Abstract
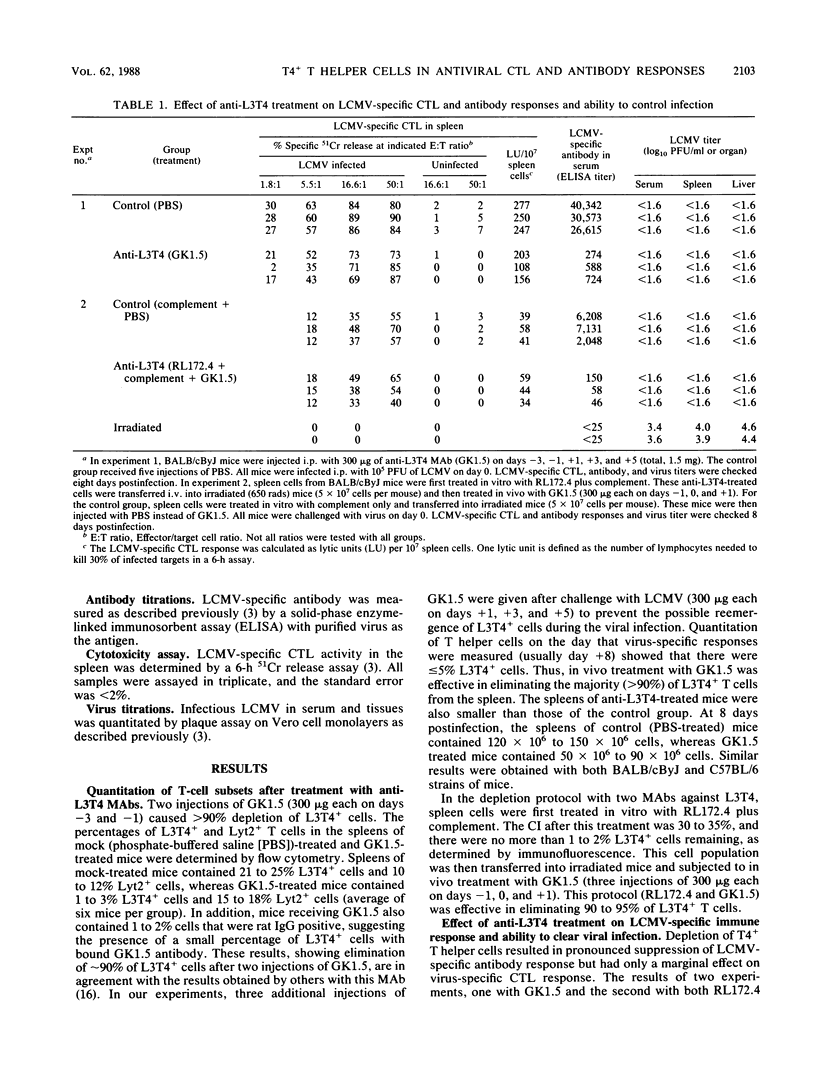
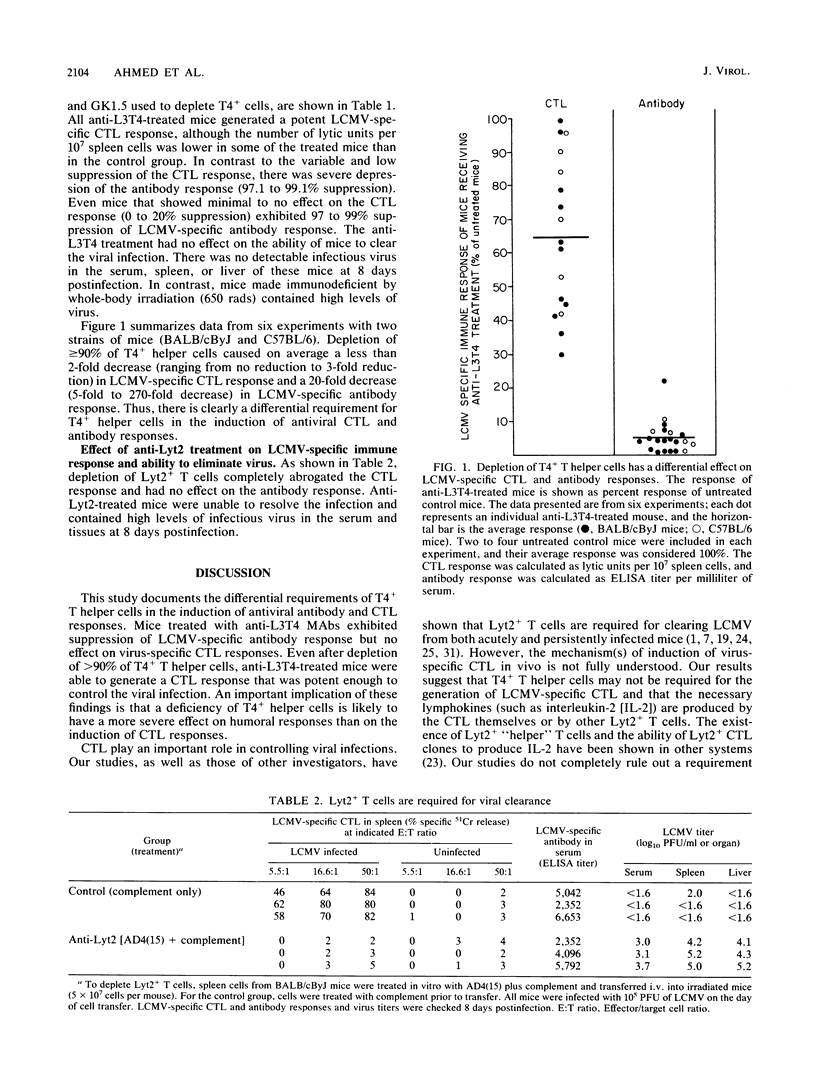
This study documents the differential requirements of T4+ T helper cells in the induction of virus-specific cytotoxic T-lymphocyte (CTL) and antibody responses during acute lymphocytic choriomeningitis virus infection. Two monoclonal antibodies (GK1.5 and RL172.4) directed against the L3T4 (T4) molecule were used for depleting T helper cells from mice. Depletion of T4+ cells caused a pronounced suppression of antiviral antibody response (20-fold decrease) but had minimal effect on virus-specific CTL response (less than 2-fold reduction). Despite the elimination of greater than 90% of T helper cells, anti-L3T4-treated mice were able to generate a CTL response of sufficient magnitude to control the viral infection. In contrast, depletion of Lyt2+ T cells abrogated the CTL response and the ability to eliminate virus. Thus, our results underscore the importance of the Lyt2+ T-cell subset in controlling infection with this virus and show that a deficiency of T4+ T cells is likely to have a more severe effect on antibody production than on CTL responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Jamieson B. D., Porter D. D. Immune therapy of a persistent and disseminated viral infection. J Virol. 1987 Dec;61(12):3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., King C. C., Oldstone M. B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987 May;61(5):1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach F. H., Grillot-Courvalin C., Kuperman O. J., Sollinger H. W., Hayes C., Sondel P. M., Alter B. J., Bach M. L. Antigenic requirements for triggering of cytotoxic T lymphocytes. Immunol Rev. 1977;35:76–96. doi: 10.1111/j.1600-065x.1977.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Ertl H. C., Finberg R. W. Characteristics and functions of Sendai virus-specific T-cell clones. J Virol. 1984 May;50(2):425–431. doi: 10.1128/jvi.50.2.425-431.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Pomerantz R. J., Kaplan J. C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987 Jul 30;317(5):278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- Jamieson B. D., Butler L. D., Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987 Dec;61(12):3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Mizuochi T., Golding H., Rosenberg A. S., Glimcher L. H., Malek T. R., Singer A. Both L3T4+ and Lyt-2+ helper T cells initiate cytotoxic T lymphocyte responses against allogenic major histocompatibility antigens but not against trinitrophenyl-modified self. J Exp Med. 1985 Aug 1;162(2):427–443. doi: 10.1084/jem.162.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Singer A., Kruisbeek A. M., Andrysiak P. M. T cell-accessory cell interactions that initiate allospecific cytotoxic T lymphocyte responses: existence of both Ia-restricted and Ia-unrestricted cellular interaction pathways. J Immunol. 1984 May;132(5):2199–2209. [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Capacity of purified Lyt-2+ T cells to mount primary proliferative and cytotoxic responses to Ia- tumour cells. Nature. 1986 Aug 7;322(6079):541–544. doi: 10.1038/322541a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker I. D., Hogarth P. M., Murray B. J., Lovering K. E., Classon B. J., Chambers G. W., McKenzie I. F. Ly antigens associated with T cell recognition and effector function. Immunol Rev. 1984 Dec;82:47–77. doi: 10.1111/j.1600-065x.1984.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]



