Abstract
Background
Vaccinia virus (VV) membrane proteins are candidates for orthopoxvirus subunit vaccines and potential targets for therapeutic antibodies. Human antibody responses to these proteins following VV vaccination have not been well characterized.
Methods
Pre- and day 26−30 post-vaccination sera from 80 VV vaccine recipients were examined for anti-B5, -A33, -A27, and -L1 specific IgG antibodies by enzyme-linked immunoassay (ELISA). Responses were compared between vaccinia-naïve and previously vaccinated (non-naïve) recipients, and between non-naïve recipients of undiluted and 1:10 diluted vaccine.
Results
VV vaccination elicited anti-A33 (100%) and -A27 (93%) antibodies in nearly all vaccinia-naïve subjects. Pre-existing antibodies were commonly detected in non-naïve subjects (anti-B5, 68%; -A33, 59%; -A27, 38%; -L1, 10%). Anti-B5 antibodies were strongly boosted by undiluted vaccine (geometric mean titer [GMT], 151 vs. 1010, p<0.001, pre- vs. post-vaccination, respectively), while anti-L1 antibody responses were less robust (31% detected, GMT, 75) in non-naïve subjects. Diluted vaccine elicited antibody responses that were similar to undiluted vaccine responses.
Conclusions
Vaccination with VV elicits long-lived specific antibody responses directed against VV membrane proteins that vary by previous vaccination status, but not by 10-fold dilution of vaccine. B5, A33 and A27 should be considered for inclusion in future human orthopoxvirus subunit vaccines.
Keywords: Smallpox, vaccination, vaccinia virus
INTRODUCTION
Despite the global eradication of smallpox nearly 30 years ago, there is renewed interest in orthopoxvirus infections. The re-emergence of smallpox is a potential threat due to intentional or accidental release of its causative agent, variola virus, as most persons living in the United States today have limited or no immunity to orthopoxviruses [1]. Furthermore, the first outbreak of monkeypox virus in the Western Hemisphere was recognized in 2003 after zoonotic importation from Africa [2], where it causes sporadic moderately severe disease. Recent vaccination with vaccinia virus (VV), a closely-related orthopoxvirus, is highly protective versus smallpox, and probably monkeypox as well, when a major cutaneous reaction is elicited [3-6]. However, widespread pre-outbreak use of VV vaccines is limited by frequent mild reactions and uncommon serious complications, mostly due to viral replication, and is thus contraindicated for a significant percentage of the United States population [1,7-9].
In response to the increased threat of orthopoxvirus infections, safer vaccines and potent antibody products are being sought. Several in vitro and animal studies have focused on evaluating VV membrane proteins as components of subunit vaccines and as targets of neutralizing monoclonal antibodies [10-13]. There are two important infectious forms of orthopoxviruses, the intracellular mature virus (MV), which is primarily responsible for virus transmission between hosts, and the extracellular enveloped virus (EV), implicated in cell-to-cell virus transmission [12,14,15]. B5 and A33 are EV-specific membrane proteins involved with EV formation [16,17], while the MV membrane-specific A27 and L1 proteins play roles in viral replication and cell attachment [18,19]. Studies in mice and non-human primates showed that vaccination with these four single proteins [10,20], or with DNA encoding for these proteins, elicited variable specific antibody responses and provided partial protection versus lethal orthopoxvirus challenge [11,21-24]. Protection was enhanced by combining EV and MV proteins and by using a DNA vaccine prime followed by protein subunit vaccine boosting. Monoclonal antibodies against these four proteins partially protect mice versus VV challenge and may be superior to vaccinia immune globulin (VIG) in this function, particularly a humanized anti-B5 monoclonal that was also shown to neutralize variola virus [12,13,25].
High titers of anti-A33, -A27, and -B5 antibodies are found in VIG; the latter are responsible for the majority of VIG's EV neutralizing activity [26-28]. In a recent study describing antibody responses to MV and EV proteins following vaccination with VV strain Lister, anti-B5 antibodies were robustly elicited in previously vaccinated (hereafter, “non-naïve”) subjects and accounted for EV neutralization, while anti-L1 antibody responses were poor [29]. We report the further characterization of specific membrane protein antibody responses in vaccinia-naïve and non-naïve human subjects following vaccination with the VV vaccine (Dryvax®, Wyeth) used in the United States.
SUBJECTS, MATERIALS, AND METHODS
Subjects and serum specimens
Stored sera were examined from vaccinia-naïve and non-naïve adults who participated in three National Institute of Allergy and Infectious Diseases -sponsored vaccine clinical trials of diluted and undiluted VV conducted at the Saint Louis University Vaccine and Treatment Evaluation Unit (Table 1) [7,30]. Sera were obtained at multiple time points during the clinical trials and stored at −70°C until assayed. Each subject provided informed consent for the future use of serum specimens. Saint Louis University and Washington University School of Medicine human experimentation guidelines were followed, and the study was approved by the review boards of both institutions.
Table 1.
Characteristics of vaccinia-naïve and previously vaccinated (non-naïve) subjects receiving undiluted or 1:10 diluted vaccinia virus vaccine.
| Undiluted Vaccine | 1:10 Diluted Vaccine | ||||
|---|---|---|---|---|---|
| Vaccinia-naïve (N = 29) | Non-Naïve (N = 29) | p* | Non-Naïve (N = 21) | p∫ | |
| DMID Study Number | 01−632¥,7; 01−65130 | 01−65130; 02−007¥ | 01−651; 02−007¥ | ||
| Age, years, mean (range) | 24.1 (18−32) | 50.6 (33−67) | <0.01 | 44.6 (35−60) | 0.02 |
| Gender, n female (%) | 14 (48.3) | 15 (51.7) | 0.79 | 12 (57.1) | 0.70 |
| Race, n non-white (%) | 4 (13.8) | 1 (3.4) | 0.35 | 0 | 1.00 |
DMID = National Institute of Allergy and Infectious Diseases Division of Microbiology and Infectious Diseases
for comparison of vaccinia-naïve vs. non-naïve recipients of undiluted vaccine using independent samples t-test and chi-square analysis
for comparison of undiluted vs. diluted vaccine amongst non-naïve recipients using independent samples t-test and chi-square analysis
convenience samples were chosen from 01−632 and 02−007, while sera from all 01−651 participants within the given categories were examined
Antibody detection
A previously described enzyme-linked immunosorbent assay (ELISA) used to measure total IgG binding antibodies to whole vaccinia virus was modified to detect antibodies versus the four proteins of interest [31]. Briefly, 96-well flat-bottom microtiter plates (MaxiSorp, Nalge Nunc International, Rochester, NY) were coated with purified baculovirus-expressed, histidine-tagged recombinant B5, A33, A27 (each at 1.0 μg/mL) and L1 proteins (at 2.0 μg/mL) [32,33] in pH 9.6 carbonate buffer. The plates were washed in phosphate buffered saline (PBS, Invitrogen, Carlsbad, CA) containing 0.1% Tween-20 (Sigma, St. Louis, MO) and blocked with PBS containing 5% nonfat milk. Serially diluted study sera were added and incubated 2 hours at 37°C. The plates were washed and peroxidase-labeled mouse anti-human IgG Fcγ (Accurate Chemical & Scientific Corporation, Westbury, NY) was added. Following a 2-hour incubation at 37°C, plates were washed and developed with a peroxidase substrate (ABTS, KPL Laboratories, Gaithersburg, MD). The optical densities were detected using a spectrophotometer at 405/490 nm dual wavelength. End point titers (EPT) were calculated as the reciprocal of the highest dilution of serum at which antibody was still detectable as extrapolated from the best-fit line using the data reduction software Unitcalc™ (Biosys Inova, Stockholm, Sweden). Individual results are reported as the arithmetic mean of duplicate EPT determinations. Paired sera from pre-vaccination and Day 26−30 post-vaccination were analyzed simultaneously. Pooled serum from unvaccinated subjects was used for a negative control. Pooled serum from vaccinated subjects with low whole vaccinia virus IgG ELISA (EVAC) antibody titers and VIG (VIGIV preparation, Center for Biologics Evaluation and Research, FDA, Rockville, MD) were used as low-titer and high-titer positive controls, respectively. Assays were repeated per the following quality control criteria: end point titer variation >20% between duplicate samples, non-linear dilution curves, negative control with optical densities above the threshold value (0.2 for A27 assay, 0.15 for the others), or VIG titers varying by >2 standard deviations from the mean titer of all assays performed.
Confirmation of low anti-L1 antibody responses
Purified L1 protein was added in increasing concentrations to VIG in order to adsorb anti-L1 antibodies. ELISAs as described above confirmed an appropriate dose-response reduction of anti-L1 antibodies following pre-incubation with specific antigen (data not shown).
Data management and statistical analysis
Subject characteristics, prior vaccination status, major cutaneous reaction status, and adverse effects data were collected previously during each of the original clinical trials. Intracellular mature virus plaque reduction neutralizing (PRNT) antibody and EVAC antibody assays were performed during the original clinical trials [7,30,34], and the results were compared to the membrane protein specific ELISA antibody responses as described above. Antibody responses were evaluated by the presence of post-vaccination antibodies, the titers of pre- and post-vaccination antibodies, and the fold-increase in antibody titers following vaccination. For data analysis purposes, specimens with undetectable antibodies were assigned a reciprocal end point titer of 50% the lower limit of detection, which was 100 for all ELISAs and 20 for PRNT antibodies. Fold increases were calculated as the post-vaccination titer divided by the pre-vaccination titer, or divided by the lower limit of detection when pre-vaccination antibodies were not detected. Summary titers were reported as geometric mean titers (GMT). Categorical variables were compared by chi-square test or Fisher's exact test (for independent variables) or McNemar's test with small sample correction (for non-independent variables). For each antibody, titers were compared between pre- and post-vaccination by paired t-tests, and between vaccinianaïve and non-naïve recipients by independent sample t-tests. Non-normally distributed continuous variables were log-transformed prior to comparisons. Non-parametric Mann-Whitney U tests were also used to compare between vaccinia-naïve and non-naïve subjects. As the statistical conclusions were similar, only results from parametric analyses were reported. For evaluation of specific antibody detection as a test to predict immunity, sensitivity, specificity, positive and negative predictive values were calculated per standard definitions [35]. Statistical Package for the Social Sciences (SPSS) version 12 was used for all analyses. A two-tailed significance level of 0.05 was used.
RESULTS
Study subjects
Sera from 80 vaccine trial participants were analyzed. One non-naïve subject yielded uninterpretable results to multiple tested antibodies because of non-linear dilution curves, leaving evaluable sera from 79 subjects. Sera from 29 vaccinia-naïve and 29 non-naïve subjects who received undiluted vaccine, and 21 non-naïve subjects who received vaccine diluted 1:10 were analyzed. Three A27 specimens (all from non-naïve subjects; one received diluted vaccine) and one L1 specimen (from a non-naïve subject who received diluted vaccine) did not meet quality control criteria and were excluded. Vaccinia-naïve subjects were younger than the non-naïve subjects due to the original trial inclusion criteria, but were otherwise similar (Table 1). Diluted vaccine recipients were slightly younger than undiluted vaccine recipients.
Antibody detection in VIG
Vaccinia immune globulin used as an ELISA positive control demonstrated consistently detectable antibodies for each of the membrane protein specific antibodies studied. Geometric mean titers were: 7631 [95% confidence interval 6679−8722], 2347 [2052−2685], 2295 [1987−2652], and 507 [421−611], for anti-B5, -A33, -A27 and –L1, respectively.
Antibody responses in vaccinia-naïve subjects
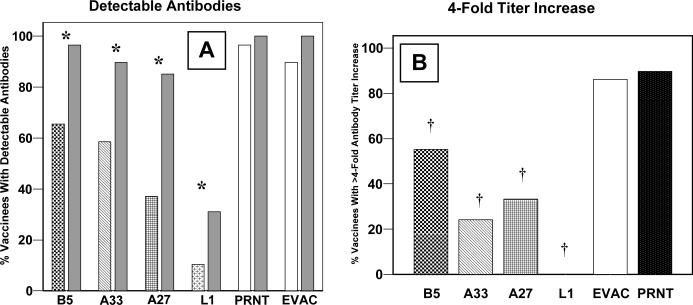
No membrane protein antibodies were detected in pre-vaccination sera from 24 of 29 (83%) vaccinia-naïve subjects. One (3%) subject had detectable antibodies to all four proteins (EPTs, anti-B5, 381; -A33, 190; -A27, 361; -L1, 186), possibly representing unrecognized prior vaccination, while four (14%) other subjects each had detectable antibodies to only one of the four proteins (EPTs, anti-B5, 506; -A33, 174; -A27, 149; -L1, 114). After vaccination, all subjects had detectable antibodies to at least one of the membrane proteins. Fifteen (52%) subjects had antibodies against all four of the tested proteins, while two (7%) subjects had antibodies against a single membrane protein (A33) only. Antibody responses to A33 were universal, and all subjects with either anti-B5 or -L1 antibodies also had both anti-A33 and -A27 antibodies. Compared to PRNT (97% detected), anti-B5 (69%, p=0.04) and –L1 (62%, p=0.01) antibodies were less frequently elicited (Figure 1A). Post-vaccination antibody titers are illustrated in Figure 1B. Titers were not associated with age, gender, or race for any of the membrane protein antibodies.
Figure 1. Vaccinia-naïve subjects.
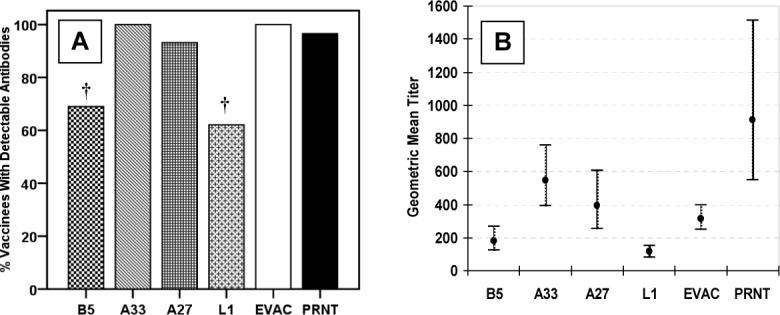
Vaccinia virus membrane protein specific IgG ELISA antibody responses, whole vaccinia virus IgG ELISA antibody responses (EVAC), and plaque reduction neutralizing (PRNT) antibodies 26−30 days following undiluted vaccinia virus vaccination in 29 vaccinia-naïve subjects. The percentage of subjects with detectable antibodies (Panel A), † = p<0.05 compared to PRNT. Geometric mean titers with 95% confidence intervals (Panel B).
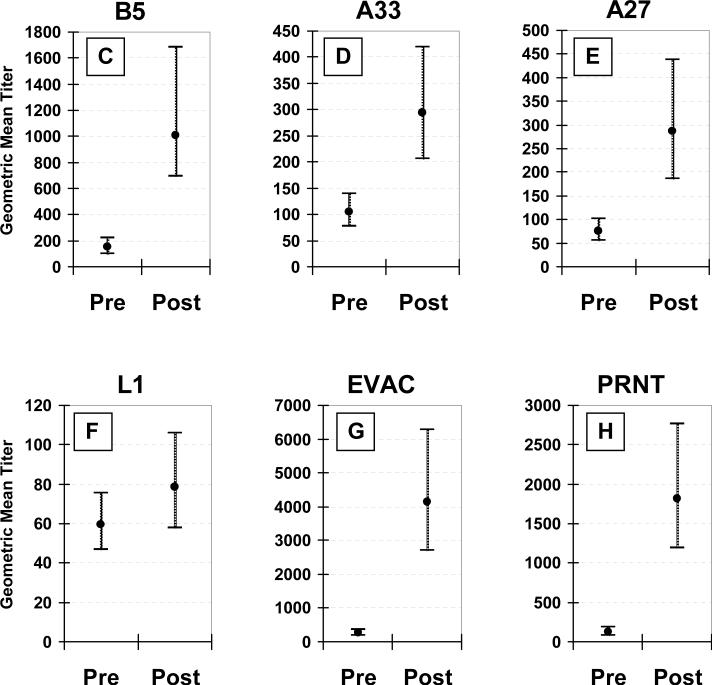
Pre-existing and elicited antibodies in non-naïve subjects
Pre-existing anti-EV (anti-B5 or -A33) antibodies were detected in 40 (80%) non-naïve subjects, and anti-MV (anti-A27 or -L1) antibodies were detected in 20 (40%) (Figure 2A). Anti-L1 antibodies were detected in only five (10%) subjects. Anti-B5 titers were negatively correlated with age (p=0.02), otherwise, none of the other specific antibody titers were associated with age, gender, or race. There was indirect evidence suggesting anti-B5 antibodies may persist with little decay, as pre-existing anti-B5 titers in non-naïve subjects (GMT, 154) were similar to vaccinia-naïve subjects' post-vaccination anti-B5 titers (GMT, 183, p=0.46).
Figure 2. Non-naïve subjects.
Vaccinia virus membrane protein specific IgG ELISA antibody responses, whole vaccinia virus IgG ELISA antibody responses (EVAC), and plaque reduction neutralizing (PRNT) antibodies following undiluted vaccinia virus vaccine in 29 previously vaccinated (non-naïve) subjects. The percentage of subjects with detectable antibodies (Panel A), patterned and white bars = pre-existing antibodies, gray bars = 26−30 days post-vaccination, * = p<0.05 for comparison of pre- vs. post-vaccination. The percentage of subjects with ≥4-fold titer increase (Panel B), † = p<0.05 for comparison to PRNT. Pre-existing versus post-vaccination geometric mean titers with 95% confidence intervals for anti-B5 (Panel C), -A33 (Panel D), -A27 (Panel E), -L1 (Panel F), EVAC (Panel G), and PRNT (Panel H) antibodies.
For the 29 non-naïve subjects receiving undiluted vaccine, all four membrane protein antibodies were detected more frequently post-vaccination compared to pre-vaccination (Figure 2A). After vaccination, nine (31%) subjects had detectable antibodies to all four of the tested proteins. One had anti-B5 antibodies only (EPT, 173), and one had no membrane protein antibodies. In both of these two latter subjects, vaccination elicited major cutaneous reactions and low titer EVAC (EPT, 384 and 311) and PRNT antibodies (titers, 113 and 230). Overall response to B5 was excellent with 28 (97%) subjects having detectable antibodies, with very high titers (GMT, 1010), and the highest post-vaccination increase (9.6-fold) of all the membrane protein specific antibodies, while anti-L1 antibody responses were poor by all measures. Only 9 (31%) subjects developed detectable anti-L1 antibodies, which were of low titer (GMT, 75), and there was minimal boosting (1.2-fold titer increase; none with a 4-fold titer increase). In comparison, EVAC and PRNT antibodies were present in all subjects, were of high titer (GMT, 1813 and 4131, for EVAC and PRNT, respectively), and more frequently exhibited a 4-fold or greater post-vaccination increase than any of the membrane protein antibodies (Figure 2B). Post-vaccination titers were significantly higher than pre-vaccination titers for all tested antibodies except anti-L1 (Figure 2C-2H).
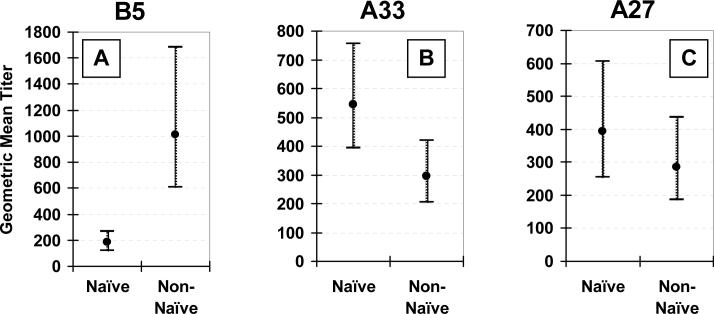
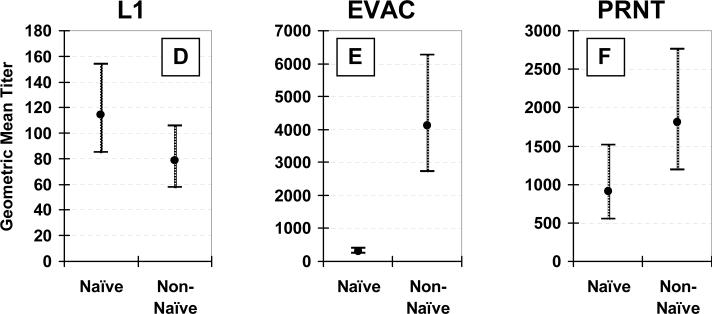
Responses in vaccinia-naïve vs. non-naïve subjects
Antibody responses to undiluted VV vaccination differed by prior vaccination status (Figure 3). Post-vaccination anti-B5 antibodies were more frequently detected (97% vs. 69% for non-naïve vs. naïve, respectively, p<0.01) and of higher titer (GMT, 1010 vs. 183, respectively, p<0.01) in non-naïve subjects (Figure 3A). Likewise, post-vaccination EVAC antibody titers were more than 10-fold higher in non-naïve subjects (GMT, 4131 vs. 314 for non-naïve vs. naïve subjects, respectively, p<0.01) (Figure 3E).
Figure 3. Effect of prior vaccination status.
Comparison of geometric mean titers and 95% confidence intervals between 29 vaccinia-naïve and 29 non-naïve subjects 26−30 days after receiving undiluted vaccinia virus vaccine. Anti-B5 (Panel A), -A33 (Panel B), -A27 (Panel C), -L1 (Panel D), and anti-whole vaccinia virus IgG (EVAC) (Panel E) ELISA, and plaque reduction neutralizing (PRNT) (Panel F) antibodies.
Effect of vaccine dilution
Among the 21 non-naïve subjects who received 1:10 diluted vaccine, the proportion of post-vaccination detectable antibodies, titers, and fold-increases for all specific antibodies were not significantly different from the 29 non-naïve subjects who received undiluted vaccine (data not shown).
Correlation of membrane protein specific antibodies with PRNT antibodies
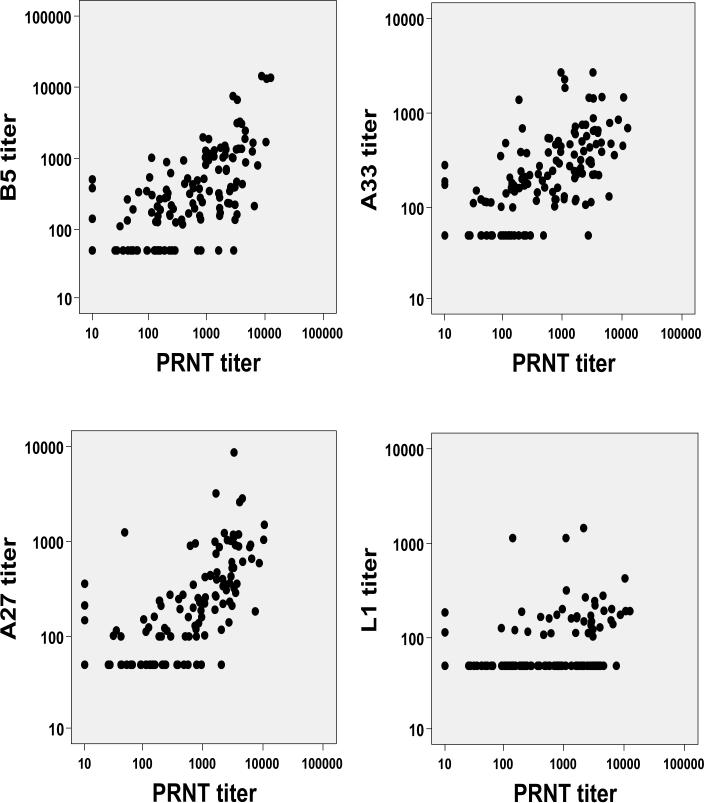
Aggregate specific antibody EPTs from all 79 subjects were examined for correlation with PRNT titers (Figure 4). Anti-B5 (r=0.74), -A33 (r=0.73), and -A27 (r=0.73) antibodies correlated moderately well with PRNT antibodies.
Figure 4.
Correlation of specific membrane protein antibody end point titers with intracellular mature virus plaque reduction neutralizing antibody (PRNT) titers.
Immunity predicted by specific antibodies
All vaccinia-naïve subjects developed a major reaction after vaccination, while three (6%) non-naïve subjects did not develop a major reaction. Two of these received diluted vaccine and one received undiluted vaccine. Neither the presence nor titer of any pre-existing antibodies was associated with developing a major reaction. Among all non-naïve subjects, post-vaccination antibody titer fold increases, but not GMTs, were significantly higher in subjects with a major reaction, compared to those without, for all antibodies except anti-A27 and -L1 (Table 2). Among non-naïve subjects no single antibody response was an accurate predictor of immunity, as indicated by development of a major reaction. The presence of anti-L1 antibodies post-vaccination had a specificity and positive predictive value of 100% for predicting a major reaction as all 15 subjects with detectable anti-L1 antibodies exhibited one, however the sensitivity and negative predictive values were extremely poor (33% and 9%, respectively). Conversely, the post-vaccination presence of all other antibodies exhibited 91−100% sensitivities and >93% positive predictive values for predicting a major reaction, but all had specificities of zero except anti-A27 (33% specificity).
Table 2.
Comparison of specific antibody responses among 50 non-naïve recipients of vaccinia virus vaccine, by presence of a major cutaneous reaction.
| Antibody | Post-vaccination GMT | Mean Titer Fold Increase | ||||
|---|---|---|---|---|---|---|
| Major Reaction N = 47 | No Major Reaction N = 3 | p* | Major Reaction N = 47 | No Major Reaction N = 3 | p* | |
| -B5 | 1034 | 395 | 0.18 | 9.2 | 1.5 | 0.02 |
| -A33 | 287 | 216 | 0.56 | 2.6 | 1.0 | <0.01 |
| -A27 | 358 | 122 | 0.08 | 4.7 | 1.6 | 0.10 |
| -L1 | 50 | 78 | 0.25 | 1.2 | 1.0 | 0.44 |
| -EVAC | 4340 | 1095 | 0.02 | 25.0 | 3.8 | 0.01 |
| -PRNT | 2167 | 693 | 0.06 | 24.4 | 3.7 | 0.02 |
= using independent samples t-tests
EVAC = Whole vaccinia virus ELISA antibodies
PRNT = Plaque-reduction neutralizing antibodies
DISCUSSION
Because of the potential risk of intentional or accidental variola virus release and the persistence of monkeypox in Africa, safer orthopoxvirus vaccines and more efficacious immunotherapeutic agents are being sought. Subunit vaccines derived from orthopoxvirus proteins are likely to be less reactogenic than currently available VV vaccines, however the optimum composition of viral proteins has not been determined. Our report provides further characterization of VV membrane protein antibody responses in vaccinia-naïve and non-naïve human subjects and confirms several important findings recently reported by Pütz and colleagues [29].
Neutralizing antibodies against both EV and MV are thought to be necessary for full protection versus orthopoxvirus infections [10,12,22,24,36,37]. Anti-B5 antibodies neutralize EV and inhibit its spread from infected cells, and may be the most important component of anti-EV responses and protective immunity [26,32,38]. This is highlighted by Chen, et al, who reported that an anti-B5 neutralizing monoclonal antibody alone may be sufficient to fully protect mice from lethal VV challenge [13]. Similar to previous reports [29], our data clearly show that anti-B5 responses are robust and relatively stronger than anti-MV responses in non-naïve individuals. The long-term persistence and vigorous boosting of anti-B5 antibodies that we observed may account for the longer duration of immunity thought to be imparted by multiple vaccinations [6]. These findings suggest that anti-B5-specific memory responses could be attributable to long-lasting anti-B5-specific memory B cells, as has been reported similarly for antibodies to H3, an MV protein [39], and provide further evidence that B5 should be a critical component of subunit vaccines.
Antibody responses to other EV proteins such as A33 may also be important for complete protection. The comet assay is a marker for release of EV from infected cells and is efficiently inhibited by anti-A33 antibodies, which do not neutralize EV in a PRNT assay [12,26]. Immunization with A33 provides partial protection in mice versus challenge with VV or the closely related ectromelia virus [20,40], suggesting that clinical protection may not be due to virus neutralization alone. Furthermore, in a recent primate monkeypox challenge study, binding antibodies to the monkeypox ortholog of A33 were inversely correlated with number of lesions [24]. In our study, anti-A33 antibodies were robustly elicited by VV vaccine in vaccinia-naïve subjects, and were moderately boosted in non-naïve subjects.
The MV-specific protein L1 seems to be poorly immunogenic, as we observed anti-L1 antibodies to be infrequently present and of low titer, consistent with Pütz, et al's recent findings [29]. Although L1 is known to be immunogenic in animals, anti-L1 antibody responses appear to be more robust after vaccination with L1 by itself than in combination with other proteins [11]. Although anti-L1 antibodies have the ability to neutralize MV, their role remains unclear in the overall humoral response to orthopoxviruses in humans. A possible explanation for poor anti-L1 antibody responses after VV vaccination is the limited quantity of L1 on the MV membrane of VV, compared to A27, which is the most abundant MV protein [41]. Although our data put into question the importance of L1 as an immunogen for eliciting humoral immunity following VV vaccination, it does not preclude the possibility this protein could be important for immunity following subunit vaccination.
We noted significant differences in the patterns of membrane protein antibody responses to VV vaccine depending on prior vaccination status. Anti-A33 and –A27 antibody responses were robust in naïve subjects. Conversely, non-naïve subjects exhibited a vigorous boosting of anti-B5 antibodies, averaging a nearly 10-fold increase in titers. These differences have implications for the optimal composition of a subunit vaccine. For example, B5 would likely be an important component when used for re-vaccination of non-naïve individuals. In addition, the pre-existing PRNT and anti-EV antibodies in non-naïve subjects, most of whom were probably vaccinated more than 30 years ago, adds to the growing body of evidence that partial humoral immunity lasts well beyond the 5−10 years thought to be the duration of reliable clinical protection imparted by vaccination [4,6,36,42].
Vaccination with VV vaccine diluted 1:10 was previously demonstrated to elicit similar major reaction rates as with undiluted vaccine, but was associated with higher PRNT antibody titers, reduced local inflammatory response and increased satellite lesions in vaccinia-naïve subjects [7,43]. However, we observed no differences in membrane protein specific or PRNT antibody responses in the subset of non-naïve subjects who received diluted vaccine, implying that the vaccine antigen dose-dependent antibody response observed in vaccinia-naïve subjects may be attenuated by pre-existing immunity.
Surrogate markers of immunity are important for estimating the efficacy of orthopoxvirus vaccines where clinical efficacy cannot be measured by disease acquisition following exposure. While the major reaction elicited by replicating VV vaccine is an excellent surrogate for clinical immunity, other markers will be necessary for evaluating subunit and other non-replicating or minimally-replicating vaccines such as Modified Vaccinia Ankara (MVA) [44]. We found that antibody responses directed against specific membrane proteins were quite variable within each of the groups studied. This phenomenon was also previously noted in animals vaccinated with VV or with subunit vaccines [23,27]. In humans, it is unclear if this represents host variability, variation in dose of vaccine delivered, or variability of viral clones in calf lymph-derived replicating VV vaccine [45]. This observed variability suggests that a single characteristic pattern of membrane protein specific antibody responses to VV vaccine may not exist. Because the equivalent MVA membrane protein genes share 98−100% homology with other vaccinia strains, it is anticipated that specific antibody responses to MVA would show similar variability [46].
Furthermore, in our study there were too few subjects without a major reaction to assess the usefulness of specific antibody responses as predictors of immunity. For example, anti-A33 antibody detection was highly sensitive for predicting immunity in vaccinianaïve subjects, but its specificity could not be determined because all of the vaccinia-naïve subjects developed a major reaction. All subjects with post-vaccination anti-L1 antibodies developed a major reaction, making this a highly specific test, but this finding is of limited value given the low percentage of subjects responding to L1. Thus, there is insufficient evidence that any of the membrane protein specific antibodies are better for predicting clinical immunity than anti-MV PRNT antibodies following vaccination with VV [6]. However, anti-MV PRNT antibodies may not be as good a predictor of protection after vaccination with a subunit vaccine. This was demonstrated by Hooper, et al, as mice vaccinated with A27 alone developed high-titer PRNT antibodies but were not reliably protected versus viral challenge [11], probably because anti-EV antibody responses were not elicited.
Our study has several recognizable limitations. Although overall immunity to orthopoxviruses is thought to be mostly dependent on humoral responses, which may be sufficient to provide full protection in some instances [5,13,36], cell-mediated and innate immune are likely important but were not addressed in this study [47-49]. Second, we examined polyclonal total IgG binding antibody responses only, and did not address functionality. Third, data regarding the number of previous vaccinations or time since vaccination in non-naïve subjects were not available, thus it was not possible to determine the relative importance of these variables on antibody titers. Finally, a more in-depth evaluation of non-responders is needed to better assess the performance characteristics of specific membrane protein ELISAs for predicting immunity.
In conclusion, we have provided further characterization of normal human antibody responses to specific VV membrane proteins following vaccination with VV. The EV and MV membrane proteins studied were variably immunogenic as vaccinia-naïve subjects responded best to A33 and A27, while non-naïve subjects exhibited vigorous boosting of B5. Anti-L1 antibody responses were poor in both groups. There is good evidence that B5, A33, and A27 could be important components of subunit vaccines and targets for monoclonal antibody products.
Acknowledgements
We kindly thank Sherri Koehm for data acquisition, Dawn-Michele Cannon for manuscript preparation assistance, Joan Cannon for regulatory assistance, and the participants in the vaccine clinical trials.
Financial Support – this study was supported by NIH Grant #1 U54 AI057160-01 (Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research) to SLS, contract# N01-AI-25464 to RBB and SEF, and grants NIH U54 AI57168 and NIH 1 UC1 AI062486 to GHC and RJE. SJL was a MRCE Biodefense Clinical/Translational Fellow.
Footnotes
Potential Conflicts of interest. F.K.N. is a consultant for VaxGen, Inc. G.H.C. and R.J.E. are on an NIH Challenge grant to Chesapeake-Perl (Savage, MD) to develop a subunit vaccine that is the topic of this paper. Their home institution (University of Pennsylvania) is pursuing a patent for the use of the vaccinia proteins of interest as a vaccine. All other authors report no conflicts of interest.
Prior Presentation - The data were presented in part at the Regional Centers of Excellence for Biodefense and Emerging Infections 2nd Annual Meeting, Galveston, TX, March 2005 and the 8th Annual Conference on Vaccine Research (Abstract #184), Baltimore, MD, May 2005.
References
- 1.Henderson DA, Inglesby TV, Bartlett JG, et al. Smallpox as a biological weapon: medical and public health management. JAMA. 1999;281:2127–37. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 2.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–50. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 3.Dixon CW. Smallpox. J&A Churchill Ltd; London: 1962. [Google Scholar]
- 4.Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–11. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 5.Edgehill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–7. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Vaccinia (smallpox) vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR. 2001;50(RR10):8. [PubMed] [Google Scholar]
- 7.Frey SE, Couch RB, Tacket CO, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346:1265–74. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 8.Casey CG, Iskander JK, Roper MH, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA. 2005;294:2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 9.Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis following smallpox vaccination among vaccinia-naïve US military personnel. JAMA. 2003;289:3283–89. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 10.Fogg C, Lustig SA, Whitbeck JC, Eisenberg RJ, Cohen CH, Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78:10230–7. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper JW, Custer DM, Thompson E. Four gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306:181–95. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal antibodies to proteins of the outer membranes of the two infections forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79:13454–62. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Earl P, Americo J, et al. Chimpanzee / human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci USA. 2006;103:1882–7. doi: 10.1073/pnas.0510598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83:2915–31. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 15.Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Wolffe EJ, Isaacs SN, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67:4732–41. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roper RL, Wolffe EJ, Weisberg A, Moss B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J Virol. 1998;72:4192–204. doi: 10.1128/jvi.72.5.4192-4204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravenello MP, Hruby DE. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J Virol. 1994;68:6401–10. doi: 10.1128/jvi.68.10.6401-6410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson CM, Hollinshead M, Smith GL. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J Gen Virol. 2000;81:47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- 20.Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254:71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 21.Pulford DJ, Gates A, Bridge SH, Robinson JH, Ulaeto D. Differential efficacy of vaccinia envelope proteins administered by DNA immunization in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004;22:3358–66. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–39. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 23.Hooper JW, Thompson E, Wilhelmsen C, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78:4433–43. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heraud J-M, Edgehill-Smith Y, Ayala V, et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177:2552–64. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez JC, Tapia E, Esteban M. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits replication efficiently under prophylactic and therapeutic conditions. J Gen Virol. 2002;83:1059–67. doi: 10.1099/0022-1317-83-5-1059. [DOI] [PubMed] [Google Scholar]
- 26.Bell E, Shamim M, Whitbeck JC, Sfyroera G, Lambris LD, Isaacs SN. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325:425–31. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Law M, Pütz MM, Smith GL. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J Gen Virol. 2005:991–1000. doi: 10.1099/vir.0.80660-0. [DOI] [PubMed] [Google Scholar]
- 28.Jones-Trower A, Garcia A, Meseda CA, He Y, Weiss C, Kumar A, Weir JP, Merchlinsky M. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343:128–40. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Pütz MM, Midgley CM, Law M, Smith GL. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat Med. 2006;12:1310–5. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- 30.Frey SE, Newman FK, Yan L, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA. 2003;289:3295–9. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 31.Frey SE, Newman FK, Cruz J, et al. Dose-related effects of smallpox vaccine. N Eng J Med. 2002;346:1275–80. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- 32.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol. 2005;79:6260–71. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, et al. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by a vaccinia virus L1R gene. Virology. 2005;34:59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Newman FK, Frey SE, Blevins TP, Mandava M, Bonifacio A, Jr., Yan L, Belshe RB. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J Clin Micro. 2003;41:3154–57. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennekens CH, Buring JE. Epidemiology in Medicine. Little, Brown & Co.; Boston/Toronto: 1987. p. 336. [Google Scholar]
- 36.Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccines. Microbes Infect. 2005;7:579–83. doi: 10.1016/j.micinf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Bray M. Henry Kempe and the birth of vaccinia immune globulin. Clin Infect Dis. 2004;39:767–9. doi: 10.1086/423005. [DOI] [PubMed] [Google Scholar]
- 38.Law M, Smith GL. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology. 2001;280:132–42. doi: 10.1006/viro.2000.0750. [DOI] [PubMed] [Google Scholar]
- 39.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 40.Fang M, Cheng H, Dai Z, Bu Z, Sigal LJ. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology. 2006;345:231–43. doi: 10.1016/j.virol.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 41.Chung C-S, Chen C-H, Ho M-Y, Huang C-Y, Liao C-L, Chang W. Vaccinia virus proteome: Identification of proteins in vaccinia virus intracellular mature virion particles. J Virol. 2006;80:2127–40. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 43.Belshe RB, Newman FK, Frey SE, Couch RB, Treanor JJ, Tacket CO, Yan L. Dose-dependent neutralizing-antibody responses to vaccinia. J Infect Dis. 2004:493–7. doi: 10.1086/380906. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–67. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 45.Artenstein AW, Johnson C, Marbury TC, et al. A novel, cell culture-derived smallpox vaccine in vaccinia-naïve adults. Vaccine. 2005;23:3301–9. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 46.Antoine G, Scheiflinger F, Dorner F, Falkner G. The complete genomic sequence of the Modified Vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–96. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 47.Tang J, Murtadha M, Schnell M, Eisenlohr LC, Hooper J, Flomenberg P. Human T-cell responses to vaccinia virus envelope proteins. J Virol. 2006;80:10010–20. doi: 10.1128/JVI.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DYM. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Chisholm SE, Reyburn HT. Recognition of vaccinia virus-infected cells by human natural killer cells depends on natural cytotoxicity receptors. J Virol. 2006;80:1125–33. doi: 10.1128/JVI.80.5.2225-2233.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]