50 cig2::ura4+ ade6-M216 leu1 ura4 |
| KJ152-9A | h90 rum1::ura4+ wee1-50 ade6-M210 leu1 ura4 |
| KJ32-2A | h90 cdc10-129 leu1 |
| KJ32-1A | h90 ste9-B36 cdc10-129 leu1 |
| KJ143-1A | h90 rad17d::ura4+ leu1 ura4-D18 |
| 6-695 | h90 ura4-294::int[ura4+, nmt-ste9+] leu1 |
| KJ141-8A | h−s cdc2-M26 |
| KJ124-3A | h−s cdc2-M26 ste9::ura4+ ura4 |
| KJ155-1A | h−s cdc2-M26 chk1::ura4+ ura4 |
| KJ238-7B | h90 ste9-B36 cig2::ura4+ rum1+-3HA ura4-D18 leu1 |
| KJ218-2B | h−s ste9::ura4+ cdc10-V50 ura4-D18 leu1 |
| Sp224 | h90 rum1::ura4+ cdc10-129 ura4-D18 leu1-32 |
| DS9-1 | h90 ste9-B36 his1-102 ade6-M216 + leu1 |
| h+s ste9-B36+ ade6-M216 ura4-D18 leu1 |
Molecular Cloning and Gene Disruption of ste9
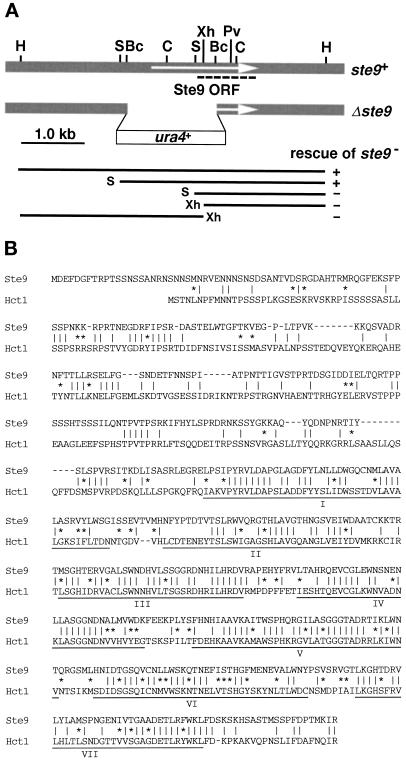
A diploid strain DS9–1, harboring the nonsense ste9-B36 mutation homozygously, was transformed by the S. pombe genomic library and plated onto SSA. Two sporulating colonies were identified by staining with iodine vapor, and plasmids were recovered from both colonies. Restriction mapping revealed that two plasmids contained the overlapping genomic DNA. A 5-kilobase (kb) HindIII fragment (Figure 1A), which was sufficient for rescue of the ste9 mutation, was integrated into a genome of the ste9 mutant. The resultant stable integrant normally conjugated and sporulated as did wild-type cells. Tetrad analysis of this integrant revealed that the integrated HindIII fragment contained the ste9+ gene itself, not a multicopy suppressor. A SalI–HindIII fragment of 3.4 kb, which complemented the ste9 mutation, was cloned into pAL-SK vector (kindly provided by Dr. K. Tanaka), and the resultant plasmid was named pAL(ste9). The nucleotide sequence of ste9+ has been submitted to the DDBJ/EMBL/GenBank databases under accession number AB001285.
Figure 1.
Molecular cloning and characterization of ste9+. (A) Restriction enzyme map of ste9+. The top shaded box indicates the genomic region around ste9+. The arrow indicates the Ste9 open reading frame. Each WD-repeat is indicated by the black box. Restriction sites: H, HindIII; S, SalI; Bc, BclI; C, ClaI; Xh, XhoI; Pv, PvuII. The second line shows the disrupted ste9 allele. The thin lines in the lower part indicate various truncated genomic fragments. Their ability to rescue the ste9 mutation is indicated by (+) and (−). The nucleotide sequence of ste9+ has been submitted to the DDBJ/EMBL/GenBank databases under accession number AB001285. (B) Alignments of Ste9 and Hct1/Cdh1. Identical amino acids between two proteins are indicated by vertical lines (‖). Residues of similar property are indicated by asterisks (*).
Chromosomal disruption of the ste9 gene was carried out by one-step gene disruption (Rothstein, 1983). The BclI–BclI fragment of 1.5 kb was replaced by the S. pombe ura4+ gene (Figure 1A; Grimm et al., 1988). Although deleted construct covered a part of the 5′-untranslated sequence, we confirmed by 5′-rapid amplification of cDNA ends analysis that this region was transcribed to ste9 mRNA. The resultant SalI-HindIII fragment containing the disrupted ste9 allele was used to transform wild-type cells, and stable Ura+ transformants were obtained from haploid and diploid strains. Successful replacement of the wild-type gene by the disrupted allele was confirmed by genomic Southern analysis.
Construction of nmt-ste9+ Gene and Ste9-overexpressing Strain
The coding region of ste9 was amplified by PCR using sense (TGAAGTCAGGGATCCTAACG) and antisense (GAGTGAATGGGATCCATTAC) oligonucleotide primers. To facilitate cloning, BamHI sites (indicated by underlining) were introduced in front of the initiation codon and behind the termination codon. After digestion with BamHI, the amplified DNA was inserted into the BamHI site of pREP1 or pREP2, which contained the thiamine-repressible nmt1 promoter (Maundrell, 1990, 1993). The strain harboring the integrated nmt-ste9+ gene was constructed as follows. One of the resultant plasmids, pREP2(ste9), was digested at the unique AatI site in the ura4+ gene and transformed yeast cells to integrate the linearized plasmid into the genomic ura4–294 locus, after which stable Ura+ integrants were isolated. The cells were maintained in the presence of 5 μg/ml thiamine. To induce expression, the cells were inoculated into thiamine-free medium after extensive washing.
Flow Cytometry and Microscopy
Cells were cultured as described in the text. For flow cytometry, cells were collected, washed once with cold water, and then fixed with 70% ethanol and stored at 4°C. After digestion by RNase A and staining with propidium iodide, the cells were analyzed by FACSCalibur (Becton-Dickinson, San Jose, CA). For microscopic observation, cells were fixed with 70% ethanol. After the cells had been washed with water, nuclei and septa were stained with DAPI and calcofluor, respectively, and inspected under a fluorescent microscope.
Immunoblotting
Cells were collected, washed once with water, resuspended in 100 μl of water, and then heated at 90°C for 5 min. Extracts were prepared as described (Masai et al., 1995). Briefly, the cells were disrupted by vigorous vortexing with glass beads in SDS sample buffer containing 4 M urea. Sixty micrograms of protein per each lane were fractionated by SDS-PAGE (8% acrylamide gel), and then transferred to nitrocellulose paper. The blot was probed with anti-Cdc13 antibody or anti-Cdc2 antibody specific for the PSTAIRE region (kindly provided by Dr. M. Yanagida and Dr. M. Yamashita, respectively). Rum1 protein tagged by triple hemagglutinin epitopes was visualized with anti-HA monoclonal antibody (12CA5, Boeringer Mannheim, Indianapolis, IN).
Assay of H1-Histone Kinase Activity
Cell extracts were prepared in HB buffer (Moreno et al., 1991) by vigorous vortexing with glass beads. Kinase reaction was performed in HB buffer with H1-histone and [γ-32P]ATP for 15 min at 30°C. Reactions were terminated by adding an equal volume of 2× sample buffer. Samples were boiled for 5 min, run on a 12% polyacrylamide gel, and then quantified with BAS1000 (Fuji film).
RESULTS
The ste9+ Gene Encodes a Homolog of Hct1/Cdh1 and Fizzy-related
To look for genes responsible for the regulation of cell cycle progression in G1-phase, especially for the cell cycle arrest, we examined whether the known sterile mutants exhibited the phenotype similar to that of the rum1 mutant. As described below in detail, the ste9 mutant conformed to this criterion and exhibited defects indistinguishable from those of the rum1 mutant. The ste9 mutant was identified previously (Leupold and Sipiczki, 1991), but its molecular characterization has not been attempted. To determine its coding product, the ste9+ gene was isolated by rescuing the sterility of its mutant. Sequencing revealed one uninterrupted large open reading frame composed of 556 amino acids (Figure 1A). A characteristic motif, called the WD-repeat, was found in its C-terminal half (Voorn and Ploegh, 1992; Neer et al., 1994). In contrast, the N-terminal half showed no characteristic sequence. Homology searching revealed that Ste9 belonged to the subfamily of WD-repeat proteins including Cdc20 and Hct1/Cdh1 (Saccharomyces cerevisiae), Fizzy and Fizzy-related (Drosophila melanogaster), p55CDC (human and rat), and Slp1 (S. pombe) (Sethi et al., 1991; Weinstein et al., 1994; Dawson et al., 1995; Matsumoto, 1997; Schwab et al., 1997; Sigrist and Lehner, 1997; Visintin et al., 1997). All proteins contain seven WD-repeats at the C-terminal region, and these WD-repeat domains of these proteins are highly conserved. In addition, several proteins of unknown function identified by genome sequencing project are also homologous, such as a hypothetical protein on the S. pombe chromosome II (genomic cosmid clone 1198, accession number U33008) and the ZK1307.6 (C. elegans). Among these proteins, Ste9 has the highest homology with the Hct1/Cdh1 (Figure 1B) and Fizzy-related (our unpublished results). As described below, mutants of these three genes exhibit similar phenotype.
The chromosomal ste9+ gene was disrupted (Figure 1A). Diploid cells harboring one wild-type and one disrupted ste9 allele were subjected to tetrad analysis. Although a slight reduction in the ste9− spore’s viability was observed, the ste9-disrupted cells grew normally with a generation time comparable to that of wild-type cultures under a standard culture condition. They exhibited neither temperature- nor cold-sensitive growth. However, the ste9 disruptant failed to conjugate in nitrogen-free sporulation medium. Furthermore, diploid cells harboring the ste9 null allele homozygously scarcely sporulated, and sporulation was also defective in the heteroallelic diploid cells harboring Δste9 and ste9-B36. Thus, disrupted ste9 allele lacked its function, and ste9+ was dispensable for proliferation but essential for cell differentiation, i.e., mating and subsequent sporulation. We used both nonsense (ste9-B36) and disrupted alleles for the experiments described below.
The ste9+ Gene Is Essential for G1 Arrest upon Starvation
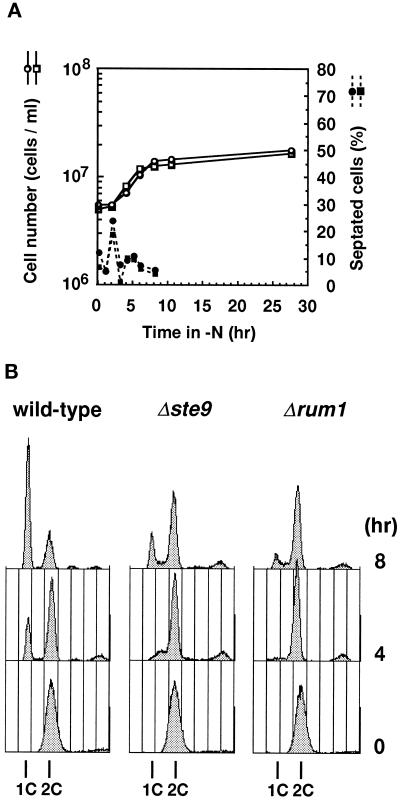
To understand the physiological role of Ste9, cell division in a nitrogen-free medium was monitored because ste9 mutant was sterile. Exponentially proliferating cells were transferred to a nitrogen-free medium. At each time indicated, a portion of the culture was taken and inspected microscopically. Profile of changes in percentage of septated cells was similar between wild-type and the ste9 mutant strains (Figure 2A). The total cell number increased 3.8 times for the wild-type strain and 4.4 times for the ste9 mutant, indicating that both strains passed through mitosis twice before they ceased to proliferate. We and others previously isolated several sterile mutants that rapidly lost viability after nutritional starvation (Kitamura et al., 1990; Devoti et al., 1991; Warbrick and Fantes, 1991; Takeda et al., 1995). To test whether the ste9 mutants normally responded to starvation, viability in long-term culture was monitored. Viability of wild-type and ste9 cells cultured in nitrogen-free medium for 9 d was 82.7% and 68.2%, respectively. Viable cells were somewhat decreased in prolonged culture in the ste9 mutant, but viability was still fairly high. Microscopic observation revealed that both wild-type and ste9 cells were small and spherical under the starved condition, indicating that both strains successfully entered into the stationary phase. Many fission yeast genes responsible for sexual development are transcriptionally induced in response to nitrogen starvation depending on the Ste11 transcription factor (Sugimoto et al., 1991). mei2 is one such gene, and its transcription occurs in a mating-type or pheromone signaling-independent manner (Shimoda et al., 1987). Northern analysis revealed that transcription of mei2 was strongly induced by nitrogen starvation in ste9 cells as well as in wild-type cells (our unpublished results).
Figure 2.
The ste9 mutants cannot arrest in the G1-phase upon nitrogen starvation. (A) Changes in cell number (open symbols) and percent of septated cells (filled symbols) of cells cultured in EMM2−N. Wild-type (L972, circle) and Δste9 (KJ100–8C, square) strains were used. (B) Wild-type (L972, left) or Δste9 (KJ100–8C, center) strains were cultured in EMM2−N for the duration indicated and analyzed by flow cytometry. The Δrum1 (KJ153–1A, right) is a control for the G1 arrest-defective strain.
The above data indicate that the ste9 mutant is competent to respond to nitrogen starvation. Nevertheless, ste9 mutants are completely sterile. In wild-type cells, starvation of nitrogen induces cell cycle arrest in the G1-phase (Costello et al., 1986). This is one of the prerequisites for fission yeast cells to commit to the developmental pathway and initiate conjugation (Nurse and Bissett, 1981). We next examined whether the ste9 mutant arrested in the G1-phase in response to nitrogen limitation. DNA content of cells cultured in nitrogen-free medium was analyzed by flow cytometry (Figure 2B). In S. pombe, most of the actively proliferating cells are in the G2-phase, and a newborn cell has already completed DNA synthesis after cytokinesis. Consistently, the majority of cells in the exponential culture had a 2C DNA content in both wild-type and ste9 strains. Upon nitrogen starvation, cells with a 1C DNA content gradually accumulated in the wild-type strain, indicating that most cells arrested in the G1-phase as they ceased to proliferate. In contrast, ste9 strain showed only a minor G1 peak and remained as cells with the 2C DNA content after 8 h (Figure 2B), or even after 24 h (our unpublished results). Consistent with the previous report (Moreno and Nurse, 1994), the control rum1 strain was also defective in the starvation induced-G1 arrest. We conclude that the ste9 mutant is defective in cell cycle arrest in the G1-phase in response to nitrogen starvation, while they are competent to enter the stationary phase from the G2-phase. Therefore, the primary cause of the sterility in the ste9 mutant is its failure to arrest in G1.
It was demonstrated that the Cig2 cyclin was a major partner of the Cdc2 kinase in the G1/S-phase in fission yeast (Fisher and Nurse, 1996; Martín-Castellanos et al., 1996; Mondesert et al., 1996). Cells harboring the deleted cig2 allele are viable but exhibit a delay in progression through G1 to the S-phase in some situations, e.g., small cells generated by the wee mutations or nitrogen starvation. We tested the possibility of whether a decrease in the G1/S cdk activity by inactivation of Cig2 restored mating ability in the ste9 mutant. This was indeed the case; mating defect was suppressed by the mutation in cig2. Efficiency of the suppression varied depending on the allele of ste9 mutation (Table 2): 6% in the double disruptant of Δste9 Δcig2, 17% in the ste9-B36 cig2-S18, and ∼50% in the ste9–59 Δcig2. The ste9–59 allele encodes a partially functional protein because mating is blocked but sporulation is hardly affected by this mutation (Kitamura and Tsujimoto, unpublished). We also investigated the effect of inactivation of other cyclin genes including puc1 (Forsburg and Nurse, 1994), cig1 (Bueno et al., 1991) or pas1 (Tanaka and Okayama, personal communication). However, the sterility of the ste9 strain was not rescued by the disruption of these cyclin genes, indicating that the relationship between cig2 and ste9 was specific. These data strongly support the previous conclusion that the cause of the sterility in the ste9 mutant is its G1-arrest defect. We noticed that about 10% of cells formed azygotic asci in the Δste9 and ste9-B36 strains in combination with the cig2 mutation. Unexpectedly, these azygotic asci were produced from haploid cells without diploidization by the preceding mating. This haploid meiosis was also observed in the rum1 cig2 strain (Kitamura, unpublished). The reason for this aberrant meiosis is currently under investigation.
Table 2.
Suppression of the mating and meiosis deficiency in the ste9 strains by the cig2 mutation
| Relevant genotype | Frequency (%)
|
||
|---|---|---|---|
| ZA | AA | V | |
| Δste9 Δcig2 | 6.1 ± 0.8 | 10.6 ± 1.0 | 83.4 ± 1.8 |
| ste9-B36 cig2-S18 | 17.1 ± 1.2 | 11.4 ± 0.6 | 71.6 ± 1.7 |
| ste9-59 Δcig2 | 48.9 ± 3.7 | 0.8 ± 0.1 | 50.4 ± 3.7 |
| ste9* | <0.2 | <0.2 | >99.6 |
All strains are homothallic mating type (h90). Each strain was cultured on MEA for 2 d at 28°C. Data are the average of four to six independent countings and expressed as mean ± SE. At least 500 cells were examined in each counting. ZA, Zygotes and zygotic asci; AA, aberrant azygotic asci derived from haploid cells; V, vegetative cells. Construction and characterization of Δste9 allele is described in the text. ste9-B36 is a nonsense allele isolated by Leupold and Sipiczki (1991). ste9-59 is a novel allele encoding partially active protein (our unpublished result, see text). ste9* represents all three ste9 alleles described above. The cig2-S18 mutation was identified as an extragenic suppressor of the sterility of ste9-B36 strain. This cig2 mutation inactivates the protein because it cannot complement the deleted cig2 allele (Kitamura, unpublished).
ste9 and wee1 Mutations Cause Synthetic Lethality
Cell cycle regulation in the G1-phase is obviously defective in the ste9 mutants, although cells completely lacking the Ste9 function still grow in a healthy manner in the rapidly proliferating phase. In rapidly proliferating wild-type cells, the size of two daughter cells born in the previous cell cycle is already beyond the critical size required for passing the Start restraint. Consequently, the length of the G1-phase is very short and the pre-Start G1 period is virtually lacking in actively proliferating cells (Nurse, 1975; Nurse and Thuriaux, 1977; Nasmyth, 1979). A protein kinase encoded by wee1+ negatively regulates G2/M-phase transition by phosphorylating Cdc2 (McGowan and Russell, 1993). Cells harboring the deleted wee1 allele are viable, but timing of the initiation of mitosis is advanced. As a result, half-size cells are generated after cell division (Russell and Nurse, 1987). In these small cells, the G1 period must be lengthened until the minimum size required for overriding the Start-restraint is attained. We next examined the role of Ste9 in such small cells. For this purpose, a temperature-sensitive wee1–50 allele (Nurse, 1975) was used. Both parental ste9 and the wee1–50 single mutant could grow at 35°C. In contrast, growth of the ste9 wee1–50 double mutant was temperature-sensitive (see below). This indicates that ste9+ was dispensable in an otherwise wild-type background but indispensable in the absence of the wee1-function. The mik1+ gene encodes a protein kinase whose function overlaps with Wee1, but disruption of the mik1 gene does not confer any significant phenotype (Lundgren et al., 1991). Accordingly, the Δste9 Δmik1 double disruptant was viable, and the phenotype of this double disruptant was identical with that of the parental Δste9 cells.
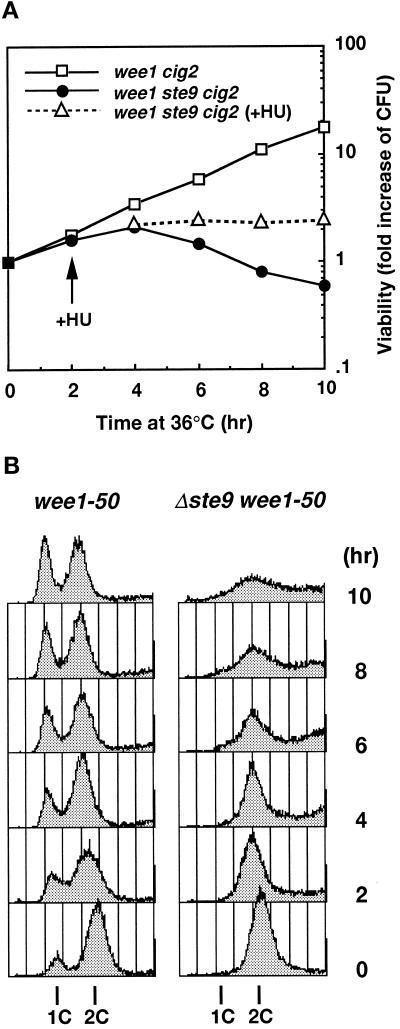
Because the sterility of the ste9 mutant was suppressed by the cig2 mutation, we examined whether the lethality of ste9 wee1 strain was also suppressed by simultaneous inactivation of cig2. However, temperature-sensitive lethality was not suppressed by the cig2 mutation, and the triple mutant also exhibited a growth defect at 35°C (Figures 3A and 7C). The growth profile of this triple mutant at the restrictive temperature was investigated in detail. Figure 3A shows the change in viability at the restrictive temperature. Control wee1–50 cells continued to proliferate and the number of viable cells exponentially increased. In marked contrast, wee1–50 cells lacking the Ste9 function began to die after 2 h. These cells did not cease cell division; the total cell number increased (our unpublished results). The DNA contents of these cells were analyzed by flow cytometry (Figure 3B). As the heat-labile Wee1 protein was inactivated, the length of the G1 period elongated. Consistently, the 1C peak corresponding to the G1 population was clearly seen in the control wee1–50 cig2 cells. In contrast, no apparent 1C peak was observed in the triple mutant of ste9 wee1–50 cig2. To emphasize 1C peak clearly, we used the cig2-disrupted strain for the experiment shown in Figure 3, but the ste9 wee1–50 double mutants also gave similar results. DAPI staining of these cells revealed that cells became small and executed aberrant septation, which led to the asymmetric nuclear segregation. These data are interpreted as follows: the wee1 ste9 double mutant could not delay DNA synthesis until the restraint of size control was cleared and could enter the S-phase immediately after previous mitosis even in the cells too small to initiate DNA synthesis. Repeats of this uncontrolled cell cycle progression led to cell death. Consistent with this hypothesis, the addition of hydroxyurea (HU), an inhibitor of DNA synthesis, at 2 h after a temperature increase, completely prevented subsequent cell death (Figure 3A).
Figure 3.
Synthetic lethality between ste9 and wee1 mutations. (A) Viability for wee1–50 Δcig2 (KJ109–5A, open square), Δste9 wee1–50 Δcig2 (KJ111–1A, filled circle), and Δste9 wee1–50 Δcig2 cells in the presence of HU (open triangle). Log-phase cultures at 23°C were shifted to 35°C at 0 h. HU was added after 2 h of incubation at a final concentration of 12 mM. (B) DNA content of wee1–50 Δcig2 (left) and Δste9 wee1–50 Δcig2 (right) at the restrictive temperature. Cells were shifted to 35°C at 0 h.
ste9 Mutants Enter the M-Phase from the Pre-Start G1-Phase
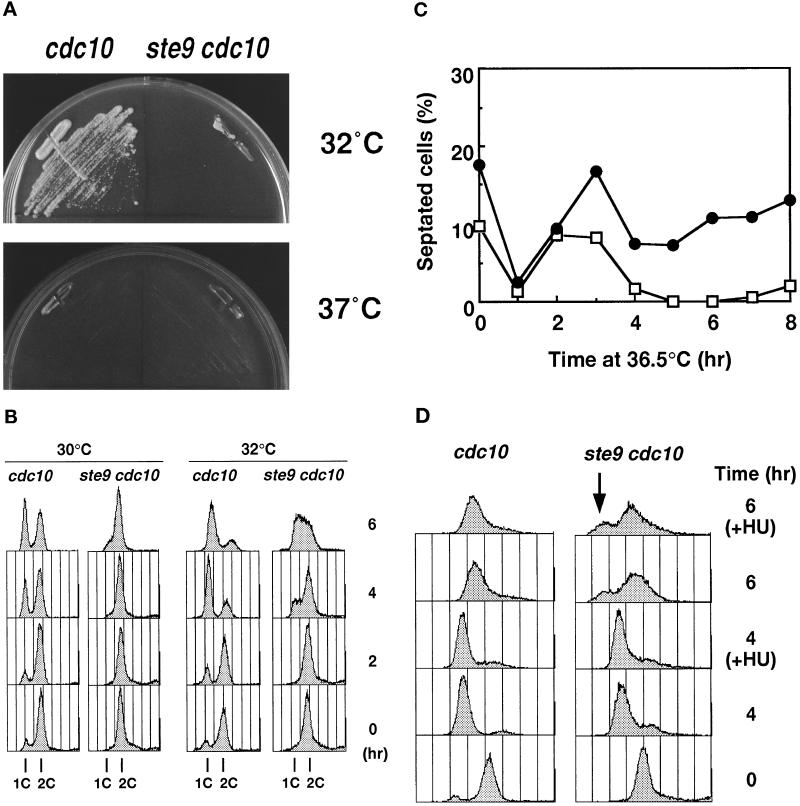
As described above, ste9+ is essential for the proper regulation of cell cycle progression in the G1-phase. The Cdc10 protein, in combination with Res1 or Res2, functions as a transcription factor for the genes that are essential for transition from the G1 to the S-phase (Lowndes et al., 1992; Tanaka et al., 1992; Caligiuri and Beach, 1993; Miyamoto et al., 1994). At the restrictive temperature, cdc10ts mutants arrest in the pre-Start period of the G1-phase (Nurse et al., 1976; Nurse and Bissett, 1981). We found that the ste9 cdc10ts double mutant rapidly lost viability compared with the cdc10ts single mutant at the restrictive temperature (36°C). Even at the semipermissive temperature (32°C), the double mutant was inviable, whereas the control cdc10ts mutant formed colonies (Figure 4A). The control ste9 strain grew normally at all temperatures examined, indicating that the cdc10ts cells were extremely sensitive to the lack of the Ste9 function. Analysis of the DNA content of the cells at 30°C revealed that the apparent 1C peak corresponding to the G1 population was seen in the cdc10ts strain, but no such population was found in the ste9 cdc10ts strain (Figure 4B). The DNA profiles of both strains at 32°C were also quite different, in that appearance of the cells in the G1/early S-phase was remarkably delayed in the double mutants (Figure 4B). These observations led to the idea that Start-promoting activity is intense in the ste9 mutant (see DISCUSSION).
Figure 4.
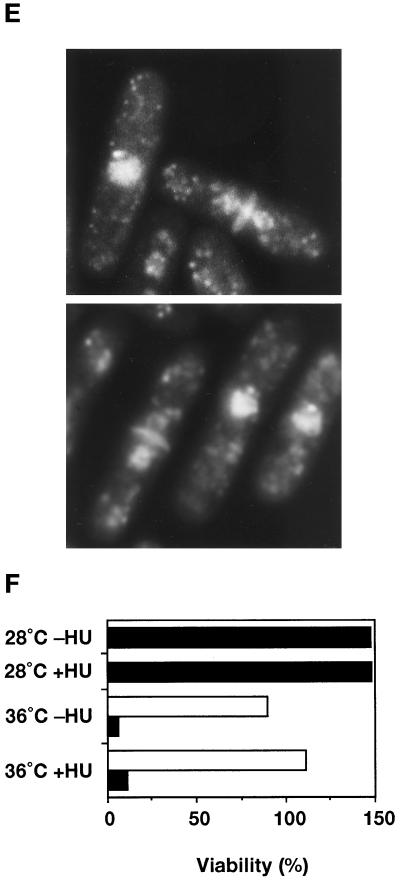
The ste9 mutant initiates mitosis from the G1-phase in the absence of S-phase. In all experiments, KJ32–2A (cdc10–129) and KJ32–1A (ste9-B36 cdc10–129) were used. (A) The double mutant of ste9 cdc10 cannot grow even at the semipermissive temperature for cdc10ts (32°C). Cells of cdc10 (left) or ste9 cdc10 (right) were cultured for 3 d at 32°C and 37°C. (B) Flow cytometric analysis of cdc10 (left) or ste9 cdc10 (right) at the semipermissive temperature. (C) Changes of the septation index. Asynchronous culture of cdc10 (open square) or ste9 cdc10 (filled circle) at the permissive temperature was shifted to 36°C. At the time indicated, cells were stained with calcofluor, and septated and nonseptated cells were counted. (D) DNA content of cdc10 (left) and ste9 cdc10 (right) mutants at 36°C. HU was added at the same time as the cells were shifted to 36°C. Addition of HU at 2 h at the restrictive temperature resulted in the same DNA profile (our unpublished observations). (E) “Cut” phenotype in the ste9 cdc10 double mutant at the restrictive temperature. Cells were incubated at 36°C for 6 h and stained with DAPI and calcofluor. (F) Lethal mitosis in the ste9 cdc10 double mutant occurred in the absence of S-phase. HU (final concentration 12 mM) was added to the cells of cdc10 (open bar) and ste9 cdc10 (black bar), which were then incubated at 28°C or 36°C for 4 h. Aliquots were plated onto YE and incubated at 25°C, and the number of colonies was counted after 3 d. Viability is expressed as the percent ratio of colony numbers of cells after 4 h of incubation relative to that of untreated cells at the beginning of the experiments (0 h). Sensitivity to HU of the cdc10 single mutant at 28°C was not examined, but the cdc10 cells were viable at 36°C in the presence of HU.
The phenotype of the ste9 cdc10ts double mutant at the restrictive temperature was examined in more detail. Asynchronous populations of cdc10 or the double mutant of ste9 cdc10 at the permissive temperature were shifted to 36°C. At the indicated time, a portion of the culture was taken for microscopic observation and flow cytometry. In both cultures, septated cells decreased after 1 h and then increased at the restrictive temperature (Figure 4C). In the cdc10 strain, septated cells decreased again by 4 h. On the contrary, 10–20% of the population still had septa in the ste9 cdc10 strain, indicating that cell division might continue even at the restrictive temperature. Figure 4D shows the DNA profile of these cells at 36°C. Both cdc10 and ste9 cdc10 cells arrested with a 1C DNA content by 4 h. After 6 h, the major peak shifted to the right, but these shifted peaks did not correspond to the 2C population. These shifts may be an artifact due to cell elongation, and the DNA profile did not alter by preventing DNA synthesis using HU (Figure 4D). We concluded that both single and double mutants arrested in the G1-phase at restrictive temperatures. DAPI staining of the ste9 cdc10 strain revealed that the nucleus was bisected by the septum at the restrictive temperature (Figure 4E). The appearance of these cells exhibiting a so-called “cut” phenotype indicated that septum formation was uncoupled with completion of DNA replication or nuclear separation. In the DNA profile of the double mutant, a small peak with less than a 1C DNA content was observed at 6 h after a temperature increase (Figure 4D, indicated by a downward arrow). This minor peak was seen even in the presence of HU but was never seen in the cdc10 single mutant. This peak might represent dead cells produced by the aberrant mitosis in the double mutant.
These observations strongly suggest that the ste9 cdc10 mutant is defective in some checkpoint process. To date, two related, but distinct, DNA structure checkpoint mechanisms that prevent premature mitosis were discovered in fission yeast (Carr, 1995; Stewart and Enoch, 1996). One is the S-phase-mitosis (S/M) checkpoint, which responds to changes in the state of DNA replication. Another is the DNA damage checkpoint, which monitors the state of DNA damage and searches for lesions in the newly replicated DNA, such as incomplete ligation. We tested whether the ste9 mutant was defective in these checkpoint mechanisms. However, ste9 cells survived in the presence of HU or methylmethane sulfonate as did wild-type cells (our unpublished results). In addition, wild-type and ste9 strains have the same sensitivity to UV light, indicating that Ste9 is apparently dispensable for the replication- and the damage-checkpoint functions.
It is possible that lethal mitosis in the cdc10 ste9 strain is caused by incomplete cdc10 arrest and premature entry into mitosis. As described, HU effectively blocks cell cycle progression in the ste9 cells because the replication checkpoint is operating. If G1 arrest by the cdc10 mutation is incomplete, it is expected that lethal mitosis is prevented in the presence of HU because of its inhibition of S-phase progression. As shown in Figure 4F, addition of HU to the ste9 cdc10 double mutant did not prevent cell death at the restrictive temperature, indicating that lethal mitosis in the cdc10 ste9 cells was not due to leakiness of cell cycle arrest. Therefore in the ste9 cdc10 strain, DNA synthesis was bypassed, and the cells entered mitosis from the pre-Start G1 period.
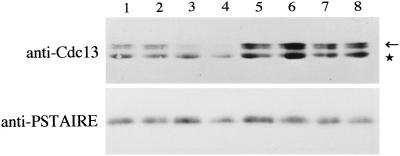
The amount of Cdc13 protein, a major mitotic B-type cyclin in S. pombe, culminates in the late G2/M and is very low in the G1-phase due to selective degradation by the proteasome (Hayles et al., 1994; Yamano et al., 1996). Strong activation of the Cdc2/Cdc13 complex in G1-arrested cells leads to mitosis in the absence of DNA synthesis (Hayles et al., 1994; Yamano et al., 1996). Because similar premature mitosis occurs in the G1-arrested ste9 cdc10 cells, we quantified the level of the Cdc13 protein at the restrictive temperature. In the cdc10 single mutant, Cdc13 almost disappeared by 4 h at 36°C as the cells arrested in the G1-phase (Figure 5). In contrast, Cdc13 did not decrease in the ste9 cdc10 double mutant (Figure 5), although the cells largely arrested in G1 by 4 h at 36°C (Figure 4D). This persistence of Cdc13 in ste9 cdc10 cells, at least in part, seems relevant to the premature mitosis. The same premature entry into M-phase from pre-Start G1 occurs also in the rum1 cdc10 double mutant (Moreno and Nurse, 1994). These data indicate that both ste9 and rum1 are essential to prevent mitosis from the pre-Start G1 period.
Figure 5.
Cdc13, a mitotic B-cyclin, is not decreased in the G1-arrested ste9 mutant. Extracts were prepared from cdc10 (lanes 1–4) or ste9 cdc10 (lanes 5–8) strains. Cells were cultured at 36°C for 0 h (lanes 1 and 5), 2 h (lanes 2 and 6), 3 h (lanes 3 and 7), and 4 h (lanes 4 and 8). Western blotting was carried out using anti-Cdc13 antibody (upper panel) or anti-PSTAIRE antibody for the loading control (lower panel). The location of Cdc13 is indicated by the arrow. The lower bands (indicated by asterisk) are unknown protein cross-reacted with the anti-Cdc13 antibody.
Overexpression of Ste9 Inhibits Mitosis and Induces an Extra Round of DNA Replication
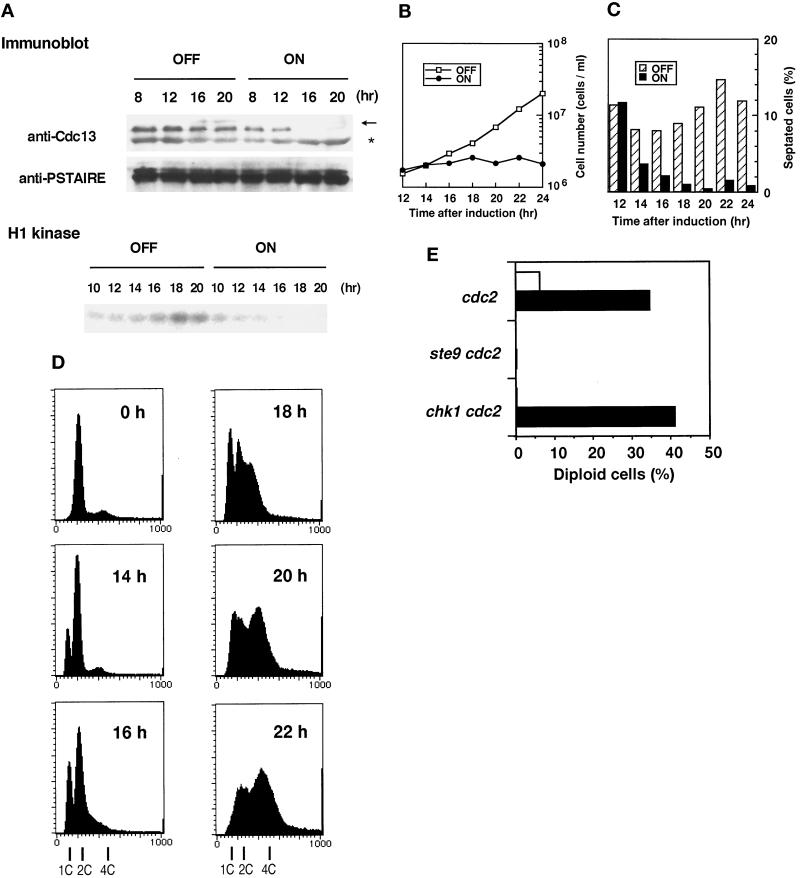
Our observation that Cdc13 persisted in the G1-arrested ste9 cells (Figure 5) suggests an intriguing possibility that Ste9 down-regulates the amount of Cdc13 protein. To confirm this assumption, the Cdc13 level in Ste9-overexpressing cells was quantified with a strain harboring the integrated nmt-ste9+ gene. In the presence of thiamine, cells exponentially proliferated (Figure 6B), and the Cdc13 protein level did not significantly change throughout the incubation (Figure 6A). In marked contrast, Cdc13 decreased when the expression of Ste9 was induced in the thiamine-free medium. Consistently, activity of total H1-histone kinase was greatly reduced in these cells.
Figure 6.
Overexpression of Ste9 prevents mitosis and induces rereplication of the genome. (A) Changes in the Cdc13 level and the H1-histone kinase activity during Ste9-overexpression. Cells harboring the integrated nmt-ste9+ gene were cultured to mid-log phase in the presence of thiamine. At 0 h, the cells were washed and then reinoculated into fresh medium without thiamine (ON) or with thiamine (OFF). Cell extracts were prepared from the cultures at the times indicated. Note that induction from the nmt promoter requires more than 10 h (Maundrell, 1990). Immunoblotting was carried out by anti-Cdc13 antibody or by anti-PSTAIRE antibody (upper two panels). Arrow and asterisk indicate the locations of Cdc13 and cross-reacted proteins, respectively. H1-histone kinase activities of uninduced (OFF) and induced (ON) cultures were also measured (lower panel). (B) Overexpression of Ste9 inhibits cell division. Cell numbers of induced (ON) and uninduced (OFF) cultures were counted at the indicated time. (C) Changes in septation index in the same cultures described in panel B. (D) Changes in the DNA content during Ste9 overexpression. Samples were taken at the indicated time for flow cytometry. The DNA profiles of the uninduced culture at each time were the same as that of 0 h. (E) The ste9 mutation prevents rereplication of DNA due to heat inactivation of the temperature-sensitive Cdc2 protein. Log-phase cells were shifted to EMM2−N and cultured at 36°C for 6 h. Cells were plated at 0 h (open bar) and at 6 h (black bar) onto YE medium containing phloxine B. Numbers of red colonies (diploid cells) and pink colonies (haploid cells) were counted, and the percent of diploid cells was calculated. Strains: cdc2-M26 (KJ141–8A); Δste9 cdc2-M26 (KJ124–3A); Δchk1 cdc2-M26 (KJ155–1A).
In the Ste9-overexpressing culture, increase in cell number stopped after 12 h of induction (Figure 6B). Ste9 overexpression requires a 12-h induction because the nmt1 promoter takes that long to induce (Maundrell, 1990). Therefore, cessation of proliferation coincided well with the kinetics of induction. Concomitant with the end of cell number increase, cells became elongated and exhibited a low septation index (Figure 6C). Less than 1% of cells contained two nuclei and exhibited mitotic figures after 14 h. In these cells, onset of the S-phase was slightly delayed, as evidenced by the appearance of the 1C peak, after which the population with a 4C DNA content corresponding to diploid cells increased (Figure 6D). The possibility that overexpression of Ste9 led to asymmetric segregation of nuclear DNA was excluded because no cells of “cut” phenotype or missegregation of the nuclei were seen during incubation. We conclude that Ste9 plays a role in the regulation of Cdc13 cyclin level during G1. Down-regulation of Cdc13 and the resultant reduction of mitotic cdk activity should lead to prevention of mitosis and the induction of an extra round of DNA replication, as demonstrated by Hayles et al. (1994). These features are quite similar to those of the Rum1-overexpressing strain (Moreno and Nurse, 1994).
In fission yeast, the state of the Cdc2 kinase determines whether cells are in G1 or G2 (O’Connell and Nurse, 1994). When cdc2ts mutants were subjected to heat shock to destroy the heat-labile Cdc2 protein, haploid cells in the G2-phase were reset to the pre-Start G1-phase. If these cells are again shifted back to permissive temperature, they rereplicate their genome and initiate a new round of cell cycle as diploid cells (Broek et al., 1991). We explored the possibility that cdc2 inactivation caused diploidization in the ste9 mutant. As shown in Figure 6E, cdc2ts single mutants indeed gave rise to diploid cells, whereas the double mutant of ste9 cdc2ts remained as a haploid. Thus, functional inactivation of ste9 completely prevents this diploidization, as reported for the rum1 mutant (Labib et al., 1995). Interestingly, similar abnormality was reported in Drosophila. Loss of Fizzy-related, a homolog of Ste9, inhibits endoreduplication in G2 cells, which normally occurs in the developing embryo (Sigrist and Lehner, 1997). Because DNA replication requires G1 cdk activity, Ste9 should be necessary to maintain the Cdc2 kinase in a pre-Start form.
Chk1 was also reported to be involved in some aspect of G1 regulation (Carr et al., 1995). We tested whether rereplication was also blocked in the double mutant of the chk1 cdc2ts strain. However, the chk1 cdc2ts strain effectively rereplicated their DNA and became diploid as well as the control cdc2ts strain (Figure 6E). If Chk1 functions as a regulator of G1, its role is quite different from that of Ste9.
Functional Relationship between Ste9 and Rum1
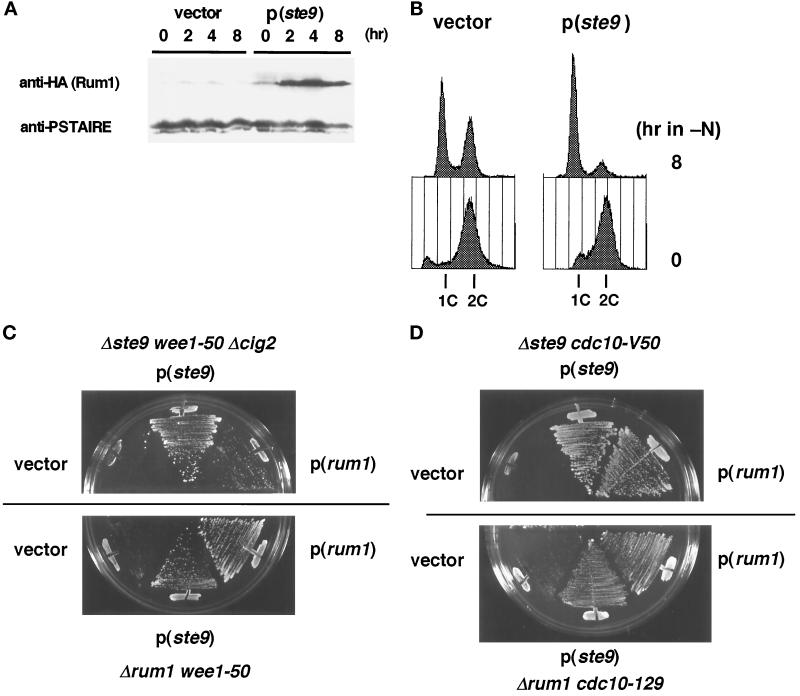
As described, various phenotypes of the ste9 mutant are indistinguishable from those of the rum1 mutant (Moreno and Nurse, 1994). The similarity of phenotypes in these mutants suggests that the roles of both proteins may be functionally related, although their primary structures are not homologous. We investigated the functional relationship between ste9 and rum1. First, the amount of Rum1 protein in the ste9 mutant was monitored. As reported earlier, Rum1 protein was very low in the asynchronous, actively proliferating cells. Upon nitrogen starvation, which induces G1 arrest in wild-type strain, considerable level of Rum1 protein was detected (Figure 7A). In contrast, such accumulation did not occur even in the ste9 cig2 cells (Figure 7A). Northern analysis revealed that the level of rum1 mRNA was induced in the same extent in both strains upon starvation (our unpublished results). This might simply reflect the fact that the ste9 mutant cannot arrest in the G1-phase (Figure 2B) and Rum1 protein is unstable except in the G1-phase (Correa-Bordes and Nurse, 1995). However, sterility was partially suppressed by the cig2 mutation (Table 2), and the G1-arrest defect was partially recovered in the ste9 cig2 cells (Figure 7B). Therefore, Ste9 might be actively involved in the production and/or stabilization of Rum1.
Figure 7.
Functional relationship between Ste9 and Rum1. (A) Changes of Rum1 protein level after starvation. The ste9-B36 Δcig2 rum1+-3HA strain (KJ238–7B) was transformed with a vector or the ste9+-plasmid. Both strains cultured in EMM2 were shifted to nitrogen-free medium. At the indicated time, samples were taken for immunoblotting. (B) DNA content of ste9+ or ste9− cells in the absence of cig2 function. Samples from the same cultures described in panel A were analyzed by flow cytometry. (C) Mutual suppression of synthetic lethality by either rum1+- or ste9+-plasmid. Cells of Δste9 wee1–50 Δcig2 (KJ111–1A, upper panel) or Δrum1 wee1–50 (KJ152–9A, lower panel) were transformed with the vector, pAL(ste9), and pREP1(rum1). Each transformant was streaked onto EMM2 plates containing 5 μM thiamine and incubated for 3 d at 34.5°C. (D) Suppression of the synthetic lethality at semipermissive temperature for cdc10 cells. Cells of Δste9 cdc10-V50 (KJ218–2B) were transformed with the vector, pAL(ste9), and pAL(rum1). Transformants were incubated at 30°C for 3 d (upper panel). Cells of Δrum1 cdc10–129 (Sp224) were transformed with the same plasmids, and then incubated at 32°C for 3 d (lower panel).
Next, the effect of the multicopy ste9+ or rum1+ gene on the suppression of the mutant phenotype was investigated. Sterility of both mutants could not be mutually suppressed by the introduction of the other gene. However, the synthetic lethality of ste9 wee1–50 cells at the restrictive temperature was weakly suppressed by the plasmid-borne rum1+ gene (Figure 7C). Conversely, rum1 wee1–50 double mutant was also viable in the presence of the ste9+-plasmid. Furthermore, growth defect in the ste9 cdc10 cells at the semipermissive temperature was effectively suppressed by the rum1+-plasmid (Figure 7D). Inviability of rum1 cdc10 cells was also suppressed by the ste9+-plasmid. These data indicate that the functions of Ste9 and Rum1 are mutually interrelated. However, both proteins do not work in a simple linear pathway, and their functions might be complementary and partly overlapping.
DISCUSSION
In this study, we demonstrated that the ste9+ gene was essential for proper regulation of cell cycle progression in the G1-phase. First, the ste9 mutant was sterile because of the failure in G1 arrest upon nitrogen starvation. Cell cycle arrest in the pre-Start G1 period is essential to enter the developmental pathway, and the sterility was partially suppressed by the inactivation of cig2, which encoded a major G1/S cyclin in S. pombe. Second, Ste9 was indispensable for the growth of the wee1 cells, which had to lengthen the pre-Start G1 period to restrain DNA synthesis until the critical size to override Start control was attained. Third, Ste9 is essential to prevent premature entry into mitosis from the pre-Start G1 period in the absence of DNA replication. Furthermore, Ste9 might be required for maintenance of the Cdc2 kinase in a pre-Start form, as suggested by the fact that overexpression of Ste9 induced rereplication of the genome due to reduction of the mitotic kinase activity of the Cdc13/Cdc2 complex, and rereplication in the cdc2ts strain was prevented by the ste9 mutation.
The ste9 gene is identical to the recently identified srw1 (Yamaguchi et al., 1997). The Ste9 is evolutionary highly conserved and is a homolog of Hct1/Cdh1 of budding yeast and Fizzy-related (Fzr) of higher organisms. The hct1/cdh1 mutant is viable, but mitotic Clb2 cyclin is highly stabilized and is not efficiently degraded in the mutant (Schwab et al., 1997; Visintin et al., 1997). Similarly, Drosophila Fzr is required for down-regulation of cyclins A, B, and B3 during G1 when the embryonic epidermal cell stops proliferation after mitosis 16 (Sigrist and Lehner, 1997). In good agreement with these reports, mitotic Cdc13 cyclin remains in the ste9 mutant arrested in G1. Therefore, all of the Ste9, Hct1/Cdt1, and Fzr are supposed to be involved in the regulation of the amount of mitotic cyclin during G1, although no biochemical function of these proteins has yet been demonstrated. Budding yeast Cdc20, a representative protein of this WD-protein family, is essential for the degradation of Pds1 and for entry into anaphase but apparently dispensable for Clb2 degradation (Visintin et al., 1997). It is proposed that Hct1 and Cdc20 might target distinct, but overlapping, sets of proteins for degradation, perhaps by recruiting these substrates to cyclosome/anaphase-promoting complex for ubiquitination (Cohen-Fix and Koshland, 1997; Hoyt, 1997). In fission yeast, Slp1 might be a homolog of Cdc20. Although the primary structures of WD-repeat domain are highly conserved between Ste9 and Slp1, the amino-terminal regions beyond WD-repeat domain are not as homologous. Both proteins exert their own role through the function of the N-terminal region. It is noteworthy if the WD-repeat regions of both proteins interact with a common protein, because the WD-repeat is involved in protein-protein interaction (Sondek et al., 1996). Although genetic interaction is demonstrated between Cdc20 and Hct1, we did not observe such interaction between Ste9 and Slp1; double mutants of Δste9 slp1–362 did not show synthetic phenotype, and the growth defect of slp1–362 was not suppressed by the ste9+-plasmid (our unpublished results). The roles of Ste9, Slp1, and the uncharacterized WD-repeat proteins of this family found by the genome-sequencing project must be further investigated.
The phenotype of the ste9 mutant is indistinguishable from that of the rum1 mutant (Moreno and Nurse, 1994). Rum1 is a potent cdk inhibitor but it is noteworthy that the Rum1 promotes proteolysis of the Cdc13 cyclin during G1-phase (Correa-Bordes et al., 1997). We also showed that both functional inactivation and overexpression of Ste9 significantly affected the level of Cdc13 protein. Therefore, Ste9 would also be involved in proteolysis of some important cell cycle regulators, one of the candidates being Cdc13 cyclin. Because sterility of ste9 mutants was suppressed by the cig2 mutation, this cyclin might be also a possible target. Inactivation of all three known B-cyclins in fission yeast (Cig1, Cig2, and Cdc13) results in lethality, and such cells arrest before DNA replication (Fisher and Nurse, 1996). Because overexpression of Ste9 induces rereplication, at least one B-cyclin functioning at the onset of DNA replication should be resistant to the effect of Ste9. High level expression of budding yeast Hct1 induces the rapid and selective disappearance of the mitotic Clb2 cyclin, probably by proteolysis, but the level of Clb5, an S-phase cyclin, does not change (Schwab et al., 1997).
In general, proteolysis of mitotic B-cyclin persists throughout G1 (Amon et al., 1994; Brandeis and Hunt, 1996). Both Ste9 and Rum1 have pivotal roles in decreasing levels and activities of the Cdc2/Cdc13 complex during G1. In the absence of this control, fission yeast cells in G1 can initiate mitosis without having undergone DNA replication. Proteolysis is indispensable for normal cell cycle progression (King et al., 1996), and expression of undegradable Cdc13 resulted in the cell cycle arrest at anaphase (Yamano et al., 1996). The fact that ste9+ and rum1+ are dispensable for normal growth is curious because Cdc13 protein remains in the ste9 or rum1 mutant arrested in G1 (Moreno and Nurse, 1994; this study). In budding yeast, HCT1 is a nonessential gene, but viability of the hct1 mutant depends on Sic1, a Clb-specific cdk inhibitor. Therefore, defects of cyclin degradation in hct1 cells is compensated by the activity of Sic1. Obviously, cdk activity must be inactivated upon exit from mitosis to G1; therefore, exit from mitosis is guaranteed either by degradation of mitotic cyclin or by the activity of cdk inhibitor. Like Hct1/Cdt1 and Sic1 in budding yeast, Ste9 and Rum1 execute an essential and overlapping function during G1. Surprisingly, a double disruptant of ste9 rum1 is still viable (Kitamura, unpublished). There are several possible explanations for the viability of the ste9 rum1 cells. 1) Both proteins have an effect on the Cdc13 proteolysis only in the G1-phase and not during mitotic exit. 2) Function of Ste9 overlaps that of other WD-repeat protein(s) belonging to the same Cdc20 subfamily. 3) cdk activity is inactivated by a mechanism other than cyclin degradation and inhibition by Rum1. Phosphorylation of Cdc2 at tyrosine 15 might be responsible for this inhibition of cdk activity because both ste9 and rum1 are synthetically lethal with the wee1 mutation. However, it is demonstrated that this inhibitory tyrosine residue is dephosphorylated in pre-Start G1 and becomes phosphorylated only after cells enter late G1 (Hayles and Nurse, 1995). In Drosophila, the roughex (rux) protein plays an analogous role to those of Ste9 and Rum1. Rux is required to establish G1-phase in the developing eye (Thomas et al., 1994). In the rux mutant, cells accumulate Cyclin A in early G1 and enter S-phase prematurely. This phenotype is suppressed by the down-regulation of Cyclin A. Furthermore, overexpression of Rux protein induces degradation of Cyclin A and inhibits S-phase entry caused by Cyclin A accumulation (Sprenger et al., 1997; Thomas et al., 1997). Thus rux shares several features with ste9 and rum1; all proteins function as a negative regulator of G1 progression. Also in fission yeast, such unidentified protein might be operating to inhibit mitotic cdk activity upon exit from mitosis. The important question of how fission yeast cells inhibit cdk activity during G1 remains to be answered.
Uncontrolled Start-promoting activity would lead to disturbance of cell cycle regulation, as in the case of cancer cells. As demonstrated in cell cycle arrest by mating pheromone (Stern and Nurse, 1997), proper regulation of the G1 cdk activity should also be important in the G1 arrest upon nutritional starvation. The following observations lead to the idea that Start-promoting activity is deregulated in the ste9 mutant. First, both wild-type and ste9 strains divide twice under nutritional starvation but only the ste9 mutant cannot arrest in the pre-Start period and initiates an additional round of DNA synthesis. Second, cells lacking ste9+ might initiate a new round of DNA replication as soon as the previous mitosis is completed, regardless of whether the cells attain the critical size. This leads to cell death in the wee1− background because cell mass is lost in every cell division as occurs in the rum1 wee1 strain (Moreno and Nurse, 1994; Sveiczer et al., 1996). Third, the population in G1 at the semipermissive temperature is considerably less in the ste9 cdc10 double mutant than in the control cdc10 strain. Because inhibition of the ubiquitin-dependent proteolytic activity overcomes Start arrest and induces DNA synthesis in fission yeast (Yamano et al., 1996), it is possible that deregulation of the Start-promoting activity is related to the proteolytic defect. In this aspect, it is intriguing that the G1 arrest induced by mating pheromone exposure is overridden in the budding yeast hct1 mutant (Schwab et al., 1997). This defect is suppressed by the deletion of the CLB2 gene. In the epidermis of Drosophila Fzr-deficient embryos, extra division preceded by DNA replication occurs after mitosis 16 instead of arresting in G1 (Sigrist and Lehner, 1997). This also might be due to the defect of cyclin degradation. Even the mitotic B-cyclin can induce DNA replication (Amon et al., 1994; Irniger et al., 1995; Fisher and Nurse, 1996); therefore, these cyclins escaping from degradation would be responsible for the promotion of the progression from G1 to S-phase.
ACKNOWLEDGMENTS
We thank Drs. U. Leupold, P. Nurse, A.M. Carr, S. Moreno, T. Matsumoto, H. Okayama, K. Tanaka, T. Ishihara, K. Maundrell, K. Okazaki, and Y. Nagai for providing S. pombe strains and plasmids; and M. Yanagida and M. Yamashita for antibodies. We also thank many participants of third UK-Japan cell cycle workshop for valuable discussion. Part of this work was supported by Grants-in-Aid for Scientific Research by the Ministry of Education, Science, Sports and Culture of Japan to C.S.
REFERENCES
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- Bueno A, Richardson H, Reed S, Russell P. A fission yeast B-type cyclin functioning early in the cell cycle. Cell. 1991;66:149–159. doi: 10.1016/0092-8674(91)90147-q. [DOI] [PubMed] [Google Scholar]
- Caligiuri M, Beach D. Sct1 functions in partnership with cdc10 in a transcriptional complex that activates cell cycle ’start’ and inhibits differentiation. Cell. 1993;72:607–619. doi: 10.1016/0092-8674(93)90079-6. [DOI] [PubMed] [Google Scholar]
- Carr AM. DNA structure checkpoints in fission yeast. Semin Cell Biol. 1995;6:65–72. doi: 10.1016/1043-4682(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Carr AM, Moudjou M, Bentley NJ, Hagan IM. The chk1 pathway is required to prevent mitosis following cell-cycle arrest at ’start’. Curr Biol. 1995;5:1179–1190. doi: 10.1016/s0960-9822(95)00234-x. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Koshland D. The metaphase-to-anaphase transition: avoiding a mid-life crisis. Curr Opin Cell Biol. 1997;9:800–806. doi: 10.1016/s0955-0674(97)80080-4. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J, Gulli M-P, Nurse P. p25rum1 promotes proteolysis of the mitotic B-cyclin p56cdc13 during G1 of the fission yeast cell cycle. EMBO J. 1997;16:4657–4664. doi: 10.1093/emboj/16.15.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Bordes J, Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Costello G, Rodgers L, Beach D. Fission yeast enters the stationary phase G0 state from either mitotic G1 or G2. Curr Genet. 1986;11:119–125. [Google Scholar]
- Dawson IA, Roth S, Atravanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoti J, Seydoux G, Beach D, McLeod M. Interaction between ran1+ protein kinase and cAMP dependent protein kinase as negative regulators of fission yeast meiosis. EMBO J. 1991;10:3759–3768. doi: 10.1002/j.1460-2075.1991.tb04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R. Mating-type genes, meiosis, and sporulation. In: Nasim A, Young P, Johnson BF, editors. Molecular Biology of the Fission Yeast. San Diego, CA: Academic Press; 1989. pp. 31–73. [Google Scholar]
- Fisher DL, Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15:850–860. [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL, Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Forsburg SL, Nurse P. Analysis of the Schizosaccharomyces pombe cyclin puc1: evidence for a role in cell cycle exit. J Cell Sci. 1994;107:601–613. [PubMed] [Google Scholar]
- Grimm C, Kohli J, Murray J, Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988;215:81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Gutz H, Heslot H, Leupold U, Loprieno N. Handbook of Genetics. Vol. 1. R.D. King, New York: Plenum Press; 1974. Schizosaccharomyces pombe; pp. 395–446. [Google Scholar]
- Hartwell L, Clotti J, Pringle JR, Reid BJ. Genetic control of the cell division cycle. Science. 1974;183:46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Hayles J, Nurse P. A pre-start checkpoint preventing mitosis in fission yeast acts independently of p34cdc2 tyrosine phosphorylation. EMBO J. 1995;14:2760–2771. doi: 10.1002/j.1460-2075.1995.tb07276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA. Eliminating all obstacles: regulated proteolysis in the eukaryotic cell cycle. Cell. 1997;91:149–151. doi: 10.1016/s0092-8674(00)80396-7. [DOI] [PubMed] [Google Scholar]
- Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–277. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Nakagawa T, Shimoda C. Novel sterile mutants of fission yeast Schizosaccharomyces pombe which are defective in their response to starvation. Curr Genet. 1990;18:315–321. [Google Scholar]
- Labib K, Moreno S. rum1: a CDK inhibitor regulating G1 progression in fission yeast. Trends Cell Biol. 1995;6:62–66. doi: 10.1016/0962-8924(96)81016-6. [DOI] [PubMed] [Google Scholar]
- Labib K, Moreno S, Nurse P. Interaction of cdc2 and rum1 regulates Start and S-phase in fission yeast. J Cell Sci. 1995;108:3285–3294. doi: 10.1242/jcs.108.10.3285. [DOI] [PubMed] [Google Scholar]
- Leupold U, Sipiczki M. Sterile UGA nonsense mutants of fission yeast. Curr Genet. 1991;15:403–405. doi: 10.1007/BF00312767. [DOI] [PubMed] [Google Scholar]
- Lowndes NF, Mclnerry CJ, Johnson AL, Fantes PA, Johonston LH. Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+ Nature. 1992;355:449–453. doi: 10.1038/355449a0. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- MacNeill SA, Fantes PA. Controlling entry into mitosis in fission yeast. In: Hutchison C, Glover DM, editors. Cell Cycle Control. Oxford: Oxford University Press; 1995. pp. 63–105. [Google Scholar]
- Martín-Castellanos C, Labib K, Moreno S. B-type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. EMBO J. 1996;15:839–849. [PMC free article] [PubMed] [Google Scholar]
- Masai M, Miyake T, Arai K. hsk1+, a Schizosaccharomyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J. 1995;14:3094–3104. doi: 10.1002/j.1460-2075.1995.tb07312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. A fission yeast homolog of CDC20/p55CDC/Fizzy is required for recovery from DNA damage and genetically interacts with p34cdc2. Mol Cell Biol. 1997;17:742–750. doi: 10.1128/mcb.17.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast: a highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell PR. Human wee1 kinase inhibits cell division by phosphorylates p34cdc2 on tyrosine 15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Tanaka K, Okayama H. res2+, a new member of the cdc10+/SWI4 family, controls the ’start’ of mitotic and meiotic cycles in fission yeast. EMBO J. 1994;13:1873–1880. doi: 10.1002/j.1460-2075.1994.tb06456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesert O, McGowan CH, Russell P. Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:1527–1533. doi: 10.1128/mcb.16.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:793–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moreno S, Nurse P. Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature. 1994;367:236–242. doi: 10.1038/367236a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth KA. A control acting over the initiation of DNA replication in the yeast Schizosaccharomyces pombe. J Cell Sci. 1979;36:155–168. doi: 10.1242/jcs.36.1.155. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P. Controls over the timing of DNA replication during the cell cycle of fission yeast. Exp Cell Res. 1977;107:365–375. doi: 10.1016/0014-4827(77)90358-5. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth KA. Genetic control of the cell division cycle in fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Nurse P. How cells know they are in G1 or G2. Curr Opin Cell Biol. 1994;6:867–871. doi: 10.1016/0955-0674(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–246. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Rothstein R. One step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sethi N, Monteagudo MC, Koshland D, Hogan E, Burke DJ. The CDC20 gene product of Saccharomyces cerevisiae, a β-transducin homolog, is required for a subset of microtuble-dependent cellular process. Mol Cell Biol. 1991;11:5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Shimoda C, Uehira M, Kishida M, Fujioka H, Iino Y, Watanabe Y, Yamamoto M. Cloning and analysis transcription of the mei2 gene responsible for initiation of meiosis in the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1987;169:93–96. doi: 10.1128/jb.169.1.93-96.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Singer PB. Crystal structure of a GA protein βγ dimer at 2.1Å resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- Sprenger F, Yakubovich N, O’Farrell PH. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr Biol. 1997;7:488–499. doi: 10.1016/s0960-9822(06)00220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern B, Nurse P. Fission yeast pheromone blocks S-phase by inhibiting the G1 cyclin B-p34cdc2 kinase. EMBO J. 1997;16:534–544. doi: 10.1093/emboj/16.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E, Enoch T. S-phase and DNA-damage checkpoints: a tale of two yeasts. Curr Opin Cell Biol. 1996;8:781–787. doi: 10.1016/s0955-0674(96)80078-0. [DOI] [PubMed] [Google Scholar]
- Su SSY, Tanaka Y, Samejima I, Tanaka K, Yanagida M. A nitrogen starvation-induced dormant G0 state in fission yeast: the establishment from uncommitted G1 state and its delay for return to proliferation. J Cell Sci. 1996;109:1347–1357. doi: 10.1242/jcs.109.6.1347. [DOI] [PubMed] [Google Scholar]
- Sugimoto A, Lino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- Sveiczer A, Novak B, Michison JM. The size control of fission yeast revisited. J Cell Sci. 1996;109:2947–2957. doi: 10.1242/jcs.109.12.2947. [DOI] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Okazaki K, Okazaki N, Ueda T, Sugiyama A, Nojima H, Okayama H. A new cdc gene required for S phase entry of Schizosaccharomyces pombe encodes a protein similar to the cdc10+ and SWI4 gene products. EMBO J. 1992;11:4923–4932. doi: 10.1002/j.1460-2075.1992.tb05599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BJ, Gunning DA, Cho J, Zipursky SL. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Zavitz KH, Dong X, Lane ME, Weigmann K, Finley RL, Brent R, Lehner CF, Zipursky SL. roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11:1289–1298. doi: 10.1101/gad.11.10.1289. [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Voorn Lvd, Ploegh HL. The WD-40 repeat. FEBS Lett. 1992;307:131–134. doi: 10.1016/0014-5793(92)80751-2. [DOI] [PubMed] [Google Scholar]
- Warbrick E, Fantes PA. The wis1 protein kinase is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991;10:4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J, Jacobsen FW, Hsu-chen J, Wu T, Baum LG. A novel mammalian protein, p55CDC, present in dividing cells is associated with protein kinase activity and has homology to the Saccharomyces cerevisiae cell division cycle proteins Cdc20 and Cdc4. Mol Cell Biol. 1994;14:3350–3363. doi: 10.1128/mcb.14.5.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Murakami H, Okayama H. A WD repeat protein controls the cell cycle and differentiaion by negatively regulating Cdc2/B-type cyclin complexes. Mol Biol Cell. 1997;8:2475–2486. doi: 10.1091/mbc.8.12.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]