Abstract
Vascular endothelial growth factor (VEGF) acts as a hierarchically high switch of the angiogenic cascade by interacting with its high affinity VEGF receptors and with neuropilin co-receptors. VEGF165 binds to both Neuropilin-1 (NP-1) and VEGFR-2, and it is believed that ligand binding forms an extracellular bridge between both molecules. This leads to complex formation, thereby enhancing VEGFR-2 phosphorylation and subsequent signaling. We found that inhibition of VEGF receptor (VEGFR) phosphorylation reduced complex formation between NP-1 and VEGFR-2, suggesting a functional role of the cytoplasmic domain of VEGFR-2 for complex formation. Correspondingly, deleting the PDZ-binding domain of NP-1 decreased complex formation, indicating that extracellular VEGF165 binding is not sufficient for VEGFR-2-NP-1 interaction. Synectin is an NP-1 PDZ-binding domain-interacting molecule. Experiments in Synectin-deficient endothelial cells revealed reduced VEGFR-2-NP-1 complex formation, suggesting a role for Synectin in VEGFR-2-NP-1 signaling. Taken together, the experiments have identified a novel mechanism of NP-1 interaction with VEGFR-2, which involves the cytoplasmic domain of NP-1.
Angiogenesis, the growth of new blood vessels from a pre-existing vascular network, plays a pivotal role during embryonic development and in numerous diseases including cancer, chronic inflammation, and vision-threatening retinopathies. Vascular endothelial growth factors (VEGF)2 are a family of mitogenic and chemotactic factors for endothelial cells (EC), which act as hierarchically high inducers of the angiogenic cascade (1). VEGFs signal through the high affinity receptor tyrosine kinases VEGFR-1, VEGFR-2, and VEGFR-3, which are almost exclusively expressed by EC. In addition to the VEGF receptors, two other molecules, namely Neuropilin-1 (NP-1) and Neuropilin-2 (NP-2), have been identified as co-receptors for VEGF (2). During development, NP-1 is expressed by arterial EC (3), whereas NP-2 is expressed by venous and lymphatic EC (4). NP-1 enhances the affinity of VEGF165 to VEGFR-2 (2) and increases its phosphorylation, thereby enhancing downstream signaling (5). Correspondingly, overexpression of NP-1 leads to the formation of excess capillaries and blood vessels (6, 7). Targeted disruption of NP-1 results in embryonic lethality and vascular defects such as impairment of neural vascularization, transposition of large vessels, and insufficient development of vascular networks in the yolk sac (7, 8).
The mechanistic analysis of the co-receptor function of neuropilins for VEGFR signaling has concentrated on the extracellular domain of the neuropilins. It was suggested that VEGF165 binds to VEGFR-2 and NP-1 to form a bridge between the extracellular domains, which results in the formation of complexes (9). However, recent work employing VEGF variants that either bind only NP-1 or bind only VEGFR-2 has suggested that the extracellular ligand-bridging model is not sufficient to account for a signal enhancing function of NP-1 in the absence of extracellular ligand binding (10). Likewise, although VEGF121 has been shown to bind NP-1 (11), stimulation with VEGF121 is not sufficient to promote complex formation between VEGFR-2 and NP-1, suggesting a further mechanism in complex formation between NP-1 and VEGFR-2.
Previous research has shown that the intracellular domain of NP-1 does not contain sequences predicted to have enzymatic activities (12). Nevertheless, the transmembrane and cytoplasmic domains share over 90% amino acid identity across species (13, 14), suggesting an evolutionary conserved role for these domains. We therefore hypothesized that the intracellular domain of NP-1 may be functionally involved in complex formation. This hypothesis was also supported by the observation that blockade of VEGFR-2 phosphorylation negatively interfered with VEGFR-2-NP-1 complex formation. The experiments of this study revealed that the PDZ-binding domain in the cytoplasmic domain of NP-1 is required for stable VEGFR-2-NP-1 complex formation. The identification of Synectin in this intracellular association supports an intracellular VEGFR-2-NP-1 association involving Synectin as a bridging molecule.
EXPERIMENTAL PROCEDURES
Materials—Antibodies against NP-1 (C-19), VEGFR-2 (C-1158) and phospho-Tyrosine (PY99) used for Western blot analysis were purchased from Santa Cruz Biotechnology. The antibody directed against NP-1 used in immunofluorescent analysis was obtained from Miltenyi Biotech. The antibody directed against VEGFR-2 (3G2) used for immunoprecipitation of human VEGFR-2 was provided by the Tumor Biology Center Freiburg (Freiburg, Germany), the antibody used for immunoprecipitation of mouse VEGFR-2 was purchased from Santa Cruz Biotechnology (C-1158), and the antibody used for immunofluorescence analysis was obtained from R&D Systems. The low molecular weight VEGFR-inhibitor PTK787/ZK 222584 was kindly provided by Novartis and used at a final concentration of 100 nm. VEGF165 was purchased from R&D Systems and used at a final concentration of 25 ng/ml.
Cell Culture—Human umbilical vein endothelial cells (HUVEC), endothelial cell growth medium, endothelial cell basal medium, and corresponding supplements were purchased from Promocell, Heidelberg, Germany. HUVEC were cultured in endothelial cell growth medium containing 10% FCS. For growth factor starvation, cells were incubated in endothelial cell basal medium supplemented with 5% FCS for 16 h. HUVEC were used between passages 2 and 6. Porcine aortic endothelial cells (PAEC) were cultured in Nutmix (Invitrogen) containing 10% FCS. For growth factor starvation, PAEC were incubated in Nutmix without FCS for 16 h. Mouse EC were isolated and cultured as described previously (15).
siRNA Transfection of HUVEC—The Synectin-specific siRNA (a) sense, 5′-AAAGGAACCCGGAUGAGCUtt-3′ and antisense, 5′-AGCUCAUCCGGGUUCCUUUtg-3′ and (b) sense, 5′-CCUGCUGGAGAGUUACAUGtt-3′ and antisense, 5′-AUGUAACUCUCCAGCAGGtc-3′ and control non-silencing siRNA were purchased from Ambion and transfected into HUVEC as described previously (16). Down-regulation of Synectin was analyzed by RT-PCR 24 h following transfection.
RNA Isolation and RT-PCR—RNA isolation and RT-PCR were performed as described previously (16).
Expression Vector, Retroviral Constructs, and Adenoviral Constructs—PAE cells were transfected using a retroviral expression system pLIB (Clontech), which was modified and kindly provided by Dr. Ralph Graeser (Tumor Biology Center, Freiburg, Germany). Full-length human VEGFR-2, human NP-1, and the human NP-1 lacking the PDZ-binding domain (NP-1ΔPDZ) were cloned into modified pLIB. Together with pVSVG, the constructs were transfected into human embryonic kidney Ampho 293 cells. Virus-containing supernatants were then used for transfection of PAEC. HUVEC were transfected with an adenoviral expression system (Invitrogen). Full-length NP-1 and NP-1ΔPDZ were cloned into pDEST according to the Gateway system manufacturer's protocol, and HUVEC were transfected with 100 infectious units/cell.
VEGF165 Binding Assay on PAEC—Specific binding experiments using iodinated VEGF165 binding to PAEC expressing recombinant NP-1 or NP-1ΔPDZ were performed as described previously (10) and analyzed using the GraphPad prism-4 software.
VEGFR-2 Phosphorylation Assay and Co-immunoprecipitation Experiments—HUVEC or PAEC were grown to 90% confluence before being starved overnight. Cells were stimulated with VEGF165 for various time points. After stimulation, cells were washed and lysed (150 mm NaCl, 50 mm Tris/HCl, pH 7.4, 1% Nonidet P-40, 10 mm EDTA, 10% glycerin, 1% protease inhibitor mixture (Serva)). For experiments with VEGFR inhibitor, cells were preincubated with 100 nm PTK787/ZK 222584 1 h prior to stimulation with VEGF165. For immunoprecipitation, extracts were incubated with an antibody against VEGFR-2 and protein-G-Sepharose overnight at 4 °C. The Sepharose beads were spun down and washed twice with ice-cold phosphate-buffered saline, 0.1% Nonidet P-40. The beads were boiled in sample buffer and loaded on an 8% SDS-PAGE, blotted onto a polyvinylidene fluoride membrane, and probed with antibodies directed against phospho-Tyrosine, VEGFR-2, and NP-1. Western blot analysis was performed using horseradish peroxidase conjugated secondary antibodies. Bound antibody was visualized using the ECL kit of Amersham Biosciences according to the instructions of the vendor.
RESULTS
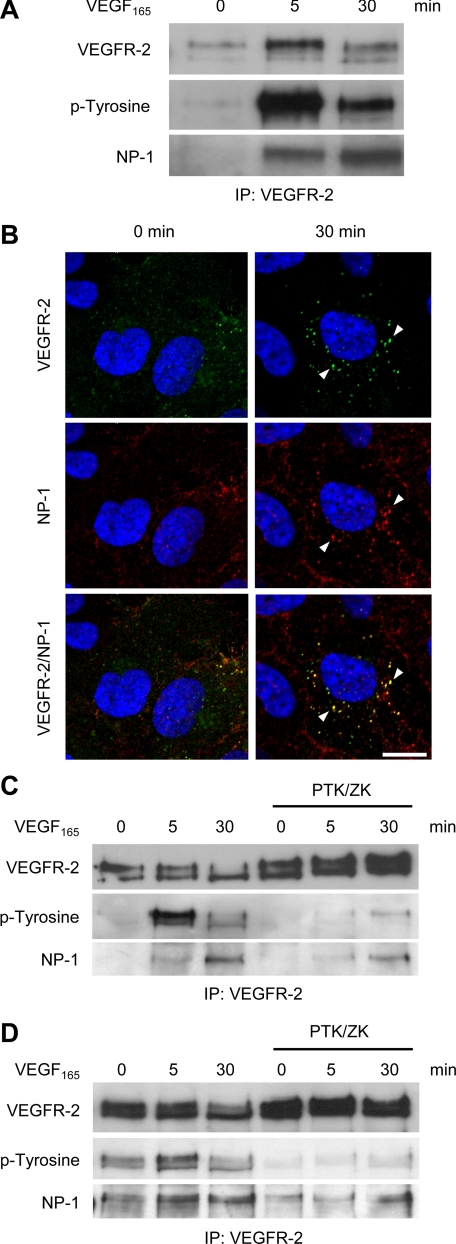
VEGFR-2 and NP-1 Associate upon Stimulation with VEGF165—Endothelial cell expressed NP-1 serves as a co-receptor for VEGF165, forming complexes with VEGFR-2 upon stimulation with VEGF165 (2). To further characterize the kinetics of this interaction, we performed co-immunoprecipitation experiments following VEGF165 stimulation for various periods of time. VEGFR-2 immunoprecipitation from primary human umbilical vein endothelial cells (HUVEC) followed by Western blot analysis revealed enhanced VEGFR-2-NP-1 complex formation after prolonged stimulation with VEGF165 (30 min versus 5 min). This did not parallel the phosphorylation level of VEGFR-2, which was strongest after 5 min of stimulation (Fig. 1A). Immunocytochemical receptor trafficking analysis showed vesicular co-internalization of VEGFR-2 and NP-1 upon stimulation with VEGF165 (Fig. 1B).
FIGURE 1.
Induction of complex formation between VEGFR-2 and NP-1 following VEGF165 stimulation and inhibition of VEGF-induced VEGFR-2-NP-1 complex formation by the VEGFR receptor blocker PTK/ZK. A, VEGFR-2 immunoprecipitation (IP) of HUVEC stimulation with VEGF165 followed by Western blot analysis revealed complex formation between NP-1 and VEGFR-2, which increased with the length of stimulation. B, double staining for VEGFR-2 (green) and NP-1 (red) and subsequent confocal analysis showed that VEGFR-2 and NP-1 were internalized together upon stimulation with VEGF165 (white arrowheads). Nuclei were stained with Hoechst (blue). Scale bar, 10μm. C, HUVEC were incubated with PTK/ZK for 1 h and stimulated with VEGF165 for the indicated time points. Thereafter, cells were lysed, and protein extracts were immunoprecipitated with an anti-VEGFR-2 antibody. VEGFR-2 immunoprecipitation revealed that PTK/ZK effectively led to a reduction of stable complexes between NP-1 and VEGFR-2 after stimulation with VEGF165. D, PAEC expressing VEGFR-2 and NP-1 were treated with PTK/ZK prior to stimulation with VEGF165. VEGFR-2 immunoprecipitation revealed that PTK/ZK effectively led to a reduction of stable complexes between NP-1 and VEGFR-2 after stimulation with VEGF165.
VEGFR-2 Phosphorylation Is Required for Association between NP-1 and VEGFR-2—The observed increase of stable VEGFR-2-NP-1 complex formation over time suggested that complex formation itself may be dependent on the activation status of VEGFR-2. We therefore performed VEGF165-dependent complex formation experiments in the presence of the VEGF receptor blocker PTK787/ZK222584 (PTK/ZK). PTK/ZK inhibits VEGFR phosphorylation but does not affect the binding of VEGF165 to its receptor (17). It thereby facilitated the selective analysis of VEGFR-2-NP-1 complex formation during suppressed VEGFR-2 phosphorylation without affecting the binding of VEGF165. Surprisingly, treatment of primary HUVEC with PTK/ZK prior to stimulation with VEGF165 led to a decrease in VEGFR-2-NP-1 complex formation (Fig. 1C). Western blot analysis of phospho-VEGFR-2 confirmed the effectiveness of PTK/ZK in inhibiting VEGFR-2 phosphorylation. To further validate these findings, we performed the same experiment with NP-1- and VEGFR-2-co-transfected PAEC. As in HUVEC, PTK/ZK inhibited the complex formation in PAEC (Fig. 1D). Taken together, these results indicate that formation of VEGFR-2-NP-1 complexes is not only dependent on ligand binding but also affected by the activation status of VEGFR-2.
The Cytoplasmic Domain of NP-1 Contributes to VEGFR-2-NP-1 Complex Formation—The observed contribution of VEGFR-2 phosphorylation suggested an intracellular contribution to VEGFR-2-NP-1 complex formation. Blockade of VEGFR-2 phosphorylation may inhibit receptor internalization, as evidenced by reports showing that VEGFR-2 phosphorylation is required for receptor internalization (18–20). In turn, the cytoplasmic domain of NP-1 is highly conserved across species, suggesting an evolutionary pressure to maintain a functional role. To examine whether the cytoplasmic domain of NP-1 is involved in VEGFR-2-NP-1 complex formation, a mutant of NP-1 lacking the cytoplasmic domain (NP-1ΔC) was cloned and expressed in PAEC expressing VEGFR-2. The expression of NP-1ΔC in PAEC was confirmed by Western blot analysis, and live staining for NP-1 validated that NP-1ΔC was expressed at the cell surface (data not shown). NP-1ΔC and VEGFR-2 expressing PAEC were stimulated with VEGF165 and lysed, and complex formation was examined by immunoprecipitation and Western blot analysis. Significantly reduced complex formation of NP-1ΔC and VEGFR-2 was observed upon stimulation with VEGF165 when compared with PAEC co-expressing full-length NP-1 and VEGFR-2 (data not shown).
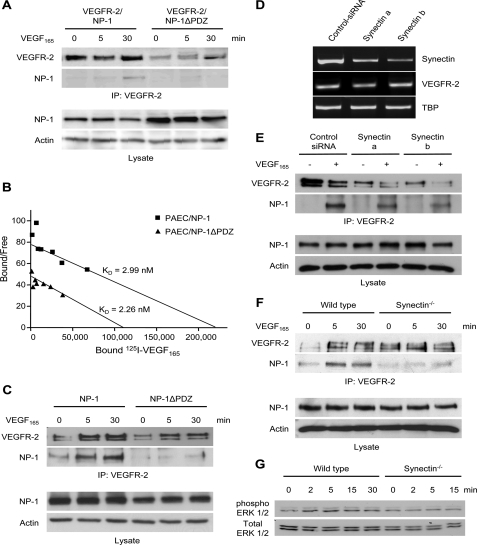
Based on these findings, a series of NP-1 cytoplasmic domain truncation mutants was cloned and co-expressed with VEGFR-2 in PAEC. When analyzing these mutants for VEGF165 ligand-induced complex formation, deletion of just the last three amino acids in the cytoplasmic domain of NP-1 (PDZ-binding domain) proved sufficient to significantly inhibit complex formation between VEGFR-2 and NP-1 (Fig. 2A). Binding experiments confirmed that NP-1ΔPDZ bound VEGF165 with the same affinity as full-length NP-1 (Fig. 2B). To extend these findings to primary EC, NP-1 and NP-1ΔPDZ were expressed in HUVEC. As in PAEC, deletion of the PDZ-binding domain in the C-terminal tail of NP-1 proved sufficient to significantly reduce ligand-induced VEGFR-2-NP-1 complex formation (Fig. 2C). Collectively, these data solidly establish a role of the PDZ-binding domain in NP-1 in contributing to VEGFR-2-NP-1 complex formation following VEGF165 stimulation.
FIGURE 2.
The PDZ-binding domain of NP-1 and Synectin are involved in the complex formation between NP-1 and VEGFR-2. A, PAEC-VEGFR-2-NP-1 and PAEC-VEGFR-2-NP-1ΔPDZ were stimulated with VEGF165 for the indicated time points. Western blot analysis of cell lysate confirmed similar expression levels of NP-1 and NP-1ΔPDZ in both cell lines. Actin was used as an internal loading control. Thereafter, the remaining lysate was immunoprecipitated (IP) with an anti-VEGFR-2 antibody. Subsequent Western blot analysis revealed that VEGFR-2 and NP-1ΔPDZ formed fewer complexes than VEGFR-2 and full-length NP-1. B, Scatchard analysis of VEGF165 showed a similar binding of increasing concentrations of bound 125I-labeled VEGF165 to PAEC-NP-1 or to PAEC-NP-1ΔPDZ. C, following transfection of HUVEC with NP-1 or NP-1ΔPDZ, cells were stimulated with VEGF165 for the indicated time points. Western blot analysis confirmed similar expression levels of NP-1 and NP-1ΔPDZ in the transfected cells. Actin was used as an internal loading control. Thereafter, the remaining lysate was immunoprecipitated with an anti-VEGFR-2 antibody. Subsequent Western blot analysis revealed that VEGFR-2 and NP-1ΔPDZ form fewer complexes than VEGFR-2 and full-length NP-1 in transfected HUVEC. D, HUVEC were transfected with siRNA directed against Synectin. mRNA was isolated 24 h following transfection, and RT-PCR of Synectin was performed to confirm silencing of Synectin on the mRNA level. RT-PCR for VEGFR-2 was performed to check whether silencing of Synectin changed its expression on the mRNA level. TATA box-binding protein (TBP) was used as an internal loading control. No difference between scramble siRNA and Synectin siRNA transfected cells could be detected. E, following silencing of Synectin, HUVEC were stimulated with VEGF165 and lysed. Western blot analysis confirmed that silencing of Synectin did not affect NP-1 expression. Actin was used as an internal loading control. Thereafter, the remaining lysate was immunoprecipitated with an anti-VEGFR-2 antibody. Subsequent Western blot analysis showed lower protein levels of VEGFR-2 following silencing of Synectin. Reprobing with NP-1 showed that less NP-1 associated with VEGFR-2 after silencing of Synectin. F, analysis of the association between NP-1 and VEGFR-2 in Synectindeficient EC. Arterial EC were freshly isolated from wild type and Synectin-deficient mice and were stimulated with VEGF165 for the indicated time points. Western blot analysis of the cell lysate confirmed similar expression levels of NP-1 in both wild type and Synectin-deficient EC. Actin was used as an internal loading control. Thereafter, the remaining lysate was immunoprecipitated with an anti-VEGFR-2 antibody. Subsequent Western blot analysis revealed that VEGFR-2 and NP-1 formed fewer complexes in Synectin-deficient EC when compared with wild type EC. G, comparative analysis of VEGF-induced ERK phosphorylation in wild type and Synectin-deficient EC. Early passage (passage 3) arterial EC from wild type and Synectin-deficient mice were stimulated with VEGF165 (25 ng/ml) for the indicated time points. Thereafter, the cells were lysed, and equal aliquots were run on parallel gels. Blotted gels were probed with phospho-ERK and ERK antibodies, respectively (1:1000; Cell Signaling). ERK phosphorylation following VEGF stimulation was reduced in Synectin-deficient EC when compared with EC isolated from wild type mice.
Effect of Synectin on the Expression of VEGFR—The only protein reported binding to the cytoplasmic domain of NP-1 was identified in a yeast two-hybrid screen (21) and termed Neuropilin-1-interacting-protein. Neuropilin-1-interacting-protein is also known as Synectin, GIPC1, or Semcap-1. Synectin is a cytoplasmic protein that binds the PDZ-binding domain of more than 25 receptors and adhesion molecules including the GTPase-activating protein RGS-GAIP (regulator of G-protein signaling Gα interaction protein) (22), the receptor tyrosine kinases TrkA and TrkB (23, 24), and the integrin α 6A subunit (25). A recent report has established an essential role of Synectin for developmental and adult formation of arteries (15). Given that Synectin is expressed in all cells and tissues, the arterial phenotype of Synectin-deficient mice suggested an involvement of one or more arterial EC selectively expressed interaction partners. The identified contribution of the PDZ-binding domain of NP-1 in VEGFR-2-NP-1 complex formation and the established arterial-specific expression of NP-1 (3) strongly suggested that Synectin may play a direct role in VEGFR-2-NP-1 complex formation. To test this hypothesis, HUVEC were transfected with siRNA to silence endogenous Synectin expression. Silencing of Synectin expression was confirmed by RT-PCR (Fig. 2D). Synectin-silenced HUVEC were stimulated with VEGF165 for 30 min and lysed, and VEGFR-2 was probed by immunoprecipitation and analyzed by Western blot analysis. Silencing of Synectin expression led to an apparent decrease of VEGFR-2-NP-1 complex formation (Fig. 2E). However, total protein levels of VEGFR-2 were also reduced (Fig. 2E), suggesting that Synectin silencing may affect VEGFR-2 expression. RT-PCR analysis of VEGFR-2 mRNA expression revealed that VEGFR-2 mRNA levels were not affected, suggesting that Synectin may play a role in the post-transcriptional control of VEGFR-2 stability (Fig. 2D). This appeared to be in contrast to a recent study demonstrating no effect of Synectin on VEGFR-2 protein expression in endothelial cells (26), although another study has established a role of Synectin in the stabilization of insulin-like growth factor I receptor (27). Taken together, the experiments suggested a role of Synectin for VEGFR-2-NP-1 complex formation.
Complex Formation of NP-1 and VEGFR-2 Is Dependent on Synectin—To circumvent the limitations of the siRNA-mediated Synectin silencing experiments, freshly isolated EC from Synectin-deficient mice were used to study the role of Synectin in complex formation between NP-1 and VEGFR-2. Synectin-deficient EC expressed VEGFR-2 and NP-1 in similar abundance as wild type EC (Fig. 2F). The difference between Synectin-deficient EC and EC transfected with Synectin siRNA could be due to long term compensatory effects. Co-immunoprecipitation experiments upon stimulation with VEGF165 in Synectin-deficient EC when compared with wild type EC revealed reduced VEGFR-2-NP-1 complex formation in mutant EC (Fig. 2F). Correspondingly, Synectin-deficient EC responded less to VEGF165, as evidenced by reduced ERK phosphorylation following short term stimulation with VEGF165 (Fig. 2G).
DISCUSSION
The data of this study support the concept that the extracellular VEGF165 ligand-bridging model between VEGFR-2 and NP-1 is not sufficient to account for the complexity of VEGF ligand interactions with the VEGF receptors and the neuropilin co-receptors. Instead, the experiments provide conclusive evidence for a functional cytoplasmic interaction between NP-1 and VEGFR-2. The findings in Synectin-deficient mouse EC establish a role of Synectin in the cytoplasmic contribution to VEGFR-2-NP-1 complex formation and support, in combination with the data obtained in HUVEC and PAEC, an interdependency model of complex formation and VEGFR-2 phosphorylation. The demonstration of a functional role of Synectin in VEGFR-2-NP-1 complex formation also provides a mechanistic model linking the arterial expression pattern of NP-1 (3) with the arterial branching morphogenesis phenotype of Synectin-deficient mice (15).
The results of this study are the first to unambiguously ascribe a distinct biochemical role to the cytoplasmic domain of NP-1 and pave the way toward a novel understanding of the VEGFR-2 co-receptor role of NP-1. Future experiments will need to decipher the contribution of extracellular and intracellular VEGFR-2-NP-1 interactions to downstream signaling events and endothelial function. Previous experiments have already hinted at a functional role of the PDZ-binding domain of NP-1. For example, silencing of NP-1 in zebra fish leads to vascular malformations, which cannot be rescued by NP-1 mRNA lacking the last three amino acids of NP-1 (28). As such, the identification of an intracellular association between VEGFR-2 and NP-1 involving a Synectin bridge also suggests that neuropilin vascular functions go beyond a simple enhancer function of VEGFR signaling.
Acknowledgments
We thank the Nikon Imaging Center at the University of Heidelberg for support in image analysis.
This work was supported by Deutsche Forschungsgemeinschaft Grant SPP1190 (Au83/9-1; to H. G. A.), the German-Israel-Foundation (to G. N. and H. G. A.), and National Institutes of Health Grant HL084619 (to M. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; NP-1, Neuropilin-1; NP-1, Neuropilin-2; PDZ, post-synaptic density, disc large, ZO-1; ERK, extracellular signal-regulated kinase; EC, endothelial cells; HUVEC, human umbilical vein endothelial cells; PAEC, porcine aortic endothelial cells; PTK787/ZK222584, PTK/ZK; RT-PCR, reverse transcription-PCR; siRNA, small interfering RNA; FCS, fetal calf serum.
References
- 1.Risau, W. (1997) Nature 386 671–674 [DOI] [PubMed] [Google Scholar]
- 2.Soker, S., Takashima, S., Miao, H. Q., Neufeld, G., and Klagsbrun, M. (1998) Cell 92 735–745 [DOI] [PubMed] [Google Scholar]
- 3.Moyon, D., Pardanaud, L., Yuan, L., Breant, C., and Eichmann, A. (2001) Development (Camb.) 128 3359–3370 [DOI] [PubMed] [Google Scholar]
- 4.Herzog, Y., Kalcheim, C., Kahane, N., Reshef, R., and Neufeld, G. (2001) Mech. Dev. 109 115–119 [DOI] [PubMed] [Google Scholar]
- 5.Becker, P. M., Waltenberger, J., Yachechko, R., Mirzapoiazova, T., Sham, J. S., Lee, C. G., Elias, J. A., and Verin, A. D. (2005) Circ. Res. 96 1257–1265 [DOI] [PubMed] [Google Scholar]
- 6.Kitsukawa, T., Shimono, A., Kawakami, A., Kondoh, H., and Fujisawa, H. (1995) Development (Camb.) 121 4309–4318 [DOI] [PubMed] [Google Scholar]
- 7.Kitsukawa, T., Shimizu, M., Sanbo, M., Hirata, T., Taniguchi, M., Bekku, Y., Yagi, T., and Fujisawa, H. (1997) Neuron 19 995–1005 [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki, T., Kitsukawa, T., Bekku, Y., Matsuda, Y., Sanbo, M., Yagi, T., and Fujisawa, H. (1999) Development (Camb.) 126 4895–4902 [DOI] [PubMed] [Google Scholar]
- 9.Soker, S., Miao, H. Q., Nomi, M., Takashima, S., and Klagsbrun, M. (2002) J. Cell. Biochem. 85 357–368 [DOI] [PubMed] [Google Scholar]
- 10.Shraga-Heled, N., Kessler, O., Prahst, C., Kroll, J., Augustin, H., and Neufeld, G. (2007) FASEB J. 21 915–926 [DOI] [PubMed] [Google Scholar]
- 11.Pan, Q., Chathery, Y., Wu, Y., Rathore, N., Tong, R. K., Peale, F., Bagri, A., Tessier-Lavigne, M., Koch, A. W., and Watts, R. J. (2007) J. Biol. Chem. 282 24049–24056 [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa, H., and Kitsukawa, T. (1998) Curr. Opin. Neurobiol. 8 587–592 [DOI] [PubMed] [Google Scholar]
- 13.Kawakami, A., Kitsukawa, T., Takagi, S., and Fujisawa, H. (1996) J. Neurobiol. 29 1–17 [DOI] [PubMed] [Google Scholar]
- 14.Takagi, S., Kasuya, Y., Shimizu, M., Matsuura, T., Tsuboi, M., Kawakami, A., and Fujisawa, H. (1995) Dev. Biol. 170 207–222 [DOI] [PubMed] [Google Scholar]
- 15.Chittenden, T. W., Claes, F., Lanahan, A. A., Autiero, M., Palac, R. T., Tkachenko, E. V., Elfenbein, A., Ruiz, d. A., Dedkov, E., Tomanek, R., Li, W., Westmore, M., Singh, J. P., Horowitz, A., Mulligan-Kehoe, M. J., Moodie, K. L., Zhuang, Z. W., Carmeliet, P., and Simons, M. (2006) Dev. Cell 10 783–795 [DOI] [PubMed] [Google Scholar]
- 16.Fiedler, U., Reiss, Y., Scharpfenecker, M., Grunow, V., Koidl, S., Thurston, G., Gale, N. W., Witzenrath, M., Rosseau, S., Suttorp, N., Sobke, A., Herrmann, M., Preissner, K. T., Vajkoczy, P., and Augustin, H. G. (2006) Nat. Med. 12 235–239 [DOI] [PubMed] [Google Scholar]
- 17.Wood, J. M., Bold, G., Buchdunger, E., Cozens, R., Ferrari, S., Frei, J., Hofmann, F., Mestan, J., Mett, H., O'Reilly, T., Persohn, E., Rosel, J., Schnell, C., Stover, D., Theuer, A., Towbin, H., Wenger, F., Woods-Cook, K., Menrad, A., Siemeister, G., Schirner, M., Thierauch, K. H., Schneider, M. R., Drevs, J., Martiny-Baron, G., and Totzke, F. (2000) Cancer Res. 60 2178–2189 [PubMed] [Google Scholar]
- 18.Dougher, M., and Terman, B. I. (1999) Oncogene 18 1619–1627 [DOI] [PubMed] [Google Scholar]
- 19.Ewan, L. C., Jopling, H. M., Jia, H., Mittar, S., Bagherzadeh, A., Howell, G. J., Walker, J. H., Zachary, I. C., and Ponnambalam, S. (2006) Traffic 7 1270–1282 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, S., Sawano, A., Nojima, Y., Shibuya, M., and Maru, Y. (2004) FASEB J. 18 929–931 [DOI] [PubMed] [Google Scholar]
- 21.Cai, H., and Reed, R. R. (1999) J. Neurosci. 19 6519–6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vries, L., Lou, X., Zhao, G., Zheng, B., and Farquhar, M. G. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 12340–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato, H., Ohno, K., Hashimoto, K., and Sato, K. (2004) FEBS Lett. 572 123–128 [DOI] [PubMed] [Google Scholar]
- 24.Lou, X., Yano, H., Lee, F., Chao, M. V., and Farquhar, M. G. (2001) Mol. Biol. Cell 12 615–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Mourabit, H., Poinat, P., Koster, J., Sondermann, H., Wixler, V., Wegener, E., Laplantine, E., Geerts, D., Georges-Labouesse, E., Sonnenberg, A., and Aumailley, M. (2002) Matrix Biol. 21 207–214 [DOI] [PubMed] [Google Scholar]
- 26.Wang, L., Dutta, S. K., Kojima, T., Xu, X., Khosravi-Far, R., Ekker, S. C., and Mukhopadhyay, D. (2007) PLoS ONE 2 e1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muders, M. H., Dutta, S. K., Wang, L., Lau, J. S., Bhattacharya, R., Smyrk, T. C., Chari, S. T., Datta, K., and Mukhopadhyay, D. (2006) Cancer Res. 66 10264–10268 [DOI] [PubMed] [Google Scholar]
- 28.Wang, L., Mukhopadhyay, D., and Xu, X. (2006) FASEB J. 20 1513–1515 [DOI] [PubMed] [Google Scholar]