Abstract
Endothelial NO synthase (eNOS) is critically modulated by kinases via the phosphorylation of its Ser1179 (bovine) or Ser1177 (human) residue. Reactive oxygen species such as H2O2 was reported to activate Akt, leading to increased eNOS Ser1179 phosphorylation and activity. But reactive oxygen species are also known to attenuate eNOS function in cardiovascular diseases. Prior studies showing H2O2-stimulated eNOS phosphorylation were performed on serum-starved cells, and only the short term effect of H2O2 was examined. Here we found that the effects of H2O2 on eNOS Ser1179 phosphorylation and function were bidirectional. With endothelial cells cultured with serum, H2O2 initially raised eNOS Ser1179 phosphorylation and activity. However, after the peak increase at 30 min, eNOS Ser1179 phosphorylation dramatically declined. Parallel to the alterations of eNOS Ser1179 phosphorylation, Akt was transiently activated by H2O2 and subsequently became dormant. In contrast, AMP-activated protein kinase (AMPK) was progressively activated in H2O2-treated cells. Blocking Akt activation abolished the initial rise of eNOS Ser1179 phosphorylation after H2O2 treatment. In long term H2O2-treated cells where Akt was deactivated, significant amounts of Ser1179-phosphorylated eNOS remained. AMPK inhibition eradicated the remaining eNOS Ser1179 phosphorylation. Taken together, these studies revealed that Akt and AMPK orchestrated a bidirectional action on eNOS Ser1179 phosphorylation in H2O2-treated cells. Long term H2O2 exposure decreased eNOS Ser1179 phosphorylation, and this might account for the loss of eNOS function in cardiovascular diseases where chronic oxidative injury occurs.
Nitric oxide generated from endothelial cells plays a key role in maintaining cardiovascular homeostasis (1–3). In endothelial cells, NO is derived from l-arginine in a reaction catalyzed by endothelial NO synthase (eNOS)2 (4). To adapt the continuously changing environment, endothelial cells employ a variety of mechanisms to modulate NO production from eNOS. Hence the function of eNOS in cells is regulated in a multifaceted manner (5). In addition to the previously established regulation via Ca2+/calmodulin, eNOS is also critically modulated by protein phosphorylation, protein-protein interactions, and subcellular localization. Among them, protein phosphorylation affords eNOS the most versatile way to respond to extracellular stimuli (6). eNOS has been found to be phosphorylated at several consensus motifs by a number of kinases. These include PKA, PKB/Akt, AMP-activated protein kinase (AMPK), PKC, Ca2+/calmodulin-dependent kinase II, etc. (6).
The best characterized pathway that modulates eNOS function through phosphorylation is the phosphatidylinositol 3-kinase (PI3K)-Akt cascade (5, 6). In the initial studies, vascular endothelial growth factor and shear stress were reported to activate Akt through PI3K, and Akt subsequently phosphorylated the Ser1179/1177 (bovine/human) residue of eNOS (7, 8). Ser1179 phosphorylation enhances eNOS activity, and remarkably, the catalytic function of Ser1179-phosphorylated eNOS no longer requires the rise of intracellular Ca2+. Since the initial reports that vascular endothelial growth factor and shear stress activated eNOS through PI3K-Akt pathway, increasing numbers of hormones or bioactive substances have been found to modulate eNOS function via Akt (6). These include insulin, insulin-like growth factor, angiopoietin-1, estrogen, leptin, sphingosine 1-phosphate, corticosteroids, etc. (5, 6). Clearly, the Akt-eNOS Ser1179 phosphorylation axis has emerged as a central mechanism in regulating NO production from endothelial cells.
Abnormalities of NO production cause diseases. For example, insufficient NO formation has been demonstrated to play an important role in the pathogenesis of hypertension and atherosclerosis (1–4). In these disease settings, it is generally thought that increased reactive oxygen species (ROS) production is the main cause of NO deficiency (9). ROS can either directly inactivate NO or impair eNOS function. Indeed, eNOS dysfunction has been reported in various chronic cardiovascular diseases (10). Interestingly, ROS such as H2O2 was also reported to activate Akt or ERK1/2 leading to increased eNOS Ser1179 phosphorylation and function (11, 12). These cell culture results appear to be in discrepancy with the fact that ROS impairs eNOS function in chronic cardiovascular diseases. It is noticed that these prior studies showing that H2O2 activated eNOS Ser1179 phosphorylation were generally conducted on serum-starved cells, and often only the transient effect of H2O2 was examined. In this study, we determined the effects of H2O2 on eNOS phosphorylation and function in endothelial cells grown in the medium with normal levels of serum. We found that the effects of H2O2 on eNOS Ser1179 phosphorylation and function were bidirectional in cells cultured with serum. Opposite to the initial activation, long term H2O2 exposure down-regulated eNOS Ser1179 phosphorylation and activity. Further studies revealed that this bidirectional action involved both Akt and AMPK. It is the integrated actions of Akt and AMPK that orchestrated the dynamic alterations of eNOS Ser1179 phosphorylation in oxidative stresses.
EXPERIMENTAL PROCEDURES
Materials—Cell culture materials were obtained from Invitrogen. Bovine aortic endothelial cells (BAECs) and growth media were purchased from Cell Systems (Kirkland, WA). 2′, 5′-ADP-Sepharose 4B was the product of Amersham Biosciences. The antibody against eNOS was purchased from the BD Transduction Laboratories. Antibodies against phospho-eNOS (serine 1179), Akt, phospho-Akt (serine 473), phospho-Akt (threonine 308), AMPK, phospho-AMPK (threonine 172) were the products of Cell Signaling Technology (Beverly, MA). The antibody against phospho-eNOS (threonine 497) was purchased from Upstate Biotechnology (Lake Placid, NY). Antibodies against 3-phosphoinositide-dependent kinase 1 (PDK1), protein phosphatase 2A (PP2A)-α/β, PP2A-B56-α, PP2A-C, phospho-ERK1/2, PI3K, and β-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). l-[14C]Arginine was purchased from PerkinElmer Life Sciences. Compound C was a gift from Merck. The protease inhibitor tablet was purchased from Roche Applied Science. Hydrogen peroxide, calmodulin, NADPH, tetrahydrobiopterin, l-arginine, N-nitro-l-arginine methyl ester, and other reagents were purchased from Sigma unless otherwise indicated.
Cell Culture—Bovine eNOS stably transfected human embryonic kidney (HEK) 293 cells were created as reported previously (13). The eNOS-HEK 293 cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Invitrogen) in a 37 °C humidified atmosphere of 95% air and 5% CO2. BAECs were cultured in endothelial growth medium supplemented with 10% fetal bovine serum, 10 μg/ml human recombinant epidermal growth factor, 1 mg/l hydrocortisone, 50 μg/ml gentamicin, 50 ng/ml amphotericin B, and 12 μg/ml bovine brain extracts. BAECs between passages 4 and 10 were used for experiments. H2O2 treatment was conducted on the cells in serum-containing medium.
Western Blotting—The cells were harvested and lysed on ice for 30 min in the modified radioimmunoprecipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 50 mm NaF, 1 mm Na3VO4,5 mm sodium pyrophosphate, and protease inhibitor tablet). Cell lysates were centrifuged at 14,000 × g for 15 min, and the supernatant was recovered. The total protein concentrations were determined by using the detergent compatible protein assay reagents (Bio-Rad). The lysates were denatured by boiling them in the SDS sample buffer. The proteins were separated by SDS/PAGE on 4–20% gradient gels (Invitrogen) and then transferred to nitrocellulose membranes by using a semidry transfer cell (Bio-Rad). After blocking, the membranes were probed with the appropriate primary antibodies. Membrane-bound primary antibodies were detected using secondary antibodies conjugated with horseradish peroxidase. Immunoblots were developed on films using the enhanced chemiluminescence technique (SuperSignal West Pico; Pierce). Densitometry was performed by using an AlphaImager 3300 gel documentation and an image analysis system.
NOS Activity Assay—eNOS activity was measured by the l-[14C]arginine to l-[14C]citrulline conversion assay (14, 15). To measure the activity of p-eNOS, the assay was performed in the presence of 10 nm Ca2+ as reported previously (7, 16). In brief, the cells were harvested in homogenate buffer (50 mm Tris-HCl, pH 7.4, 2 mm dithiothreitol, 50 mm NaF, 1 mm Na3VO4, and protease inhibitor mixture) and homogenated by pulse sonication. After centrifugation (14,000 × g for 15 min at 4 °C), the pellets were recovered, washed, and resuspended in homogenate buffer. The cell lysates (45 μg of protein) were added to the reaction mixture containing 50 mm Tris-HCl, pH 7.4, 0.5 mm NADPH, 10 nm CaCl2,10 μg/ml calmodulin, 10 μm tetrahydrobiopterin, 5 μm l-[14C]arginine, and 45 μm l-arginine. After a 1-h incubation at 37 °C, the reactions were terminated by ice-cold stop buffer (20 mm HEPES and 2 mm EDTA, pH 5.5). l-[14C]Citrulline was separated by passing the reaction mixture through the Dowex AG 50W-X8 (Na+ form, Sigma) cation exchange column and quantitated by liquid scintillation counting.
Pull-down Assay—The cells were harvested and lysed on ice for 30 min in lyses buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 0.5% Nonidet P-40, 50 mm NaF, 1 mm Na3VO4, 5 mm sodium pyrophosphate, and protease inhibitor tablet). The cell lysates were centrifuged at 14,000 × g for 15 min, and the supernatants were recovered. Supernatants containing equal amounts of proteins were incubated with 2′,5′-ADP-Sepharose 4B resins (50 μl in 50% slurry) overnight at 4 °C (13). The resins were washed twice with regular washing buffer (50 mm Tris-HCl, 100 mm NaCl, 1 mm EDTA, and 0.5% Nonidet P-40), three times with high salt washing buffer (50 mm Tris-HCl, 500 mm NaCl, 1 mm EDTA, and 0.5% Nonidet P-40), and another time with regular washing buffer. Pulled down proteins were then eluted by 5 min of boiling of the beads in SDS sample buffer and analyzed by Western blotting.
Statistical Analysis—The data are expressed as the means ± S.E. Comparisons were made using a two-tailed Student's paired or unpaired t test. Differences were considered statistically significant at p < 0.05.
RESULTS
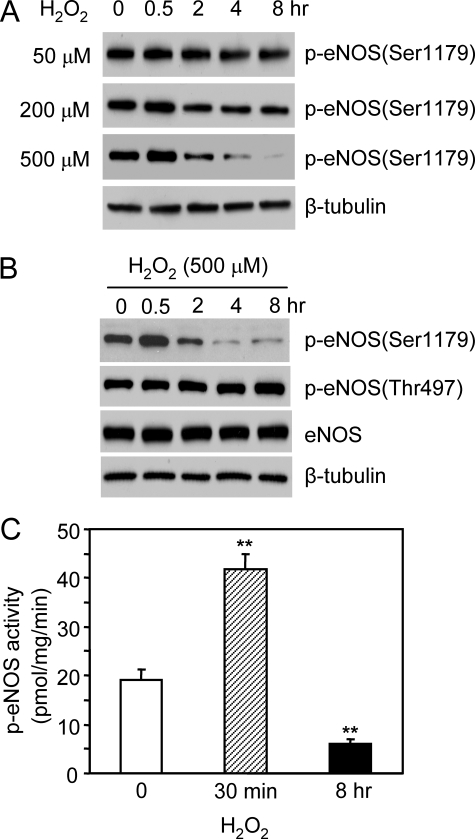
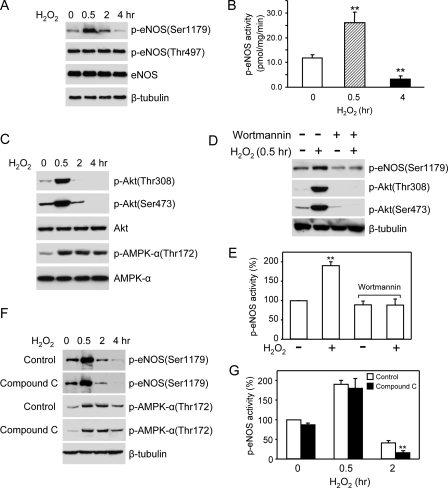
To determine the effect of H2O2 on eNOS phosphorylation, we grew the bovine eNOS stably transfected HEK293 cells in the medium containing 10% serum and subjected them to H2O2 treatment. Under this circumstance, significant amounts of Ser1179-phosphorylated eNOS were detected in resting cells (Fig. 1A). Adding H2O2 (50–500 μm) caused a dose-dependent bidirectional effect on eNOS Ser1179 phosphorylation (Fig. 1A). Initially, H2O2 (500 μm) up-regulated eNOS phosphorylation. The magnitude of eNOS Ser1179 phosphorylation was increased by 175 ± 6.6% in cells at 30 min of H2O2 treatment (p < 0.001, n = 5). Accordingly, p-eNOS activity was elevated (19.7 ± 2.5 versus 41.8 ± 2.5 pmol/mg/min before and after 30 min of H2O2 treatment, p < 0.01, n = 5; Fig. 1C). However, after the peak increase at 30 min, eNOS Ser1179 phosphorylation dramatically declined. At 8 h of H2O2 treatment, the levels of Ser1179-phosphorylated eNOS were reduced more than 3-fold (p < 0.01, versus control, n = 5), and p-eNOS activity was largely diminished (6.1 ± 0.9 pmol/mg/min, n = 5). This time-dependent bidirectional action of H2O2 was specific to Ser1179 because neither eNOS Thr497 phosphorylation nor the total eNOS levels in cells were changed (Fig. 1B). Taken together, these data revealed that with cells cultured in the presence of serum, H2O2 modulated eNOS Ser1179 phosphorylation and function in a bidirectional manner. After a transient activation, long term H2O2 exposure attenuated eNOS Ser1179 phosphorylation leading to decreased enzymatic activity.
FIGURE 1.
Bidirectional effects of H2O2 on eNOS Ser1179 phosphorylation and function. A, the effects of H2O2 (50–500 μm) on eNOS Ser1179 phosphorylation in eNOS-HEK293 cells grown in medium containing 10% fetal bovine serum. B, the effects of H2O2 (500 μm) on eNOS expressions and the phosphorylation status of eNOS Ser1179 and Thr497. As shown, H2O2 selectively caused a bidirectional effect on eNOS Ser1179 phosphorylation. C, effects of H2O2 on eNOS activity. The cells were subjected to H2O2 (500 μm) treatment for 30 min or 8 h. Phospho-eNOS activity was assayed by measuring the l-[14C]arginine to l-[14C]citrulline conversion in the present of 10 nm Ca2+. The data are shown as the means ± S.E. (**, p < 0.01, versus control, n = 5).
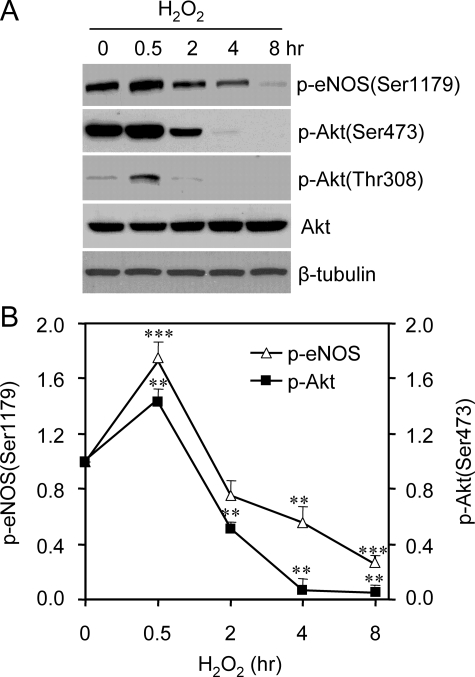
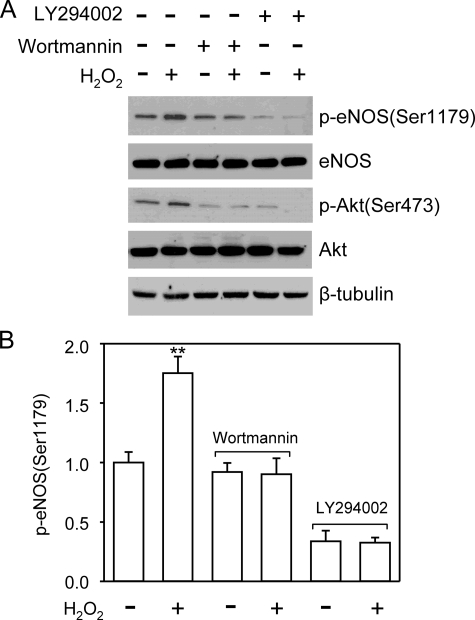
Akt has been shown to be a kinase responsible for eNOS Ser1179 phosphorylation under various conditions. We therefore investigated whether changed Akt activity accounted for the alteration of eNOS Ser1179 phosphorylation. Akt is activated by the phosphorylation of its Thr308 and Ser473 residues. As shown in Fig. 2, the alteration of Akt phosphorylation also exhibited a bidirectional pattern in H2O2-treated cells. The time course of p-Akt levels was largely parallel to that of eNOS Ser1179 phosphorylation. To determine whether Akt activation was responsible for the early rise of eNOS Ser1179 phosphorylation, we treated the cells with PI3K inhibitor wortmannin (1 μm) or LY294002 (20 μm) prior to H2O2 exposure (7, 8). Wortmannin or LY294002 pretreatment prevented H2O2-induced Akt activation. As a result, the early rise of eNOS Ser1179 phosphorylation in H2O2-treated cells was abolished (Fig. 3). These results indicated that H2O2-induced transient elevation of eNOS Ser1179 was mediated by PI3K-Akt pathway. We noticed that PI3K inhibition also reduced basal eNOS Ser1179 phosphorylation, suggesting that Akt was the primary kinase maintaining eNOS Ser1179 phosphorylation in the resting cells grown in the medium containing serum.
FIGURE 2.
Alterations of Akt activation in H2O2-treated cells. A, effects of H2O2 (500 μm) on Akt Ser473 and Thr308 phosphorylation. Consistent with the alteration of eNOS Ser1179,H2O2 treatment also changed the phosphorylation status of Akt Ser473 and Thr308 in a bidirectional manner. Representative blots were shown from six independent experiments. B, quantitative densitometry analyses of the effects of H2O2 of eNOS Ser1179 and Akt Ser473 phosphorylation. The data were shown as the means ± S.E. (*, p < 0.05; **, p < 0.01; ***, p < 0.001 versus the levels prior to H2O2 treatment, n = 6).
FIGURE 3.
Akt inhibition prevented the H2O2-induced increases of eNOS Ser1179 phosphorylation. A, cells were pretreated with PI3K inhibitor wortmannin (1 μm) or LY294002 (20 μm), and the levels of eNOS Ser1179 phosphorylation were examined after 30 min of H2O2 (500 μm) treatment. As shown, PI3K inhibition blocked the H2O2-elicited Akt activation. As a result, the early rise of eNOS Ser1179 phosphorylation was prevented. Representative blots are shown from three independent experiments. B, quantitative analyses of the effects of Akt inhibition on eNOS Ser1179 phosphorylation. The data are shown as the means ± S.E. (**, p < 0.01, versus the levels before H2O2 treatment, n = 3).
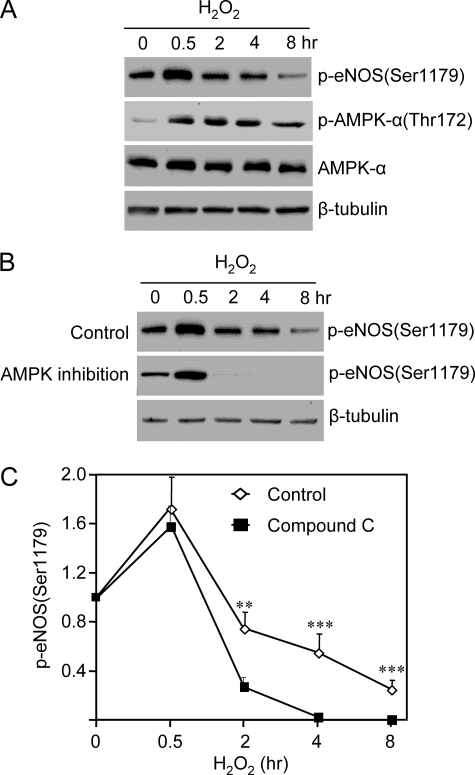
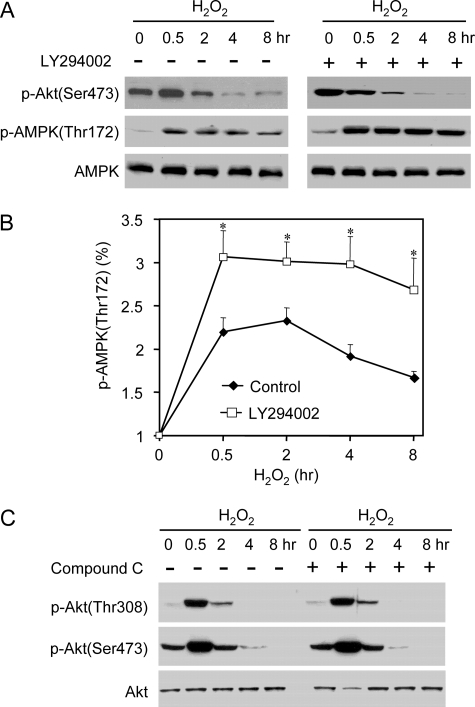
After the initial elevation, the magnitude of eNOS Ser1179 phosphorylation quickly declined below the levels in cells before H2O2 treatment. We noticed that in the cells subjected to long term H2O2 treatment (4–8 h), small yet significant amounts of eNOS Ser1179 phosphorylation remained, whereas p-Akt (Thr308/Ser473) was undetectable (Fig. 2A). This suggested that other kinases might also contribute to the phosphorylation of eNOS Ser1179 in the later phase of H2O2 treatment. To determine the possible involvement of AMPK, we measured AMPK activation by monitoring the alteration of its Thr172 phosphorylation. As expected, H2O2 induced AMPK Thr172 phosphorylation (Fig. 4A). In contrast to the bidirectional manner of Akt activation, AMPK activation sustained up to 8 h after H2O2 treatment. In the 8-h H2O2-treated cells, p-AMPK levels were slightly reduced but still significantly higher than those before H2O2 treatment. To determine whether AMPK contributed to eNOS Ser1179 phosphorylation, we treated the cells with Compound C (10 μm), a well established specific AMPK inhibitor (17–19). As shown in Fig. 4 (B and C), AMPK inhibition did not alter the initial rise of eNOS Ser1179 phosphorylation induced by H2O2. However, the eNOS Ser1179 phosphorylation in the later phase of H2O2 treatment was abolished by AMPK inhibition. These data suggested that AMPK contributed to eNOS Ser1179 phosphorylation in long term H2O2-treated cells.
FIGURE 4.
AMPK contributed to the action of H2O2 on eNOS Ser1179 phosphorylation. A, effects of H2O2 on AMPK Thr172 phosphorylation and expressions in cells. H2O2 triggered a sustained AMPK activation without significantly affecting AMPK expressions. B, effects of AMPK inhibition on eNOS Ser1179 phosphorylation. As shown, AMPK inhibition with Compound C (10 μm) did not significantly affect the initial rise of eNOS Ser1179 phosphorylation. However, the eNOS Ser1179 phosphorylation in the late phase of H2O2 treatment was abolished by AMPK inhibition. Representative data were shown from six independent experiments. C, quantitative comparison of the time course of the effect of H2O2 on eNOS Ser1179 phosphorylated in the absence (control) and presence of AMPK inhibitor compound C. The data are shown as the means ± S.E. (**, p < 0.01; ***, p < 0.001 versus control at the same time, n = 6).
To ascertain that the above findings from eNOS-HEK 293 cells occurred in native endothelial cells, we conducted similar experiments in BAECs. As shown in Fig. 5 (A and B), H2O2 indeed induced bidirectional effects on eNOS Ser1179 phosphorylation and function in BAECs. Similar to what was observed in eNOS-HEK 293 cells, Akt in BAECs was also transiently activated by H2O2 and then became deactivated. On the other hand, AMPK was activated during the entire course of H2O2 treatment (Fig. 5C). Blocking Akt activation with wortmannin prevented the early rise of eNOS Ser1179 phosphorylation in H2O2-treated cells (Fig. 5D). As a result, H2O2-induced increases of p-eNOS activity were also abolished (Fig. 5E). Compound C competitively inhibited AMPK function without affecting AMPK Thr172 phosphorylation (Fig. 5F). AMPK inhibition significantly attenuated eNOS Ser1179 phosphorylation and function in BAECs at the later phase of H2O2 treatment (Fig. 5G). Collectively, these data confirmed that H2O2 caused bidirectional effects on eNOS Ser1179 phosphorylation and function in native endothelial cells. This bidirectional alteration involved both Akt and AMPK.
FIGURE 5.
Bidirectional effects of H2O2 on eNOS Ser1179 phosphorylation and function in BAECs. A, the effect of H2O2 (500 μm) on eNOS Ser1179 phosphorylation in BAECs grown in the medium containing serum. B, effects of H2O2 (500 μm) on eNOS function in BAECs. C, the time courses of Akt and AMPK activation in H2O2-treated cells. D, Akt inhibition abolished the increases of eNOS Ser1179 phosphorylation in 30-min H2O2-stimulated BAECs. E, Akt inhibition eradicated the elevations of p-eNOS activity in the BAECs after 30 min of H2O2 treatment (**, p < 0.01, versus the levels before H2O2 treatment, n = 3). F, effects of AMPK inhibition on eNOS Ser1179 phosphorylation in H2O2-treated BAECs. As shown, AMPK inhibition with Compound C (10 μm) significantly attenuated the eNOS Ser1179 phosphorylation in the late phase of H2O2 treatment. As a competitive inhibitor, Compound C had no effect on AMPK Thr172 phosphorylation. Representative data are shown from three independent experiments. G, effects of AMPK inhibition on eNOS function in H2O2-treated BAECs. AMPK inhibition significantly reduced p-eNOS activity in the BAECs exposed to H2O2 for 2 h. The data are shown as the means ± S.E. (**, p < 0.01, versus control, n = 5).
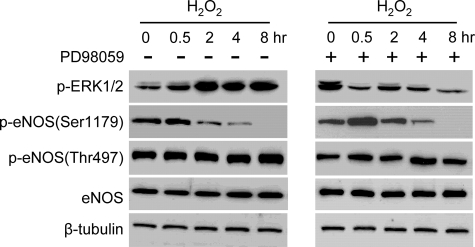
With endothelial cells subjected to serum starvation, MAPK ERK1/2 has been reported to be involved in the phosphorylation of eNOS Ser1179 in the presence of H2O2 (12). We also explored whether ERK1/2 participated in eNOS Ser1179 phosphorylation in cells cultured in the presence of serum. Under this condition, significant levels of p-ERK1/2 were detected in quiescent cells. The addition of H2O2 triggered further ERK1/2 activation, and this was blocked by MEK1/2 (upstream kinase of ERK1/2) inhibition using PD98059 (20 μm) (Fig. 6). However, ERK1/2 inhibition did not significantly change H2O2-elicited bidirectional alterations of eNOS Ser1179 phosphorylation. These results suggested that under the cell culture condition with serum, ERK1/2 did not appear to significantly contribute to the effect of H2O2 on eNOS Ser1179 phosphorylation.
FIGURE 6.
Role of ERK1/2 in the effect of H2O2 on eNOS Ser1179 phosphorylation. The effects of H2O2 (500 μm) on eNOS Ser1179 phosphorylation were measured in the absence and presence of ERK1/2 inhibitor PD98059 (20 μm). Although PD98059 prevented ERK1/2 activation, the bidirectional action of H2O2 on eNOS Ser1179 phosphorylation was not significantly changed by PD98059. Representative data were shown from three independent experiments.
Previous studies reported that Akt can negatively regulate AMPK phosphorylation in hearts (20). We therefore studied whether a cross-talk existed between Akt and AMPK pathways in H2O2-treated cells. We first determined the possible effect of Akt on AMPK activation. As shown in Fig. 7A, PI3K inhibitor LY294002 blocked Akt activation in H2O2-treated cells. Intriguingly, H2O2-induced increases of p-AMPK in Akt-inhibited cells were markedly higher than that in the control cells without LY294002 treatment (Fig. 7, A and B). On the other hand, AMPK inhibition with Compound C had no significant effect on the time course or magnitude of Akt activation in H2O2-treated cells (Fig. 7C). These data suggested that Akt also negatively modulated AMPK activation in cells under oxidative stresses.
FIGURE 7.
Interactions between Akt and AMPK in H2O2-treated cells. A, effects of Akt inhibition on AMPK activation in H2O2-treated cells. The cells were pretreated with PI3K inhibitor LY294002 (20 μm) and then exposed to H2O2 (500 μm). As shown, the levels of p-AMPK were significantly higher in Akt-inhibited cells than that in control cells. B, quantitative analyses of the alterations of p-AMPK in H2O2-treated cells in the absence and presence of LY294002. The data are shown as the means ± S.E. (*, p < 0.05, versus the levels in control cells, n = 3). C, effects of AMPK inhibition (Compound C 10 μm) on Akt activation in H2O2-treated cells. Representative blots are shown from three independent experiments.
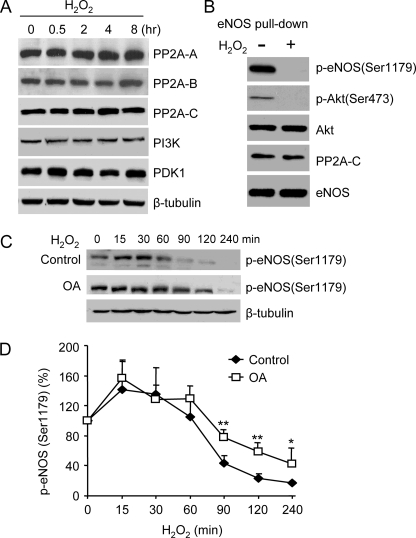
Lastly, we explored the possible roles of protein phosphatases in the bidirectional effects of H2O2 on Akt and eNOS Ser1179 phosphorylation. Both Akt and eNOS Ser1179 have been reported to be dephosphorylated by PP2A (21, 22). To determine whether the loss of Akt and eNOS Ser1179 phosphorylation was due to the increases of PP2A, we measured the effect of H2O2 on PP2A expressions. PP2A is a heterotrimeric enzyme consisting of a catalytic subunit C, a structural subunit A, and a regulatory subunit B (22). As shown in Fig. 8A, none of the PP2A subunit expression was changed in H2O2-treated cells. These data indicated that Akt deactivation as well as the loss of eNOS Ser1179 phosphorylation in long term H2O2-treated cells was not due to elevated PP2A expressions. We also examined the expression levels of Akt upstream kinase PI3K and PDK1. Neither PI3K nor PDK1 expressions were altered by H2O2 treatment. We recently reported that Akt and PP2A associate with eNOS (13). There was a possibility that increased PP2A associations caused Akt and eNOS Ser1179 dephosphorylation. To determine whether this occurred, we pulled down eNOS from control cells and the cells after 8 h of H2O2 treatment and measured eNOS-associated Akt and PP2A. As shown in Fig. 8B, whereas p-Akt and p-eNOS (Ser1179) were both lost, the amounts of Akt and PP2A associated to eNOS were not significantly changed by H2O2 treatment. These results indicated that the loss of Akt and eNOS Ser1179 phosphorylation after long term H2O2 treatment was not due to the increased PP2A associations to eNOS. Finally, we determined whether PP2A blockade prevented the loss of eNOS Ser1179 phosphorylation. BAECs were pretreated with 100 nm okadaic acid (OA). OA at this concentration has been shown to only inhibit PP2A (13, 22). As shown in Fig. 8 (C and D), PP2A inhibition significantly preserved eNOS Ser1179 phosphorylation in the late phase of H2O2 treatment. However, PP2A inhibition was only partially effective because the overall levels of eNOS Ser1179 phosphorylation were still reduced in long term H2O2-treated cells.
FIGURE 8.
Roles of PP2A in the effects of H2O2 on eNOS Ser1179 phosphorylation. A, effects of H2O2 (500 μm) on PP2A, PI3K, and PDK1 expressions in cells. As shown, there was no significant change in the levels of PP2A, PI3K, and PDK1 in cells before and after H2O2 treatment. Representative blots are shown from three independent experiments. B, effects of H2O2 on the associations of Akt and PP2A to eNOS. After 8 h of H2O2 treatment, eNOS in cells was pulled down with the 2′,5′-ADP-Sepharose beads, and eNOS-associated proteins were measured by Western blotting with respective antibodies. Consistent with the results with cell lysates, long term H2O2 treatment diminished Akt (Ser473) and eNOS (Ser1179) phosphorylation. However, with equal amounts of eNOS input, the levels of Akt and PP2A associated to eNOS were not significantly changed by H2O2 treatment. All of the data shown are representatives of three independent experiments. C, effects of PP2A inhibition on the alterations of eNOS Ser1179 phosphorylation in H2O2-treated BAECs. BAECs were pretreated with OA (100 nm) and then exposed to H2O2 (500 μm). As shown, OA partially protected eNOS Ser1179 phosphorylation in H2O2-treated cells. D, quantitative analyses of the effect of H2O2 on eNOS Ser1179 phosphorylated in the absence (control) and presence of OA. The data are shown as the means ± S.E. (*, p < 0.05; **, p < 0.01, versus control at the same time, n = 3).
DISCUSSION
The key finding of the present study is that H2O2 affects eNOS Ser1179 phosphorylation and function in a bidirectional manner. In endothelial cells after serum starvation, H2O2 was found to stimulate eNOS Ser1179 phosphorylation and increase NO formation (11, 12). This does not appear to reconcile with the fact that ROS is the primary cause of eNOS dysfunction in cardiovascular diseases (9, 10). Although serum starvation facilitates detecting the changes of protein phosphorylation in cells, the effect of H2O2 on eNOS phosphorylation still needs to be characterized in cells under normal serum present conditions. In addition, only the short term (15–60 min) effect of H2O2 on eNOS phosphorylation in endothelial cells was documented in prior studies. In the present study, we delineated the entire time course of the effect of H2O2 on eNOS phosphorylation and function with the cells grown in the medium containing normal levels of serum. Consistent with the results in prior studies (11, 12), we detected a similar transient elevation of eNOS Ser1179 phosphorylation after H2O2 treatment. However, a progressive decline of eNOS Ser1179 phosphorylation was seen afterward. Corresponding to these alterations of Ser1179 phosphorylation, a time-dependent bidirectional change of eNOS activity was measured. It appears that the transient activation of eNOS Ser1179 phosphorylation represents an acute response of endothelial cells to oxidative stresses. Long term H2O2 treatment reduces eNOS Ser1179 phosphorylation and function. These findings now reconcile with the longstanding observation that ROS attenuates eNOS function in chronic vascular diseases (9, 10, 23–25).
eNOS Ser1179 was first reported to be phosphorylated by Akt (7, 8). Although Akt remains the key kinase in regulating eNOS Ser1179 phosphorylation under various circumstances (26), many other kinases are found to also phosphorylate eNOS Ser1179. These include AMPK, PKG, PKA, ERK1/2, Ca2+/calmodulin-dependent kinase II, etc. (5, 6, 27). Previously, the role of these kinases in regulating eNOS Ser1179 phosphorylation was often characterized individually. To facilitate the characterization of a particular kinase, cells are often preconditioned with certain pharmacological treatments or serum starvation. Moreover, many stimuli are known to activate several kinase pathways. For example, H2O2 treatment has been reported to trigger Akt, AMPK, and ERK1/2 activation (12, 26, 28, 29). The relative contribution of each kinase to the effect of H2O2 on eNOS Ser1179 phosphorylation in complicated pathophysiological settings has rarely been accessed. Our data suggested that Akt interacted with AMPK in cells exposed to H2O2. Furthermore, the present study revealed that these kinases functioned preferentially in regulating the magnitude and time course of eNOS Ser1179 phosphorylation in oxidative stresses. At the early phase of H2O2 treatment, despite AMPK being activated, Akt appeared to play a dominant role in raising eNOS Ser1179 phosphorylation because Akt inhibition prevented the initial increases of eNOS Ser1179 phosphorylation. The mechanism underlying this Akt preference remains to be determined. One possible explanation is that Akt may have higher affinity to eNOS than AMPK does. Another possibility is that Akt has been reported to associate with eNOS through the intermediation of heat shock protein 90 (15, 30). It is conceivable that the close proximity between Akt and eNOS may render Akt priority to phosphorylate eNOS in acute responses to stresses. However, in later phase of H2O2 treatment where Akt was deactivated, significant levels of eNOS Ser1179 phosphorylation still existed. Blocking AMPK abolished the remaining eNOS Ser1179 phosphorylation. Thus, AMPK played a main role in preserving eNOS Ser1179 phosphorylation in sustained oxidative stress. Collectively, these findings indicated that Akt and AMPK together orchestrated the bidirectional alteration of eNOS Ser1179 phosphorylation in H2O2-treated cells.
With serum-starved cells, ERK1/2 has been reported to partially mediate H2O2-induced increases of eNOS Ser1179 phosphorylation because the increases were blocked by ERK1/2 inhibition with PD98059 (12). With the cells grown in the presence of serum, H2O2 treatment also triggered ERK1/2 activation. However, the addition of PD98059 affected neither the basal eNOS Ser1179 phosphorylation nor H2O2-elicited bidirectional changes. These different results may be due to whether or not cells were subjected to serum starvation. Under the condition of serum starvation, where Akt is largely latent, H2O2 triggered a rapid ERK1/2 activation, which may play a significant role in modulating eNOS Ser1179 phosphorylation. However, the present studies failed to demonstrate the significant contribution of ERK1/2 in controlling eNOS Ser1179 phosphorylation when oxidative stresses occurred to cells grown in the medium containing normal levels of serum.
The phosphorylation status of a protein is determined by the balanced action of kinases and phosphatases. It has been reported that eNOS Ser1179 was dephosphorylated by PP2A (13, 21). We have examined whether increased PP2A expressions accounted for the loss of eNOS Ser1179 in H2O2-treated cells. We did not detect any significant change of the expressions of all three PP2A subunits. Nor was the amount of PP2A associated with eNOS altered by H2O2. These results indicated that the loss of eNOS Ser1179 phosphorylation was primarily because of the diminishment of upstream kinase activities rather than increased phosphatase expressions. Future studies are still needed to elucidate the mechanism on how Akt deactivation occurred in long term H2O2-treated cells. However, our studies at least ruled out the possibility that changes of PI3K or PDK1 expressions rendered Akt inactivation. In addition, PP2A blockade only partially protected eNOS Ser1179 phosphorylation in long term H2O2-treated cells. These results suggested that in addition to PP2A, other protein phosphatases might also be involved in the process of eNOS Ser1179 dephosphorylation in prolonged oxidative stresses.
In summary, with the cells cultured in the presence of serum, H2O2 affected eNOS Ser1179 phosphorylation and activity in a bidirectional manner. Although H2O2 initially caused transient eNOS Ser1179 activation, long term H2O2 diminished eNOS Ser1179 phosphorylation and function. The effects of H2O2 on eNOS Ser1179 phosphorylation involved both Akt and AMPK. It is the integrated actions of these two pathways that dictate the dynamic changes of eNOS phosphorylation in H2O2-treated cells. Coordination of these two pathways may represent a crucial mechanism by which endothelial cells motivate eNOS to cope with oxidant stresses.
This work was supported, in whole or in part, by National Institutes of Health Grants HL77575 and HL86965. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: eNOS, endothelial nitric-oxide synthase; ROS, reactive oxygen species; Akt/PKB, protein kinase B; AMPK, AMP-activated protein kinase; ERK1/2, extracellular signal-regulated kinase 1/2; PP2A, protein phosphatase 2A; OA, okadaic acid; PI3K, phosphatidylinositol 3-kinase; BAEC, bovine aortic endothelial cell; PDK1, 3-phosphoinositide-dependent kinase 1; HEK, human embryonic kidney.
References
- 1.Moncada, S., Palmer, R. M., and Higgs, E. A. (1991) Pharmacol. Rev. 43 109–142 [PubMed] [Google Scholar]
- 2.Bredt, D. S., and Snyder, S. H. (1994) Annu. Rev. Biochem. 63 175–195 [DOI] [PubMed] [Google Scholar]
- 3.Félétou, M., and Vanhoutte, P. M. (2006) Am. J. Physiol. Heart. Circ. Physiol. 291 H985–H1002 [DOI] [PubMed] [Google Scholar]
- 4.Griffith, O. W., and Steuhr, D. J. (1995) Annu. Rev. Physiol. 57 707–736 [DOI] [PubMed] [Google Scholar]
- 5.Fleming, I., and Busse, R. (2003) Am. J. Physiol. 284 R1–R12 [DOI] [PubMed] [Google Scholar]
- 6.Fulton, D., Gratton, J. P., and Sessa, W. C. (2001) J. Pharmacol. Exp. Ther. 299 818–824 [PubMed] [Google Scholar]
- 7.Fulton, D., Gratton, J. P., McCabe, T. J., Fontana, J., Fujio, Y., Walsh, K., Franke, T. F., Papapetropoulos, A., and Sessa, W. C. (1999) Nature 399 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R., and Zeiher, A. M. (1999) Nature 399 601–605 [DOI] [PubMed] [Google Scholar]
- 9.Cai, H., and Harrison, D. G. (2000) Circ. Res. 87 840–844 [DOI] [PubMed] [Google Scholar]
- 10.Papaharalambusm, C. A., and Griendling, K. K. (2007) Trends Cardiovasc. Med. 17 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas, S. R., Chen, K., and Keaney, J. F., Jr. (2002) J. Biol. Chem. 277 6017–6024 [DOI] [PubMed] [Google Scholar]
- 12.Cai, H., Li, Z. M., Davis, M. E., Kanner, W., Harrison, D. G., and Dudley, S. C. (2003) Mol. Pharmacol. 63 325–331 [DOI] [PubMed] [Google Scholar]
- 13.Wei, Q., and Xia, Y. (2006) J. Biol. Chem. 281 21652–21659 [DOI] [PubMed] [Google Scholar]
- 14.Bredt, D. S., and Snyder, S. H. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 9030–9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia, Y., and Zweier, J. L. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 12705–12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei, Q., and Xia, Y. (2005) J. Biol. Chem. 280 18081–18086 [DOI] [PubMed] [Google Scholar]
- 17.Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., Musi, N., Hirshman, M. F., Goodyear, L. J., and Moller, D. E. (2001) J. Clin. Investig. 108 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoki, K., Zhu, T., and Guan, K. L. (2003) Cell 115 577–590 [DOI] [PubMed] [Google Scholar]
- 19.Kim, E. K., Miller, I., Aja, S., Landree, L. E., Pinn, M., McFadden, J., Kuhajda, F. P., Moran, T. H., and Ronnett, G. V. (2004) J. Biol. Chem. 279 19970–19976 [DOI] [PubMed] [Google Scholar]
- 20.Kovacic, S., Soltys, C. M., Barr, A. J., Shiojima, I., Walsh, K., and Dyck, J. R. B. (2003) J. Biol. Chem. 278 39422–39427 [DOI] [PubMed] [Google Scholar]
- 21.Greif, D. M., Kou, R., and Michel, T. (2002) Biochemistry 41 15845–15853 [DOI] [PubMed] [Google Scholar]
- 22.Millward, T. A., Zolnierowicz, S., and Hemmings, B. A. (1999) Trends. Biochem. Sci. 24 186–191 [DOI] [PubMed] [Google Scholar]
- 23.Liu, S., Premont, R. T., Kontos, C. D., Zhu, S., and Rockey, D. C. (2005) Nat. Med. 11 952–958 [DOI] [PubMed] [Google Scholar]
- 24.Musicki, B., Kramer, M. F., Becker, R. E., and Burnett, A. L. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 11870–11875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du, X. L., Edelstein, D., Dimmeler, S., Ju, Q., Sui, C., and Brownlee, M. (2001) J. Clin. Investig. 108 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiojima, I., and Walsh, K. (2002) Circ. Res. 90 1243–1250 [DOI] [PubMed] [Google Scholar]
- 27.Michell, B. J., Chen, Z., Tiganis, T., Stapleton, D., Katsis, F., Power, D. A., Simi, A.T., and Kemp, B. E. (2001) J. Biol. Chem. 276 17625–17628 [DOI] [PubMed] [Google Scholar]
- 28.Choi, S. L., Kim, S. J., Lee, K. T., Kim, J., Mu, J., Birnbaum, M. J., Soo, Kim. S., and Ha, J. (2001) Biochem. Biophys. Res. Commun. 287 92–97 [DOI] [PubMed] [Google Scholar]
- 29.Zhang, M., Dong, Y., Xu, J., Xie, Z., Wu, Y., Song, P., Guzman, M., Wu, J., and Zou, M. H. (2008) Circ. Res. 102 328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontana, J., Fulton, D., Chen, Y., Fairchild, T. A., McCabe, T. J., Fujita, N., Tsuruo, T., and Sessa, W. C. (2002) Circ. Res. 90 866–873 [DOI] [PubMed] [Google Scholar]