Abstract
Seeking to better understand how membrane trafficking is coordinated with phospholipid synthesis in yeast, we investigated lipid synthesis in several Sec- temperature-sensitive mutants, including sec13-1. Upon shift of sec13-1 cells to the restrictive temperature of 37 °C, phospholipid synthesis decreased dramatically relative to the wild type control, whereas synthesis of neutral lipids, especially triacylglycerol (TAG), increased. When examined by fluorescence microscopy, the number of lipid droplets appeared to increase and formed aggregates in sec13-1 cells shifted to 37 °C. Electron microscopy confirmed the increase in lipid droplet number and revealed that many were associated with the vacuole. Analysis of lipid metabolism in strains lacking TAG synthase genes demonstrated that the activities of the products of these genes contribute to accumulation of TAG in sec13-1 cells after the shift to 37 °C. Furthermore, the permissive temperature for growth of the sec13-1 strain lacking TAG synthase genes was 3 °C lower than sec13-1 on several different growth media, indicating that the synthesis of TAG has physiological significance under conditions of secretory stress. Together these results suggest that following a block in membrane trafficking, yeast cells channel lipid metabolism from phospholipid synthesis into synthesis of TAG and other neutral lipids to form lipid droplets. We conclude that this metabolic switch provides a degree of protection to cells during secretory stress.
The endoplasmic reticulum (ER)2 plays a central role in the synthesis and transport of the proteins and lipids that constitute the entire endomembrane system. Following their synthesis and insertion into the ER, proteins destined for the secretory pathway are folded and sorted into COPII vesicles that are delivered to their ultimate cellular locations via cellular membrane trafficking pathways. The ER is also a site of synthesis of the major membrane bilayer forming phospholipids, including phosphatidic acid (PA), precursor to all phospholipids (Fig. 1), as well as phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylcholine (PC) (1). Given that phospholipids are the main structural constituents of membrane-bound organelles, membrane growth and homeostasis must require continuous phospholipid synthesis that is coupled to early membrane trafficking events. Indeed, induction of proliferation of ER-like membranes in yeast, following expression of a heterogeneous ribosome receptor, requires the activity of Ino2p, a transcriptional activator of phospholipid biosynthetic gene expression (2). However, the mechanism of coordination of lipid synthesis with COPII vesicle formation and/or ER membrane proliferation remains elusive.
FIGURE 1.
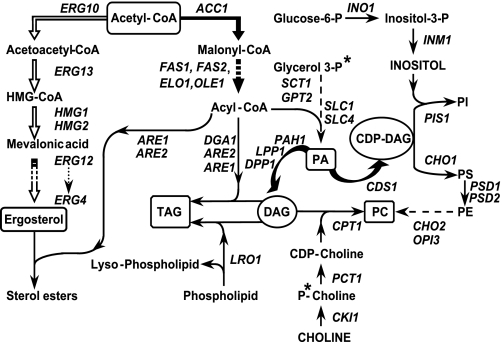
Metabolic pathways for the synthesis of phospholipids and neutral lipid classes in S. cerevisiae. PA serves as a precursor to CDP-DAG, precursor to PI and PS, which serves as precursor to PE. PC is synthesized either via methylation of PE or from CDP-choline and DAG via the Kennedy pathway. DAG, produced by dephosphorylation of PA, serves as a precursor to TAG, as well as PC via the Kennedy pathway. When PC is synthesized via the Kennedy pathway, the 32P label enters via phosphorylation of choline. In contrast, when PC is synthesized via methylation of PE, 32P is derived from PA. Metabolites in boxes denote key intermediaries and/or end products that are relevant in the discussion of this study. Metabolites in ovals are derived from PA. The bold arrows indicate the multiple steps of fatty acid synthesis, and the block arrows indicate the mevalonate pathway for the synthesis of ergosterol. The asterisks indicate the two places where 32P label enters into the pathways for the synthesis of the major phospholipids shown here.
It has been proposed that the unfolded protein response (UPR) plays a role in coordinating membrane growth and phospholipid metabolism in both yeast (3) and mammalian cells (4, 5). The UPR is a highly conserved ER-localized stress response pathway, which is activated when the load of unfolded proteins entering the ER exceeds its protein folding and secretory capacity (6-12), leading to the increased expression of target genes (13). Interestingly, hyper-activation of the UPR leads to the proliferation of the ER membrane in yeast (14) and mammalian cells (4, 5). However, the UPR response is not required for proliferation of ER-like membranes under all conditions, including overexpression of a heterogeneous ribosome receptor protein (15) or hydroxymethylglutaryl-CoA reductase (16). Thus, it remains unclear if UPR signaling is sufficient to drive ER expansion under physiological conditions.
Moreover, the UPR is activated in wild type yeast growing in medium lacking inositol (3, 17), a precursor in the synthesis of PI from cytidine diphosphate-diacylglycerol (CDP-DAG) (Fig. 1). A large number of coregulated genes involved in phospholipid metabolism are derepressed under these conditions (18, 19), including INO1, the most highly regulated of the genes responding to inositol limitation (18-20). It is now clear that INO1 and other coregulated genes of lipid metabolism that are activated by the Ino2p-Ino4p complex and repressed by Opi1p, are not under the control of the UPR (18, 19). Instead, under these growth conditions, the UPR may be responding to reduced secretory capacity caused by changes in phospholipid composition or rate of synthesis or, alternatively, to signals in the ER directly related to PI levels or metabolism in the ER (19).
Indeed, the UPR is activated in yeast temperature-sensitive Sec- mutants defective in a wide range of membrane trafficking steps (9, 10). For example, a sec13-1 mutant, which is defective in COPII vesicle trafficking from the ER (21-23), exhibits high levels of UPR activation when grown at the semi-permissive temperature of 30 °C (9) and is an inositol auxotroph at that temperature (24). Sec13p is a soluble protein that forms a complex with Sec31p to bind to the Sec23/24 complex in the ER, as the final step in COPII vesicle formation (25). Letts and Dawes (26) reported that a temperature-sensitive mutant, which proved to be allelic with sec13, exhibited a rapid decrease in the rate of phospholipid synthesis after a shift to its restrictive temperature. These observations suggest that synthesis of phospholipids, particularly PI, is influenced by, or influences, membrane trafficking from the ER.
Seeking to better understand how membrane trafficking pathways are coordinated with phospholipid synthesis, we investigated the status of membrane lipid synthesis in several Sec- mutants, including sec13-1, shifted to restrictive growth conditions. In this study, we report that phospholipid synthesis decreased dramatically in sec13-1 cells following a shift to the restrictive temperature, whereas synthesis of triacylglycerol (TAG) increased. Double or triple mutant strains, containing mutations in TAG synthesis in combination with the sec13-1 mutation, exhibited a reduction in their ability to grow at temperatures that are permissive for sec13-1, suggesting that the shift from phospholipid synthesis to TAG synthesis in sec13-1 cells has physiological significance under conditions of impaired COPII vesicle formation. These results are discussed in the context of regulation of lipid metabolism in coordination with membrane trafficking.
EXPERIMENTAL PROCEDURES
Yeast Strains and Culture Conditions—The Saccharomyces cerevisiae strains used in this study are listed in Table 1. Cultures were maintained on YPD (1% yeast extract, 2% peptone, 2% glucose, 2% agar) media plates. All experiments were conducted using cultures grown to mid-logarithmic phase at 25 °C on a rotary shaker (New Brunswick Scientific Co., Inc.) at 200 rpm using chemically defined synthetic media as described by Jesch et al. (18). Unless otherwise stated, all cells were grown in 50-ml batches of complete synthetic media with 75 μm inositol (I+ medium) at 25 °C until mid-logarithmic phase and shifted to 37 °C to follow changes in lipid content and synthesis. Synthetic medium lacking inositol is referred to as I- medium.
TABLE 1.
List of yeast strains used in this study
| Strain | Genotype | Source or Ref. |
|---|---|---|
| BY4742 | MATα his3 leu2 lys2 ura3 | Invitrogen |
| CKY8 | MATα leu2-3 115 ura 3-52 | Chris Kaiser |
| CKY555 | MATasec31-2 leu2-3 115 ura3-52 | Chris Kaiser |
| JBY318 | MATasec6-4 his4 leu2 ura3 | Jeff Brodsky |
| LGY113 | MATα sec13-1his3 ura3 leu2 | This study |
| LGY 248 | MATα lro1Δ::HIS3 his3 leu2 ura3 | This study |
| LGY 267 | MATα dga1Δ::kanMX his3 leu2 ura3 | This study |
| LGY 273 | MATα lro1Δ::HIS3 dga1Δ:: kanMX his3 leu2 ura3 | This study |
| LGY 249 | MATα sec13-1lro1Δ::HIS3 his3 leu2 ura3 | This study |
| LGY 270 | MATasec13-1dga1Δ:: kanMX his3 leu2 ura3 | This study |
| LGY 268 | MATα sec13-1lro1Δ::HIS3 dga1Δ:: kanMX his3 leu2 ura3 | This study |
| LGY 276 | MATα sec13-1lro1Δ::HIS3 dga1Δ:: kanMX are2Δ::LEU2 his3 ura3 leu2 | This study |
| LGY 210 | MATa TGL3-GFP (HIS3) his3 leu2 ura3 met15 | This study |
| LGY 218 | MATa TGL3-GFP (HIS3) sec13-1 his3 leu2 ura3 | This study |
Deletion mutant strains for LRO1 and DGA1 were generated in wild type and sec13-1 cells by PCR-mediated gene replacement as described previously (27). The plasmids pFA6a-His3MX6 and pFA6a-kanMX6 were used as a template to generate PCR fragments for the LRO1 and DGA1 gene disruptions. The entire open reading frame of the LRO1 gene was replaced with the HIS3 marker gene. Histidine prototrophs were screened by colony PCR to verify integration at the correct genetic locus. Likewise, the entire open reading frame of the DGA1 gene was replaced with the KanMX6 marker gene. Transformants were selected on YPD medium containing 200 mg/liter geneticin (YPD + G418; Calbiochem) and were screened by colony PCR to verify integration at the correct genetic locus.
In addition, the plasmid pRS315 was used as a template to generate a PCR fragment for the ARE2 gene disruption. The entire open reading frame of the ARE2 gene was replaced with the LEU2 marker gene. Leucine prototrophs were screened by colony PCR to verify integration at the correct genetic locus.
Pulse Labeling of Phospholipids—To analyze de novo synthesis of glycerophospholipids following the temperature shift, wild type and sec13-1 cells from overnight cultures grown at 25 °C in I+ medium were diluted back into I+ medium at 25 °C to a culture density of approximately A600 nm = 0.1-0.2. These cells were then allowed to grow at 25 °C until they reached a culture density of A600 nm = 0.5. At this cell density each culture was divided in two. One-half of each culture was maintained at 25 °C, and the other half was shifted to 37 °C. 100 μCi/ml [32P]orthophosphate (specific activity of isotope was 13.51 mCi/mmol phosphate) was immediately added to the cultures maintained at 25 °C, and these cells were harvested 20 min later. This sample is referred to as the 0 time point for both wild type and sec13-1. 32P was added in similar fashion to wild type and sec13-1 cultures at 60, 120, and 180 min following the temperature shift. For each time point, samples were harvested by centrifugation 20 min after addition of label. Lipids were extracted from the pellet and quantified by two-dimensional chromatography, as described by Atkinson et al. (28).
To determine phosphate uptake, wild type and sec13-1 cultures were grown, pulse-labeled with 32P, and harvested exactly as described above for pulse labeling of lipids. However, the pellet, in this case, instead of being extracted was immediately washed in 2 volumes of distilled water, resuspended in 500 μl of water, and added to scintillation vials containing 5 ml of Ecoscint scintillation solution (National Diagnostics). In both wild type and sec13-1 cultures, uptake of 32P per A600 nm unit of culture following a 20-min pulse was ∼1.8 × 106 cpm at 0 and 60 min after the shift to 37 °C. Uptake declined in both cultures to ∼1.4 × 106 cpm per A600 nm units of culture by the 120- and 180-min time points. Despite this decline in apparent 32P uptake in both cultures at 37 °C, there was no significant difference at any of the time points when sec13-1 and wild type were compared.
Cell Growth and Viability—Wild type and sec13-1 cultures were grown as described above for pulse labeling at 25 °C in I+ medium to A600 nm = 0.5 and divided in half. One-half of each culture was shifted to 37 °C. Growth was monitored in all experiments by following the increase in A600 nm of each culture. Cell growth and viability were also assessed in selected experiments by colony forming ability. Samples were collected at 60, 120, and 180 min following the temperature shift, and culture samples were subjected to serial dilution into sterile distilled water and plated onto I+ medium at an expected density of 200 colonies per plate. After 2 days of incubation at 25 °C, colonies were counted. Cell number and viability were also followed in selected experiments by staining with the vital stain methylene blue and counting stained versus unstained cells on a hemocytometer.
The A600 nm of wild type cultures increased with a doubling time of 2-2.5 h following the shift to 37 °C. The A600 nm of sec13-1 cultures increased identically to wild type for the first 60 min of incubation at 37 °C, but, in contrast to wild type, A600 nm of sec13-1 cultures leveled off after 60 min. No increase in A600 nm was observed between 60 and 180 min in sec13-1 cultures. All experiments involving labeling of cells to assess lipid metabolism were normalized to the A600 nm of the culture.
Colony-forming units and cell count in the sec13-1 and wild type cultures increased similarly and in parallel with the rate of increase in A600 nm in the first 60 min of incubation at 37 °C. Wild type cultures experienced close to a 2-fold increase between 0 and 120 min and continued at an equivalent rate through 180 min. Between 60 and 120 min, the number of colony-forming units remained unchanged in sec13-1 cultures. The number of colony-forming units dropped by ∼30% in sec13-1 cultures between 120 and 180 min, and ∼20% of the sec13-1 cells were stained with methylene blue at 180 min. No significant staining with methylene blue was seen at any of the other time points in either strain. The fact that a fraction of the sec13-1 cells may have lost viability by 180 min is discussed under “Results” in conjunction with labeling experiments.
CPY Pulse-Chase Labeling and Immunoprecipitation—Strains were grown in I+ medium at 25 °C to mid-logarithmic growth phase as described for pulse labeling. At A600 nm = 0.5, cultures were split, harvested by filtration, and resuspended in synthetic labeling media lacking methionine, cysteine, and ammonium sulfate at room temperature or prewarmed to 37 °C. After preincubation for 15 min at either 25 or 37 °C, cells were pulse-labeled with 50 μCi per 1 A600 nm unit Tran 35S-labeling reagent (MP Biochemicals, Irvine, CA) for 5 min at the respective temperature, chased by adding a 1/100 volume of solution containing 0.1 m ammonium sulfate, 0.3% cysteine, and 0.4% methionine, and incubated for 0, 5, 10, 20, or 40 min at 25 or 37 °C. A total of 5 A600 nm units was collected at each time point by centrifugation in chilled tubes containing sodium azide at a final concentration of 0.1 m. Protein extracts were prepared by trichloroacetic acid precipitation of cell pellets, followed by resuspension and boiling with glass beads in lysis buffer (50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 1% SDS, 1 mm phenylmethylsulfonyl fluoride). After a 5-fold dilution with IP dilution buffer (60 mm Tris-HCl, pH 7.5, 6 mm EDTA, 190 mm NaCl, 1.25% Triton X-100), samples were precleared by centrifugation to remove insoluble material. Carboxypeptidase Y (CPY) was immunoprecipitated from each sample by incubating with rabbit anti-CPY antibodies (Rockland, Gilbertsville, PA) and protein A-Sepharose CL-4B (Sigma) in IP buffer (50 mm Tris-HCl, pH 7.5, 5 mm EDTA, 150 mm NaCl, 1% Triton X-100, 0.2% SDS). Washed immunoprecipitates were eluted with sample buffer, separated on 8% SDS-polyacrylamide gels, and visualized by autoradiography.
RNA Isolation and Northern Blotting—Wild type and sec13-1 strains were grown in I+ medium at 25 °C to mid-logarithmic growth phase as described above. At A600 nm = 0.5 cultures were shifted to 37 °C and continued to grow at the elevated temperature. Samples were harvested by filtration at 0, 30, 60, 120, and 180 min following the temperature shift, flash-frozen in dry ice, and stored at -80 °C. Total RNA was isolated from samples using RNeasy® mini kit (Qiagen, Valencia, CA) according to manufacturer's instructions. 1 μg of total RNA was fractionated on 1% glyoxal-agarose gels and transferred to Nytran SuPerCharge nylon membrane (Whatman) in 20× SSC as described (29). Strand-specific 32P-labeled riboprobes were synthesized from linearized plasmids pJH310-INO1 (30) and pGEM-HAC1 (9) and hybridized to membranes in formamide hybridization buffer as described (18). Quantitation was performed following scanning on a STORM 860 PhosphorImager and analyzed using ImageQuant software (GE Healthcare).
Total Lipid Composition Assessed by [14C]Acetate Steady State Labeling—Cultures of wild type, sec13-1, and other indicated strains were grown at 25 °C in I+ medium in the presence of 1 μCi/ml of [1-14C]acetate (specific activity, 57 mCi/mmol) overnight. The next day, cultures were diluted to A600 nm = 0.1 maintaining label at 1 μCi/ml of [1-14C]acetate and were then allowed to grow until mid-logarithmic phase (A600 nm = 0.5). The cultures were then shifted to 37 °C and incubated for an additional 180 min. Samples were taken at 0, 60, 120, and 180 min following the shift to 37 °C. Lipids were extracted and analyzed as described by Gaspar et al. (31).
Flow Cytometric Analysis—Overnight cultures of wild type cells, sec13-1, sec31-2, and sec6-4 mutants were diluted to A600 nm = 0.1 and grown in I+ medium at 25 °C until they reached A600 nm = 0.5. Half of each culture was maintained at 25 °C, and the remaining half was shifted to 37 °C. The cells were allowed to grow for 120 min, and 5-ml samples were withdrawn and fixed for 1 h at room temperature with 3.7% formaldehyde. Formaldehyde-fixed cells were permeabilized by treatment with 0.1% Triton X-100 in PBS, pH 8.0, for 10 min. The cells were washed twice in PBS and were incubated with 10 μm BODIPY® 493/503 (4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a, 4a-diaza-s-indacene; Invitrogen; catalog number D-3922). The density of each cell suspension was quickly adjusted so that the final culture density was approximately A600 nm = 0.5. A negative control consisting of cells with no dye was also prepared. The stained cells were analyzed immediately on a BD Biosciences FACS Aria high speed flow cytometer/cell sorter.
Fluorescence Microscopy Analysis—Wild type and sec13-1 cultures were grown overnight as described above at 25 °C in I+ medium. Prior to microscopy, overnight cultures were diluted to A600 nm = 0.1 and were allowed to regrow to mid-logarithmic phase in I+ medium at 25 °C. They were then diluted once more to A600 nm = 0.1 in 2 ml of I+ media in multiwell plates and incubated at 25 °C in an Eppendorf incubator/mixer. After reaching A600 nm = 0.5, cultures were shifted to 37 °C, and aliquots removed at time = 0, and 120 min, briefly fixed with 1% formaldehyde in 1 m sorbitol and stained with 0.5 μg/μl BODIPY® 493/503 (Invitrogen), prior to fluorescence microscopy. Cells were mounted as described previously (32). BODIPY® 493/503 fluorescence was excited at 488 nm, using 10% laser output to reduce bleaching, and emission was detected with a BP525/50 band pass filter on a Leica TCS4d confocal microscope. Using this experimental setup, BODIPY® 493/503 uptake and staining efficiency are maximized and independent of growth phase and mutant background (32). BODIPY® 493/503 fluorescence was typically recorded using eight optical sections through the specimen preparation, and images were combined in a single projection. Transmission images were recorded using differential interference contrast (Nomarski) optics. Glycogen accumulation in cells grown as described above was visualized by staining with 10% Lugol solution (Sigma) (33) using transmission microscopy (488 nm laser).
Electron Microscopy Analysis—For transmission electron microscopy, wild type and sec13-1 cells were grown as described above in I+ medium at 25 °C overnight. Cultures were diluted to A600 nm = 0.1 and allow to grow at 25 °C in I+ medium to A600 nm = 0.5. Cultures were then split and then incubated for an additional 120 min at either 25 or 37 °C as described above. Cells were then fixed, dehydrated, and embedded according to the methods described by Wright (34). Thin sections were cut with the Porter-Blum MT-2 ultramicrotome and viewed with a Philips/FEI Morgagni transmission electron microscopy at 80 kV. To quantitate the average number of lipid droplets per cell, their association with the vacuole was counted as follows: all cells within a grid space were counted without bias; lipids droplets were counted as being either associated with vacuoles or not associated; the fraction of lipid droplets associated with vacuoles is clearly underestimated because lipid droplets could have been associated with vacuoles outside the plane of the section.
RESULTS
32P Pulse Labeling Reveals a Decrease in Total Phospholipid Synthesis in the sec13-1 Mutant and a Substantial Increase in PI Synthesis in Wild Type Cells following a Temperature Shift to 37 °C—At its semi-permissive temperature of 30 °C, the sec13-1 mutant is an inositol auxotroph (Ino-) (24), a phenotype often associated with defects in the regulation of phospholipid metabolism (20). Another conditional sec13 mutant was reported previously to exhibit a defect in phospholipid synthesis upon shift to its restrictive temperature (26). Because of these reports, we first analyzed phospholipid synthesis in the sec13-1 strain shifted to its restrictive temperature of 37 °C.
Rates of phospholipid synthesis were assessed in sec13-1 and wild type cells growing in inositol containing (I+) medium by pulse labeling with 32P for 20 min, as described under “Experimental Procedures.” An aliquot of each culture was pulse-labeled at 25 °C (0 time point, Fig. 2), and the cultures were shifted to 37 °C. Further aliquots were pulse-labeled for 20 min commencing at 60, 120, and 180 min following the temperature shift. 32P enters the phospholipid biosynthetic pathway via glycerol 3-phosphate, or choline phosphate, as indicated by the asterisks in Fig. 1. The timing of appearance of 32P into specific lipids reflects both the relative position of the phospholipid in the pathway and its relative rate of synthesis from precursors earlier in the pathway, as described in Kelley et al. (35).
FIGURE 2.
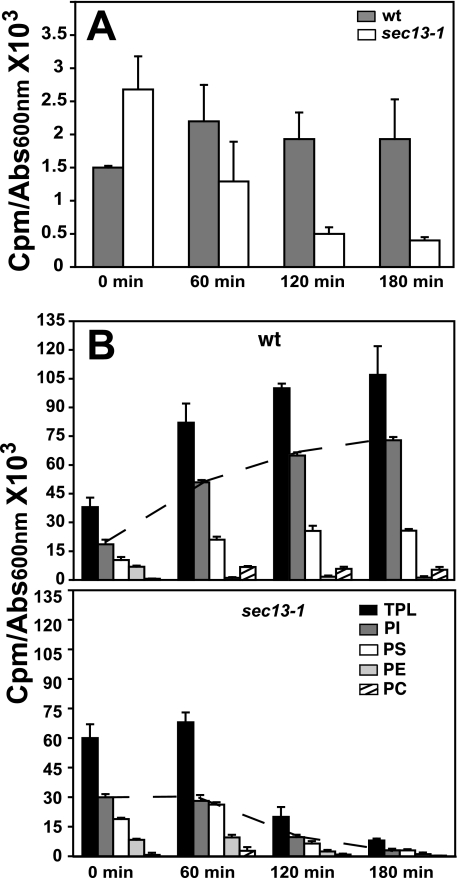
Pulse labeling of phospholipids with 32P in wild type (wt) and sec13-1 cells following a shift from 25 °C to the sec13-1 restrictive temperature of 37 °C. Cells were grown in I+ medium at 25 °C until mid-logarithmic phase of growth (A600 nm = 0.5). One aliquot was maintained at 25 °C, and the reminder was shifted to 37 °C. A 5-ml sample of each culture was maintained at 25 °C and immediately labeled with 100 μCi/ml [32P]orthophosphate for 20 min (0 min time point). Samples from the cultures shifted to 37 °C were labeled for 20 min at 60, 120, and 180 min after the temperature shift. Lipids were extracted and analyzed as described under “Experimental Procedures.” Data are expressed as counts of 32P radiolabel incorporated into total and individual phospholipids per A600 nm unit in the cell culture. A, 32P label associated with phosphatidic acid (PA). B, 32P label associated with TPL = total label associated with the sum of the following phospholipids: PI, PS, PE, and PC. The dotted line traces the increased rate of PI synthesis in wild type cells and the decrease in PI synthesis in sec13-1 cells, following the shift to 37 °C.
32P label first enters the lipid soluble pool via PA and is rapidly transferred into downstream lipids. Consequently, only a very small proportion of label is retained in PA in a 20-min pulse (Fig. 2A; Table 2). However, essentially all 32P label recovered from the phospholipids shown in Fig. 2B, with the exception of PC, is derived from PA (Fig. 1). The absolute amount of label recovered from PA per A600 nm unit of culture after a 20-min pulse at 25 °C was about 40% higher in the sec13-1 culture than in wild type (Fig. 2A, compare sec13-1 and wt, 0-min time point). However, the proportion of label recovered from PA as a function of total phospholipid-associated label was not significantly different in the two cultures after pulse labeling at 25 °C (Table 2, compare % PA in sec13-1 to wild type at 0 time point). Within 60 min following the shift to 37 °C, the amount of label recovered from PA per A600 nm unit of culture increased by 25-30% in wild type and remained at this level throughout the experiment (Fig. 2A). Despite the increase in total labeling, however, the proportion of label in PA actually declined in wild type cells as a percentage of total phospholipid after the shift to 37 °C (Table 2).
TABLE 2.
Pulse labeling of phospholipids in wild type and sec13-1 cells
Wild type, and sec13-1 cells were pre-grown to A600 nm = 0.5 in I+ medium at 25 °C and divided into two cultures. One culture was maintained at 25 °C, and the other was shifted to 37 °C. 100 μCi /ml [32P]orthophosphate was immediately added to the cultures maintained at 25 °C, and these cells were harvested 20 min later. 32P was added in similar fashion to wild type and sec13-1 cultures at 60, 120, and 180 min following the temperature shift. For each time point, samples were harvested 20 min after addition of label. Lipids were extracted and quantified by two-dimensional chromatography, as described under “Experimental Procedures.” Values represent the percentage of total lipid-associated 32P incorporated into each phospholipid species. “Other” represents the pooled percentages of CDP-DAG, phosphatidyl-monomethylethanolamine, and phosphatidyldimethylethanolamine.
|
32P-Phospholipid
| |||||
|---|---|---|---|---|---|
|
25 °C, 0 min
|
37 °C
|
||||
| 60 min | 120 min | 180 min | |||
| % total | |||||
| Wild type | |||||
| PI | 48.1 ± 6.2 | 60.9 ± 0.1 | 63.7 ± 1.6 | 67.2 ± 1.2 | |
| PS | 27.0 ± 3.9 | 25.2 ± 1.8 | 25.1 ± 1.9 | 23.6 ± 0.3 | |
| PE | 17.9 ± 1.5 | 1.3 ± 0.3 | 1.7 ± 0.9 | 1.1 ± 0.1 | |
| PC | 1.5 ± 0.8 | 8.0 ± 0.6 | 5.7 ± 0.1 | 4.9 ± 0.4 | |
| PA | 3.8 ± 0.1 | 2.6 ± 0.6 | 1.9 ± 0.6 | 1.8 ± 0.8 | |
| Other | 1.7 ± 0.4 | 2.0 ± 0.5 | 1.9 ± 0.1 | 1.4 ± 0.1 | |
| sec13-1 | |||||
| PI | 48.9 ± 2.3 | 40.9 ± 2.1 | 47.1 ± 4.9 | 37.5 ± 3.7 | |
| PS | 30.8 ± 1.3 | 38.2 ± 1.7 | 31.4 ± 5.1 | 37.4 ± 0.5 | |
| PE | 13.6 ± 0.8 | 13.8 ± 2.7 | 12.1 ± 3.3 | 13.8 ± 2.8 | |
| PC | 0.3 ± 0.1 | 4.0 ± 0.5 | 4.3 ± 2.0 | 4.4 ± 0.2 | |
| PA | 4.3 ± 0.7 | 1.8 ± 0.5 | 2.4 ± 0.6 | 4.9 ± 0.4 | |
| Other | 2.1 ± 0.3 | 1.3 ± 0.1 | 2.7 ± 0.5 | 2 ± 0.2 | |
In contrast, the amount of 32P label recovered from PA per A600 nm unit of sec13-1 culture declined steadily after the shift to 37 °C and had decreased by 120 min to about 20% of the level observed at 25 °C (Fig. 2A). In a pulse-labeling experiment, a decline in PA labeling could result from a decrease in its rate of synthesis or an increase in the rate of synthesis of lipids for which it serves as a precursor, including neutral lipids such as DAG and TAG, which do not retain 32P (Fig. 1). As a percentage of total 32P accumulated in phospholipid in a 20-min pulse (Table 2), label associated with PA declined by about 50% by 120 min in both wild type and sec13-1 cultures. In sec13-1 cultures by 180 min, despite the overall low level of labeling of PA (Fig. 2A), the proportion of label in PA rebounded to about 5% of total 32P associated with phospholipids in a 20-min pulse (Table 2).
Examination of the total labeling of phospholipids with 32P in wild type and sec13-1 cultures (Fig. 2B and Table 2) provides quite a different perspective on phospholipid synthesis than PA labeling alone. Within 60 min following a shift to 37 °C, total 32P label associated with the major phospholipids depicted in Fig. 2B (TPL) more than doubled in a 20-in pulse in the wild type strain and reached even higher levels at the 120- and 180-min time points. In contrast, in sec13-1 cultures, label associated with TPL did not increase in the first 60 min after the shift to 37 °C and had declined by 64% at the 120-min time point (Fig. 2B). Labeling of TPL had decreased by 84% at the 180-min time point (Fig. 2B). However, this further decline in labeling of phospholipids could be due, at least in part, to the observed drop in cell viability by 180 min, as we will discuss below. The overall decrease in 32P labeling of phospholipids seen by 120 min could be due to a decline in PA synthesis or alternatively to increasing dephosphorylation of PA to form DAG, which serves as precursor to TAG.
A most striking difference between the wild type and sec13-1 cultures was the effect of the temperature shift on PI labeling. In the wild type culture, the amount of 32P associated with PI, after a 20-min pulse, more than doubled by 60 min and by 180 min PI labeling had increased more than 3-fold, representing a substantial fraction of the total increase in labeling of phospholipids. In the sec13-1 culture, in contrast, total labeling of PI per A600 nm unit of culture remained constant at 60 min after the shift to 37 °C and declined by more than 3-fold at 120 min after the shift to 37 °C. As a percentage of total 32P incorporated into phospholipids in the sec13-1 culture, however, PI did not decline dramatically (Table 2). Indeed, the percentage of 32P incorporated into PI in a 20-min pulse in the sec13-1 cells decreased insignificantly from a level of about 49% at 25 °C to about 47% after 120 min of incubation at 37 °C (Table 2). This is a reflection largely of the fact that the synthesis of all the major phospholipids showed a comparable decline in synthesis over this time period (Fig. 2B), and the rate of PI synthesis declined more or less in proportion to the other major phospholipids in the first 120 min in sec13-1 cells. In the wild type culture, in contrast, 32P label associated with PI rose as a percentage of total phospholipid-associated label in a 20-min pulse from 48% at 25 °C to about 64% after 120 min at 37 °C (Table 2). This increase in the proportion of label in PI in the wild type strain after the shift to 37 °C is a reflection of the fact that the rate of PI synthesis increased more dramatically than that of any other phospholipid in response to increasing temperature (Fig. 2B).
Other phospholipids also showed changes in their patterns of synthesis in the wild type culture after the shift to 37 °C. For example, labeling of PE with 32P in a 20-min pulse dropped precipitously in the wild type culture after the shift (Fig. 2B; Table 2), whereas label associated with its precursor, PS, actually increased, as did labeling of PC, which can be derived from PE by methylation (Fig. 1). However, PC can also be derived from CDP-choline (Fig. 1). Thus, it is unclear whether the increase in labeling of PC in wild type cells is because of choline produced by increased turnover of PC and reincorporated via the CDP choline pathway or by increased methylation of PE. Nevertheless, it is clear from these data that the pattern of phospholipid synthesis changes markedly in wild type cells following a shift to 37 °C.
In contrast, in sec13-1 cells, the major change upon shift to 37 °C was an overall decline in 32P labeling of total phospholipid, reflected in labeling of essentially all phospholipids (Fig. 2B). However, in contrast to wild type cells, the percentage of total phospholipid associated 32P recovered from PI, PS, and PE changed very little after the shift to 37 °C. Overall labeling of PC with 32P also declined in sec13-1 cells after the shift to 37 °C (Fig. 2B). However, the decrease in labeling of PC was proportionately less than the decrease in the other major phospholipids in the sec13-1 cells. Consequently, the percentage of label associated with PC actually increased from 1% at 25 °C to 4.4% of total phospholipid associated label at 120 min after the shift 37 °C (Table 2).
We questioned whether the decrease in incorporation of 32P into phospholipids might be due simply to loss of viability of sec13-1 cells. However, as described under “Experimental Procedures,” there was no loss of viability of sec13-1 cells during the first 120 min following the temperature shift, and yet labeling of phospholipids with 32P had declined substantially by this time. At 180 min, ∼70-80% of sec13-1 cells retained viability. However, as we discuss below, splicing of HACi RNA appeared unaffected at 180 min, and incorporation of [14C]acetate into neutral lipid in sec13-1 cultures between 120 and 180 min after the shift substantially exceeded that of wild type cultures per A600 nm unit, as we will document below. We also questioned whether the decrease in phospholipid labeling might be the result of failure to take up 32P. This was not the case, however, as total uptake of 32P into sec13-1 and wild type cultures was not significantly different at 25 °C or after the shift to 37 °C (as described under “Experimental Procedures”). At 25 °C, ∼2.0% of total 32P taken up in wild type, and 3.5% in sec13-1 cultures, was incorporated into phospholipids in a 20-min pulse (data not shown). In sec13-1 cultures, this proportion dropped to 0.9% by 180 min, as a result of declining phospholipid synthesis (Fig. 2B). In contrast, in wild type cultures, this proportion rose to 7.6% in the same time period, because of increasing phospholipid synthesis (Fig. 2B).
CPY Processing in sec13-1 Cells following the Shift to 37 °C—Previous studies have shown that Sec13p is rapidly inactivated in the sec13-1 strain following a temperature shift to 37 °C (36, 37). To confirm that a secretory block occurs prior to the alterations we observed in phospholipid metabolism in the sec13-1 mutant, we monitored the kinetics of CPY processing in a pulse-chase experiment. CPY is a post-translationally proteolyzed glycoprotein that is used to monitor trafficking steps between the ER, Golgi, and vacuole compartments of the secretory pathway. It has been shown previously that processing of CPY in wild type cells is unaffected by 1 h of preincubation at 37 °C (37). In sec13-1 cells incubated at the permissive temperature of 25 °C, newly synthesized CPY, as expected, is first detected in the 67-kDa ER form, is rapidly converted to the 69-kDa Golgi form, and subsequently proteolytically cleaved in the vacuole to its mature 61-kDa form. As shown in Fig. 3, ∼95% of newly synthesized CPY reached the vacuole after 40 min, whereas 5% remained in the ER form. In contrast, following a preincubation of sec13-1 cells at 37 °C for only 15 min, greater than 95% of the newly synthesized CPY was recovered in the ER form after 40 min (Fig. 3). Because a decline in total phospholipid synthesis was not observed until 120 min after the shift to 37 °C, the block in the ER-to-Golgi trafficking step in the sec13-1 strain significantly precedes the inhibition of de novo phospholipid synthesis (Fig. 2, A and B).
FIGURE 3.
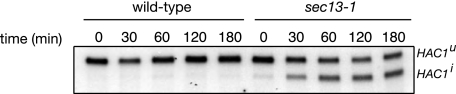
Comparison of CPY processing in sec13-1 mutant strain at permissive and restrictive temperatures. sec13-1 cells were preincubated for 15 min at 25 °C (permissive temperature) or 37 °C (restrictive temperature), pulse-labeled with [35S]methionine/cysteine for 5 min, and chased with an excess of cold methionine and cysteine at 25 or 37 °C. Cell lysates from samples collected at indicated time points were immunoprecipitated with anti-CPY antibodies followed by SDS-PAGE analysis and autoradiography. Migration of ER-glycosylated (p1) and Golgi-modified (p2) precursors and vacuolar mature form (mCPY) are indicated.
UPR Is Rapidly Activated in sec13-1 Cells following a Shift to 37 °C—Previously, we showed that the UPR pathway is also activated in sec13-1 cells grown at the semi-permissive condition of 30 °C (9). However, the kinetics of UPR activation following inactivation of Sec13p has not been measured in any previous study. Given that sec13-1 cells experience both secretory stress and alterations in lipid metabolism (Fig. 2), we wanted to determine whether UPR activation in sec13-1 cells occurs in response to the block in ER-to-Golgi transport or instead to the changes occurring in lipid metabolism. Therefore, we measured the rate at which the UPR is activated in sec13-1 cells following a temperature shift from 25 to 37 °C. We reasoned that if the UPR is activated with rapid kinetics, then it is likely responding to secretory stress produced by the block in ER-to-Golgi transport. On the other hand, if the activation of the UPR occurred with timing similar to the changes observed in phospholipid metabolism, then the UPR could be responding to the slowing in phospholipid metabolism.
To monitor activation of the UPR, Northern blotting of cellular RNA was performed to measure the level of HAC1 mRNA splicing. During UPR activation, the HAC1u (uninduced HAC1) transcript is spliced by an unconventional mechanism involving the endonuclease Ire1p and the tRNA ligase Trl1p to produce the form of HAC1i (induced HAC1) transcript, which is subsequently translated to form the active Hac1p transcription factor (38). As shown in Fig. 4, the UPR is rapidly activated following a temperature shift from 25 to 37 °C in the sec13-1 strain. By 30 min, ∼25% of the total HAC1 mRNA transcript was present in the HAC1i spliced form and increased to 50% by 60 min (Fig. 4), well before major changes in phospholipid synthesis were observed in sec13-1 cells (Fig. 2). This high level of splicing was maintained throughout the time course of the experiment, indicating that despite the trafficking block and shift in phospholipid metabolism, the cells are capable of maintaining a vigorous UPR stress response. As a control, wild type cells shifted to 37 °C did not show a significant change in HAC1 splicing.
FIGURE 4.
Splicing of HAC1 mRNA is initiated in the sec13-1 mutant shortly after elevation to 37 °C. Strains were grown to mid-logarithmic growth phase in I+ medium at 25 °C. RNA was isolated from yeast cells immediately prior to and following temperature shift to 37 °C at indicated time points. HAC1 transcript abundance in both wild type and sec13-1 cells was analyzed by Northern blotting. The appearance of the spliced HAC1i form indicates activation of the UPR pathway. Ethidium bromide staining of 25 S and 18 S rRNA served as loading controls (not shown).
These results suggest that the UPR is responding to a buildup of secretory proteins in the ER, resulting from the rapid block in ER-to-Golgi transport induced by the temperature shift to 37 °C. However, these data do not rule out the possibility that changes in phospholipid metabolism can also contribute to UPR activation in this strain.
The Lipid Composition of Sec- Mutants Is Altered following a Shift to 37 °C—To determine overall lipid composition, as opposed to the rate of phospholipid synthesis (as shown in Fig. 2), cells were labeled to steady state with [14C]acetate at 25 °C and shifted to 37 °C while maintaining constant label. [14C]Acetate is incorporated into all carbon-containing metabolites and, unlike 32P, labels both phospholipids and neutral lipids. The proportionate distribution of [14C] in lipids in cells labeled in this fashion provides an assessment of overall lipid composition at the time of harvest.
This analysis was extended to strains carrying the sec31-2 and sec6-4 mutations, to determine whether the changes in lipid metabolism observed in sec13-1 were unique or shared with other Sec- mutants. The sec31-2 mutant, like sec13-1, is defective in COPII vesicle formation (39). Sec13p and Sec31p are soluble proteins, which form a complex that binds to the Sec23/24 complex in the final step in formation of the COPII vesicle coat (25). The sec6-4 mutant is defective in vesicle transport from the Golgi to the plasma membrane. Sec6p is a part of a multicomponent complex called the exocyst, which localizes to the tip of the growing bud (40).
In wild type cells, prior to the temperature shift, the predominant phospholipids observed were PI and PC, with [14C]acetate label distributed almost equally to these two major phospholipids (Fig. 5A, 0 time point). As expected, label associated with PE and PS was less than that associated with PI or PC in wild type cells labeled with [14C]acetate at 25 °C (Fig. 5A). This labeling pattern is comparable with the steady state phospholipid composition reported by Gaspar et al. (31), for wild type cells grown at 30 °C in inositol-containing medium. The overall amount of [14C]acetate label associated with the total neutral lipid fraction was comparable with the label associated with either PI or PC in wild type cells (Fig. 5A).
FIGURE 5.
Alteration in total lipid composition of wild type, sec13-1, and sec31-2 and sec6-4 cells following a shift to 37 °C. Cells were labeled to steady state with [1-14C]acetate (1 μCi/ml) in I+ medium at 25 °C. Cultures were allowed to grow to mid-logarithmic phase and were then shifted to 37 °C for 180 min. Samples were taken at 0, 60, 120, and 180 min after the elevation of temperature. The results represent the average of two independent experiments for sec31-2 and sec6-4. The data for wild type and sec13-1 represent the average of four and three independent experiments, respectively. Experimental error was less than 10% in all cases. Error bars are not shown for clarity of presentation. The lipids indicated are as follows: PI, solid squares; PC, solid circles; PS, open circles; PE, open triangles; NL, open diamonds. A, wild type cells; B, sec13-1; C, sec31-2 cells; D, sec6-4 cells.
The lipid composition of each of the Sec- mutants grown at 25 °C differed somewhat from wild type. In all three mutants, sec13-1, sec31-2, and sec6-4, the amount of label associated with PI was higher than that associated with PC (Fig. 5, B-D, 0 time point). The relative elevation of PI over PC content was most pronounced in the sec13-1 and sec6-4 strains. In sec13-1 cells grown at 25 °C (Fig. 5B, 0 time point), this effect was particularly pronounced, with PI content being almost twice the level of PC. In wild type cells, following a shift from 25 to 37 °C, PC and neutral lipid content per culture A600 nm unit increased slightly, whereas PI levels rose almost 40% after 3 h of growth at 37 °C (Fig. 5A). This increase in PI content in wild type cells is consistent with the dramatic increase observed in the rate of PI synthesis in wild type cultures shifted to 37 °C (Fig. 2).
In sec13-1 cells during the first 60 min of incubation at 37 °C, PI levels increased ∼35%, maintaining a level of PI per A600 nm of culture about 30% higher than that of wild type cells. PC levels also increased significantly during the first 60 min, as did the level of label associated with both PE and PS, although not as significantly as PC (Fig. 5B). In sec13-1 cultures, the increase in label associated with total neutral lipids per A600 nm unit of culture was comparable with the increase in PC for the first 60 min. However, by 120 min, label associated with PI had begun to decline and label associated with PC essentially plateaued. PI levels continued to drop between 120 and 180 min following the shift to 37 °C, whereas label in PC remained relatively constant. Most dramatically, label accumulated continuously in total neutral lipids in the sec13-1 culture. An increase of over 3-fold in 14C derived from acetate in neutral lipids was observed over the course of 3 h following the shift of sec13-1 cultures to 37 °C (Fig. 5B). In contrast, a much more modest increase in neutral lipids was observed in wild type cells, where an increase of about 30% occurred in the first 60 min. However, by 180 min, the level of [14C]acetate in neutral lipids in wild type cultures was only marginally higher than the level observed at 25 °C (Fig. 5A).
The sec31-2 and sec6-4 strains each showed unique patterns of accumulation of the various phospholipids in response to the temperature shift. Unlike sec13-1, the levels of PI per A600 nm unit remained constant in sec6-4 cells throughout the entire experiment, whereas a 2-fold increase in PS and a relative drop in PE levels at 120 min were observed following the shift to 37 °C (Fig. 5D). However, PC content increased almost 3-fold during the first 120 min of incubation of sec6-4 cells at 37 °C, leveling off thereafter (Fig. 5D). The sec31-2 strain showed increases in both PI and PC content for the first 120 min, followed by a decline in the levels of both of these lipids by 180 min from the peak achieved at 120 min (Fig. 5C).
Strikingly, all three Sec- strains, sec13-1, sec31-2, and sec6-4, showed dramatic increases in total neutral lipids upon a shift to 37 °C, as compared with wild type cells (Fig. 5). This subject is discussed in greater detail below.
Abundance of Several Neutral Lipid Classes Increases in Sec- Mutants following a Shift to the Restrictive Temperature—Wild type, sec13-1, and sec31-2 cells exhibited similar neutral lipid composition at 25 °C (Fig. 6, A-C, 0 time point), whereas sec6-4 cells exhibited somewhat higher free sterol levels (Fig. 6D, 0 time point). Increasing the incubation temperature from 25 to 37 °C had little effect on the neutral lipid composition of wild type cells, with the exception that free sterols increased slightly (Fig. 6A) per A600 nm unit. In contrast, all of the Sec- mutants showed pronounced increases in total neutral lipid content following the shift to 37 °C (Fig. 5, B-D, and Fig. 6, B-D). By 180 min following the shift to 37 °C, the free fatty acid fraction had increased by 8-fold in sec13-1, 5-fold in sec31-2, and 3-fold in sec6-4 cells per A600 nm unit (Fig. 6, B-D), whereas wild type cells showed a slight increase in free fatty acids (Fig. 6A) after the shift to 37 °C. The free sterol fraction also increased markedly in sec6-4, and to a lesser degree in sec31-2 and sec13-1 cells following the shift to 37 °C (compare Fig. 6D with B and C). DAG levels increased slightly in sec13-1 cultures and doubled in both sec31-2 and sec6-4 cultures. The levels of TAG increased by 11-fold in sec13-1 and 5-fold in both sec31-2 and sec6-4 cultures after 180 min of incubation at 37 °C (Fig. 6, B-D). The increase in TAG levels was already apparent in sec13-1 cells by 120 min (Fig. 6), coincident with the dramatic decrease in labeling of phospholipids with 32P seen in Fig. 2. The accumulation of label in TAG in sec13-1 cells during the interval from 120 and 180 min continued at a linear rate, comparable with that observed between 60 and 120 min (Fig. 6B), despite indications of some loss of cell viability by 180 min. Because label accumulation is normalized to A600 nm, the actual rate of increase in [14C]acetate incorporation into TAG per viable cell is underestimated by these results if a fraction of the sec13-1 population was not metabolizing during this interval.
FIGURE 6.
Effect of high temperature on the neutral lipid content of wild type, sec13-1, sec31-2, and sec6-4 cells. The cells were grown and labeled to steady state with [1-14C]acetate (1 μCi/ml), as described in Fig. 5. Experimental error was less than 10%. (Error bars are not shown.) The lipids indicated are as follows: free fatty acids, solid squares; triacylglycerols, open triangles; free sterols, open circles; diacylglycerols, solid circles; sterol esters, open diamonds. A, wild type cells; B, sec13-1; C, sec31-2 cells; D, sec6-4 cells. (The data for wild type and sec13-1 are also repeated in Fig. 12 on expanded scales.)
TAG is derived from DAG, which is produced from PA (Fig. 1). Therefore, an increase in accumulation of TAG, at a time when phospholipid synthesis is declining, suggests that PA is continuing to be synthesized, but rather than serving as precursor to phospholipid synthesis, it appears to be diverted via DAG to increased TAG synthesis.
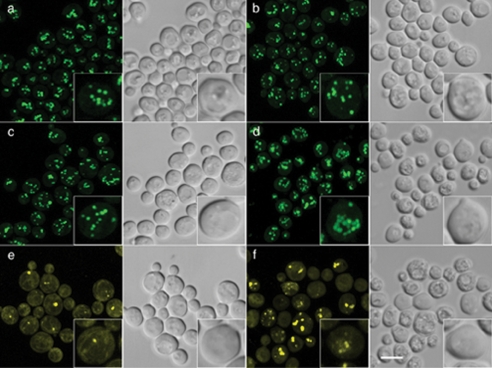
Fluorescence Microscopy Reveals Accumulation of Lipid Droplets in sec13-1 Cells following a Shift to Their Restrictive Temperature—The observed increase in TAG and other neutral lipids in Sec- mutants following the shift to 37 °C suggested that cells of these strains might show an increase in lipid droplet formation in comparison with wild type cells. To explore this hypothesis, cells of wild type and sec13-1 were stained with the neutral lipid dye BODIPY® 493/503, and examined by fluorescence microscopy, as described under “Experimental Procedures.” It is important to note that these cells, growing at 25 °C, are in mid-logarithmic phase at the time of the temperature shift to 37 °C and have not been stimulated to form lipid droplets, for example, by fatty acid loading (41, 42). However, during this growth phase, lipid droplet pools are mostly replenished after following the early phase of lipolysis (43). The distribution of lipid droplets in wild type cells at both 25 and 37 °C (Fig. 7, A and B) was normal for cells grown under these conditions (43). sec13-1 cells grown at 25 °C contained lipid droplets similar in size and distribution to those seen in wild type cells at both 25 and 37 °C (compare Fig. 7c to a and b). However, in sec13-1 cells harvested 120 min following a shift to 37 °C (Fig. 7d), the number of BODIPY®-stained lipid droplets appeared to be increased compared with sec13-1 at 25 °C or wild type at either temperature. In sec13-1 cells, in some cases, the droplets appeared to aggregate and to be partially fused, compared with those seen in wild type cells at 25 or 37 °C (Fig. 7, a and b) or to sec13-1 cells at 25 °C (Fig. 7c). As expected, the sec13-1 dga1Δlro1Δare2Δ strain lacking almost all DAG acyltransferase activity was largely devoid of lipid droplets when grown at the permissive temperature of 25 °C (Fig. 7e and confirmed by electron microscopy; data not shown). Interestingly, more prominent BODIPY®-stained but slightly red-shifted fluorescent structures appeared in this strain after incubation at 37 °C, which therefore could represent phospholipid membrane aggregates rather than lipid droplets (32) (Fig. 7f). Less condensed, but still significant, staining of intracellular membranes with the lipophilic dye was apparent in this strain, even under permissive growth conditions.
FIGURE 7.
Fluorescence microscopic analysis of lipid droplets. Prior to microscopy, cultures were grown in I+ medium at 25 °C as described under “Experimental Procedures” and were then diluted to A600 nm = 0.1 in 2 ml of I+ medium in multiwell plates, and incubated at 25 °C in an Eppendorf incubator/mixer. After reaching A600 nm = 0.5, cultures were shifted to 37 °C, and aliquots removed at time 0 and 120 min, fixed and stained with BODIPY® 493/503, and analyzed by fluorescence microscopy. a, wild type cells at 25 °C (zero time at 37 °C); b, after 120 min of incubation at 37 °C; c, sec13-1 cells at 25 °C (zero time at 37 °C); d, after 120 min of incubation at 37 °C; e, sec13-1lro1Δdga1Δare2Δ cells at 25 °C (zero time at 37 °C); and f, after 120 min incubation at 37 °C. Note that fluorescence emitted from BODIPY® that is dissolved in membrane aggregates, as opposed to lipid droplets, is red shifted, as indicated by a more yellowish fluorescence in e and f. Insets are 2-fold magnifications of representative cells. Bar = 5 μm.
Flow Cytometric Analysis of the Neutral Lipids in sec13-1 sec31-2 and sec6-4 Cells—To quantitate lipid droplet accumulation, wild type, sec13-1, sec13-2, and sec6-4 strains were grown at 25 °C to mid-logarithmic phase. Half of each culture was maintained at 25 °C for 120 min, as a control, and the remaining half was shifted to 37 °C for 120 min. All cultures were stained for with the fluorescent neutral lipid dye BODIPY® 493/503 and analyzed by flow cytometry. The results are shown in Fig. 8. The level of fluorescence intensity in the Sec- mutants at 25 °C was very similar to the levels observed in wild type cells grown at the same temperature with the exception of sec13-1, which exhibited somewhat higher fluorescence intensity than the other strains, even at 25 °C (Fig. 8).
FIGURE 8.
Flow cytometric analysis of lipid droplet content of wild type (wt), sec13-1, sec31-2, and sec6-4 cells at 25 and 37 °C. Yeast cells were grown until mid-logarithmic phase in I+ medium at 25 °C and split in two cultures; one of the cultures was shifted to 37 °C, and the other one was kept at 25 °C as a control. After 120 min of incubation, samples were withdrawn and fixed with 3.7% formaldehyde. Formaldehyde-fixed cells were permeabilized by treatment with 0.1% Triton X-100 in 1× PBS buffer, pH 8, for 10 min in and incubated with 10 μm BODIPY® 493/503 for 15 min to label the lipid droplets and were analyzed as described under “Experimental Procedures” by flow cytometer. Open bars illustrate the strains incubated at 25 °C; solid bars represent the strains incubated at 37 °C. The results represent the mean of three independent experiments.
The level of fluorescence detected in cultures of both wild type cells and Sec- mutants incubated at 37 °C for 120 min was higher than that of cultures incubated at 25 °C (Fig. 8). However, the relative fluorescence intensity varied among the various mutants. For example, sec6-4 cells incubated at 37 °C for 120 min showed fluorescence intensity only slightly higher than wild type cells grown under identical experimental conditions (Fig. 8). In contrast, sec31-2 and sec13-1 cells exhibited significantly higher fluorescence intensity than wild type cells, with sec13-1 cultures exhibiting the largest increase following a shift to 37 °C.
Electron Microscopy Reveals the Buildup of Lipid Droplets and Glycogen in sec13-1 Cells Shifted to 37 °C—Electron microscopy confirmed normal appearing lipid droplets in wild type cells grown at 25 °C or shifted from 120 min to 37 °C (Fig. 9, A and B), as well as in sec13-1 cells grown at 25 °C (thin arrows, Fig. 9C). These structures presumably correspond to the lipid droplets seen by fluorescence microscopy (Fig. 7). In addition, a significant fraction of the sec13-1 cells shifted to 37 °C for 120 min appeared to have fully formed lipid droplets that were closely associated with the vacuole. At higher magnifications, lipid droplets were frequently found tightly bound to the vacuole (Fig. 10, A-D), in some cases apparently caught in the process of being “internalized” into the vacuole (Fig. 10D). To quantitate the average number of droplets per cell section and to determine the average number of lipid droplets associated with the vacuole lipid, droplets were counted, as described under “Experimental Procedures” (Table 3). Sections of sec13-1 cells that had been shifted to 37 °C for 120 min, contained a significantly higher number of lipid droplets, and a higher fraction of these lipid droplets were associated with the vacuole than in either wild type cells shifted to 37 °C or sec13-1 cells grown at 25 °C (Table 3).
FIGURE 9.
Electron microscopy reveals lipid droplets associated with vacuoles in sec13-1 cells. Cells were grown as in Fig. 6, and then processed for transmission electron microscopy as described under “Experimental Procedures.” A, wild type (WT) cells at 25 °C; B, wild type cells after 120 min at 37 °C; C, sec13-1 cells at 25 °C; D-F, sec13-1 cells after 120 min at 37 °C. Arrows point to lipid droplets adjacent to the ER (A-C and E) or tightly associated with vacuoles (D and F). The arrowhead in F points to electron lucent glycogen clusters found in sec13-1 cells after 120 min at 37 °C. Vac = vacuole. Bars = 0.5 μm.
FIGURE 10.
Higher magnification views of sec13-1 cells after 120 min at 37 °C reveal lipid droplets in close association with the vacuole. Electron microscopy performed as in Fig. 9. Lipid droplets are seen in association with the vacuoles (arrows in A-D), and in some cases were apparently being internalized into the vacuole (arrows in C and D). Also, the electron lucent clusters of glycogen (arrowheads) are interconnected and found under the cortical ER (E and F). Vac = vacuole; bars = 0.2 μm.
TABLE 3.
Quantitation of lipid droplets observed in transmission electron microscopy thin sections
Lipid droplets were counted, and their association with the vacuole was assessed by visual inspection of grids of yeast cell images from thin sections examined by transmission electron microscopy as described under “Experimental Procedures.” The cell images were obtained from two different experiments for wild type cells grown at 25 °C and shifted for 120 minat 37 °C. Data for sec13-1 cells represent three separate experiments in which sec13-1 cells were grown at 25 °C and shifted for 2 h to 37 °C. Abbreviations used are as follows: LD, lipid droplets; LD vac, lipid droplets associated with the vacuole; LD non-vac, lipid droplets not associated with the vacuole.
|
Number
|
Percentage (%)
|
||||
|---|---|---|---|---|---|
| Cells | LD | LD/cell | LD vac | LD non-vac | |
| sec13-1 25 °C | 747 | 188 | 0.25 | 13.3 | 86.7 |
| sec13-1 37 °C | 609 | 849 | 1.39 | 65.4 | 34.6 |
| Wild type 37 °C | 560 | 239 | 0.42 | 10.5 | 89.5 |
Moreover, in sec13-1 cells incubated at 37 °C for 120 min, unusual large grape-like clusters of electron lucent particles that did not appear to contain a membrane surface layer were seen (Fig. 9F, arrowhead). These clusters were often found adjacent to the cortical ER and appeared to be interconnected (Fig. 10, E and F). Based on similar depictions in the literature, we speculated that these structures might represent accumulations of glycogen. Lugol staining, which detects glycogen accumulation, revealed lightly stained patches, which were scattered in wild type cells incubated at 37 °C for 120 min (Fig. 11A), and similar staining patterns were observed in both wild type and sec13-1 cells grown at 25 °C (data not shown). However, in the sec13-1 strain shifted to 37 °C for 120 min, distinctive, heavily condensed, and deeply stained patches appeared in about 50% of the cells (Fig. 11B). We believe that the condensed Lugol-staining patches observed in sec13-1 cells incubated at 37 °C correspond to the electron lucent grape like structures seen by electron microscopy (Fig. 9F; Fig. 10, E and F).
FIGURE 11.
Lugol staining of wild type and sec13-1 strains grown at 37 °C for 2 h. Cells were grown at 25 °C in I+ medium and then shifted to 37 °C prior to microscopy as described in Fig. 7 and stained as described under “Experimental Procedures.” a, wild type cells; b, sec13-1 cells. Bars = 5 μm.
Deletion of Genes Involved in TAG Biosynthesis in the sec13-1 Strain Leads to a Decrease in TAG Accumulation and a Compensating Increase in Phospholipid and Sterol Content after a Shift to 37 °C—To determine whether specific enzymes are responsible for the elevated TAG accumulation observed in sec13-1 cells shifted to 37 °C, genes encoding TAG synthases (Fig. 1) were systematically deleted in the sec13-1 genetic background. The strains created include sec13-1lro1Δ, sec13-1lro1Δdga1Δ, and sec13-1lro1Δdga1Δare2Δ (see Table 1 for full genotypes), as well as the following control strains: lro1Δ, dga1Δ, and lro1Δdga1Δ in the SEC13 background. The position of the reactions catalyzed by the products of each of the TAG synthase genes is shown in Fig. 1. Strains carrying TAG synthase structural gene deletions in various combinations in the sec13-1 and SEC13 genetic backgrounds were labeled to steady state with [14C]acetate, as described above, to analyze the changes in lipid composition following a shift to 37 °C.
Both before and after the shift to 37 °C, SEC13lro1Δ cultures had a neutral lipid composition similar to wild type except for a decreased TAG content (Fig. 12). The SEC13 dga1Δ strain, in contrast, had somewhat elevated TAG levels, whereas the SEC13 lro1Δdga1Δ strain exhibited elevated DAG levels, both before and after the shift to 37 °C. These differences could be due to the two different routes by which Dga1p and Lro1p catalyze synthesis of TAG and the relative importance of these routes in logarithmic phase growth versus stationary phase where many studies of lipid droplets and TAG synthesis have been conducted (44). The sec13-1 and wild type data provided for comparison on Fig. 12 are identical to that in Fig. 6, except that the data are displayed on different scales.
FIGURE 12.
Deletion of the LRO1, DGA1, and ARE2 genes in the sec13-1 genetic background results in a decrease in accumulation of neutral lipids, compared with the sec13-1 parent, following elevation to 37 °C. Cells were grown as described for steady state labeling in the presence of 1 μCi/ml [1-14C]acetate in I+ medium at 25 °C as described under “Experimental Procedures” and Figs. 5 and 6. At the mid-logarithmic phase of growth, the cultures were shifted to 37 °C. Samples were taken for lipid analysis at 0, 60, 120, and 180 min after the shift to 37 °C. Note that the data for wild type and sec13-1 are identical to (but are displayed on a different scale) the data shown in Fig. 6. Data are representative of at least two independent experiments. Experimental error was less than 10%. Error bars are not shown for clarity of presentation. The neutral lipids depicted are as follows: free fatty acids, solid squares; triacylglycerols, open triangles; free sterols, open circles; diacylglycerols, solid circles. Note that the free sterols (open circles) are depicted on a different scale than the other lipids.
As shown in Fig. 12, TAG accumulation in the sec13-1lro1Δ strain shifted to 37 °C was slower per A600 nm unit of culture in comparison with the sec13-1 parent. Free sterol accumulation was also slightly increased compared with sec13-1. Compared with the sec13-1 strain, TAG accumulation was further decreased, and free sterol accumulation was further increased in the sec13-1lro1Δdga1Δ and sec13-1lro1Δdga1Δare2Δ strains. In addition, DAG levels in these two strains were ∼2-fold higher than in either the wild type or sec13-1 strains, even at 25 °C, before the temperature shift (Fig. 12, 0 time point). Following the temperature shift, DAG levels increased further in the sec13-1lro1Δ, sec13-1lro1Δdga1Δ, and sec13-1 lro1Δdga1Δare2Δ strains, leading to levels of DAG that were at least 3-fold higher than in the sec13-1 or wild type strains. The sec13-1lro1Δdga1Δare2Δ strain also accumulated the least amount of TAG, whereas free sterol accumulation was 4-fold higher than the wild type strain and more than double that observed in sec13-1. In the sec13-1lro1Δ and sec13-1lro1Δdga1Δare2Δ strains, as well as in the sec13-1 parent, free fatty acid levels, which were similar to wild type at 25 °C, increased more than 4-fold after the temperature shift.
Each of the strains carrying deletions of the TAG synthase genes also showed increased phospholipid accumulation in comparison with sec13-1 following a shift to 37 °C (Fig. 13). In the sec13-1 strain, net accumulation of both PI and PC as a function of A600 nm unit of culture ceased after the 60-min time point at 37 °C (Figs. 5 and 13), coincident with the drop in 32P incorporation into total phospholipids and PI, which occurred between the 1st and 2nd h after the temperature shift (Fig. 2). In contrast, the sec13-1 lro1Δ strain continued accumulating 14C label into both PI and PC in an almost linear fashion as a function of A600 nm unit of culture throughout 180 min of labeling following the shift to 37 °C. In the sec13-1lro1Δdga1Δ and sec13-1 lro1Δdga1Δare2Δ strains (Fig. 13), net PC accumulation as a function of A600 nm unit of culture continued for the full 180 min, reaching levels significantly higher than in the sec13-1 parent strain, whereas PI synthesis plateaued after 60 min. However, in each of these strains, the overall level of both PC and PI accumulation reached levels considerably higher than the sec13-1 parent. The total accumulation of PC was greatest in the sec13-1 lro1Δdga1Δare2Δ strain, reading levels 2-fold higher than in the sec13-1 strain (Fig. 13).
FIGURE 13.
Deletion of the LRO1, DGA1, and ARE2 genes in the sec13-1 genetic background leads to an increase in PC and PI content, relative to the sec13-1 parent strain, following a shift to 37 °C. Changes in the accumulation of phospholipids in response to an increase in temperature in wild type and sec13-1 cells carrying deletions in some steps of the TAG formation was assessed by labeling the cells in the presence of [1-14C]acetate under conditions identical to those described in Figs. 6, 7, and 12. Experimental error was less than 10%. Error bars are not shown for clarity of presentation. The lipids indicated are as follows: PI, solid squares; PC, solid circles; PS, open circles; PE, open triangles.
These results suggest that the gene products of all three genes, LRO1, DGA1 and ARE2, contribute to the accumulation of TAG in the sec13-1 strain following a shift to 37 °C. The increase in phospholipid accumulation compared with the sec13-1 parent, in the strains lacking TAG synthases, supports the hypothesis that the increase in TAG in the parental sec13-1 strain occurs by diverting PA from phospholipid synthesis into production of TAG. These strains also exhibited elevated accumulation of free sterols in comparison with their sec13-1 parent. These results will be presented under “Discussion.”
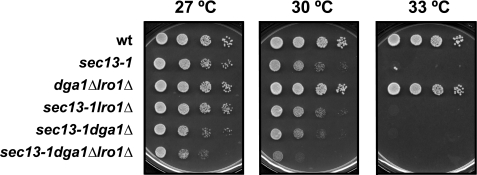
The sec13-1 Mutation Exhibits Partial Synthetic Lethality with Mutations in the Last Step of the TAG Synthesis—To explore whether elevated TAG synthesis plays a role in the survival of the sec13-1 mutant under semi-permissive growth conditions, growth of the strains carrying sec13-1 in combination with mutations in genes involved in TAG synthesis were tested by plating strains on different media and at various growth temperatures. Phenotypes obtained following growth on YPD plates at the permissive, semi-permissive, and nonpermissive growth temperatures for the sec13-1 mutant are shown in Fig. 14. The sec13-1lro1Δ and sec13-1 dga1Δ double mutant strains had phenotypes very similar to the sec13-1 parent. No change was detected in the growth pattern of these strains in comparison with sec13-1 on YPD media (Fig. 14). However, the sec13-1lro1Δdga1Δ strain grew poorly on YPD medium at 30 °C, a temperature that is permissive for sec13-1 and lro1Δdga1Δ strains under this growth condition (Fig. 14). The sec13-1lro1Δdga1Δ-are2Δ strain exhibited reduced growth, similar to the sec13-1 lro1Δdga1Δ strain on YPD medium at 30 °C (data not shown).
FIGURE 14.
The lro1Δ and dga1Δ mutations lower the permissive temperature of strains carrying the sec13-1. Overnight cultures were diluted to A600 nm = 0.1 in 25 ml of YPD medium and allowed to grow to mid-logarithmic phase at 25 °C. Each sample was diluted in multiwell plates by 1:10 serial dilutions, and 4 μl of cells from each dilution were spotted on YPD plates and allowed to grow at the designated temperatures for 2 days.
Growth of various strains carrying sec13-1 in combination with mutations blocking TAG synthesis was also tested on synthetic complete medium with inositol (I+) or without inositol (I-) (Fig. 15). All the strains exhibited growth phenotypes on I+ medium at 27 °C comparable with the sec13-1 parent strain (Fig. 15). Also, consistent with the report of Gilstring et al. (24), all strains carrying the sec13-1 mutation failed to grow in I- medium at 30 °C (Fig. 15). However, in contrast to the sec13-1 parent strain, the sec13-1lro1Δdga1Δ and sec13-1 lro1Δdga1Δare2Δ strains all grew poorly in I- medium at 27 °C, whereas the sec13-1 parent showed a comparable defect only at 30 °C and above (Fig. 15). The probable role of inositol supplementation in this phenotype will be presented under “Discussion.”
FIGURE 15.
The lro1Δ, dga1Δ, and are2Δ mutations lower the permissive temperature of strains carrying the sec13-1 mutation on synthetic complete medium lacking inositol. Overnight, cultures were diluted to A600 nm = 0.1 in 25 ml of I+ or I- medium and allowed to grow to mid-logarithmic phase at 25 °C. Each sample was diluted in multiwell plates by 1:10 serial dilutions, and 10 μl of cells from each dilution were spotted on I+ and I- plates and allowed to grow at the designated temperatures for 2 days.
The above results indicate that defects in TAG biosynthesis have a synthetic lethal interaction with the sec13-1 mutation. This suggests that the metabolic switch to increased synthesis of TAG at the expense of phospholipid synthesis, in the sec13-1 strain, following the shift to 37 °C, is physiologically significant and provides protection under conditions of secretory stress, particularly in the absence of inositol.
DISCUSSION
We have shown that arrest of membrane trafficking in yeast cells results in a metabolic switch from phospholipid synthesis to neutral lipid synthesis. Our study focused primary attention on changes in lipid metabolism occurring in sec13-1, a temperature-sensitive mutant defective in COPII vesicle formation. However, similar changes were observed in sec31-2, another mutant affecting COPII vesicle formation and in sec6-4, a mutant defective in a distal step of the secretory pathway at the plasma membrane. Although the changes in lipid metabolism in these strains were not as extreme as those observed in sec13-1, all three Sec- mutants accumulated elevated levels of neutral lipids in comparison with the wild type control, following a shift from 25 °C to the restrictive temperature of 37 °C. In the sec13-1 and sec31-2 strains, the most striking change was the accumulation of TAG, but levels of free sterol also increased. In sec6-4, a smaller increase in TAG was observed, whereas a major increase in free sterols took place. These increases in neutral lipids were also reflected in an increase in fluorescence detected by FACS following BODIPY® staining. In sec13-1, concurrent changes in lipid droplet accumulation and morphology were detected by both fluorescence and electron microscopy.
The fact that changes in lipid metabolism were detected in two mutants defective in COPII vesicle trafficking and in a mutant defective in distal secretion suggests that this process is not specific to a single membrane trafficking step, but may, similar to induction of the UPR, be generally associated with a partial (9, 10) or total (Fig. 3) block in membrane transport. Consistent with this hypothesis, Szymanski et al. (45) detected changes in lipid droplet morphology in several mutants affecting retrograde trafficking from the Golgi to the ER (45). However, the kinetics of the changes in lipid metabolism and the magnitude of the effect on specific lipids varied (Figs. 5 and 6) in the different Sec- mutants we examined, particularly with respect to sec6-4, as compared with sec13-1 and sec31-2. This suggests that mutations affecting different steps in membrane trafficking may result in somewhat different patterns of changes in lipid metabolism, a subject that will require further investigation. Because the majority of this study concerned sec13-1, the remainder of this discussion will focus on the effects seen in strains carrying this mutation.
Block in Membrane Trafficking in the sec13-1 Mutant Precedes Changes in Lipid Metabolism—The original isolation of Sec- mutants was based on density gradient centrifugation to select for cells of increased density, following a shift to the restrictive temperature of 37 °C (21). Indeed, Sec- mutants all exhibited increased density following a shift to their restrictive temperature. Mutants auxotrophic for inositol (Ino- phenotype), which cannot synthesize PI when exogenous inositol is absent, also increase in density when they are shifted to medium lacking inositol (46). Inositol-starved ino1Δ cells were also shown to cease membrane expansion while continuing to metabolize within a static volume (46, 47), a characteristic shared by Sec- mutants (21). The phenotype of increased cellular density was used independently by Letts and Dawes (26) with the intention of enriching for mutants defective in overall phospholipid synthesis. Letts and Dawes (26) succeeded in isolating a temperature-sensitive mutant that showed a fairly rapid cessation of phospholipid synthesis, while continuing to synthesize fatty acids, but this mutant proved to be allelic to the sec13-1 mutant isolated by Novick et al. (21). Letts and Dawes (26) reported that, upon shift to the restrictive temperature, phospholipid synthesis was blocked in strains carrying their sec13 allele prior to any effect on secretion of invertase into the periplasmic space. However, several reports indicate that transport of proteins out of the ER is rapidly blocked in sec13-1 strains shifted to the restrictive temperature of 37 °C (36, 37). Furthermore, Ramirez et al. (48) reported that phospholipid synthesis continued in a wide range of Sec- strains following cessation of membrane expansion.
Although strains carrying the allele of sec13 as studied by Letts and Dawes (26) are no longer available for comparison, the results obtained with the sec13-1 strain in our study clearly confirm that, following a shift of sec13-1 to the restrictive temperature of 37 °C, a block in ER to Golgi trafficking occurred within 15 min (Fig. 3), well before the major changes in lipid metabolism (Figs. 2 and 5). Furthermore, UPR activation, indicative of ER stress, occurred within 30 min after elevation of sec13-1 cells to 37 °C (Fig. 4), again preceding major changes in phospholipid and neutral lipid synthesis (Figs. 2, 5, and 6). Therefore, the changes in lipid metabolism observed in sec13-1 cells could be a consequence, either direct or indirect, of the primary defect in membrane trafficking.
Arrest of Membrane Trafficking Leads to Profound Changes in Lipid Metabolism, Including a Dramatic Slowing of Synthesis of Bilayer-forming Phospholipids and a Simultaneous Increase in Accumulation of Neutral Lipids—Phospholipid synthesis declined rapidly in sec13-1 cells between 60 and 120 min following the shift to 37 °C and was accompanied by a simultaneous increase in neutral lipid accumulation (Figs. 5, 6, and 12). Levels of TAG increased dramatically in sec13-1 cells between 60 and 120 min and continued to increase in linear fashion out to 180 min after the shift to 37 °C (Figs. 6 and 12). Similar patterns of TAG accumulation with different kinetics were observed in sec13-2 and sec6-4 cultures (Figs. 5 and 6). All three strains showed an increase in neutral lipid content (Figs. 5 and 6), suggesting that a rapid metabolic switch from phospholipid synthesis to neutral lipid accumulation is a general effect following a block in membrane trafficking in yeast.
In contrast, the most dramatic change in wild type cells following a shift to 37 °C was an increased rate of synthesis and accumulation of PI (Figs. 2 and 6), suggesting that at higher growth temperatures yeast cells have an elevated requirement for PI and/or inositol-containing metabolites for which PI serves as a precursor. This may explain, in part, the observation that a number of mutants such as sac1Δ and scs2Δ, which are weak inositol auxotrophs at 30 °C (49, 50), show strong inositol auxotrophy at 37 °C. In other words, an underlying deficiency in inositol caused by insufficient de novo production of inositol would have greater physiological impact at 37 °C because of increased demand for PI synthesis.
At the permissive temperature of 25 °C, sec13-1 cells exhibited elevated rates of synthesis of both PI and PS (Fig. 2) and accumulated levels of a higher PI content per A600 nm unit of culture (Fig. 5) than wild type cells. This suggests that, even at 25 °C, the mutant version of Sec13p encoded by sec13-1 is partially defective and that its subtle dysfunction results in changes in lipid metabolism relative to wild type cells. This speculation is consistent with the observation that sec13-1 cells require supplementation with inositol at the semi-permissive temperature of 30 °C (Fig. 15) (24). The Ino- phenotype of the sec13-1 strain indicates that its requirement for PI synthesis at 30 °C exceeds its capacity to synthesize inositol de novo. This could result either from reduced capacity to synthesize inositol or from increased demand for inositol at higher temperatures, or a combination of the two effects.
Cells growing in the absence of inositol are forced to rely on de novo synthesis of inositol, the rate-limiting step catalyzed by the product of the INO1 gene (51). Chang et al. (9) reported that the level of INO1 expression in sec13-1 at 30 °C, although diminished compared with wild type, was at a level equivalent to that reported in other strains capable of growing in the absence of inositol. Because the signal that controls INO1 expression is the level of PA in the ER (52), diversion of PA to support synthesis of TAG could result in lower levels of expression of INO1, causing diminished de novo inositol production. This, combined with a higher demand for PI synthesis seen in the sec13-1 mutant even at 25 °C (Figs. 2 and 5), could explain the inositol auxotrophy of sec13-1 at 30 °C. The introduction of mutations in TAG synthesis into the sec13-1 genetic background forces yet a greater requirement for PI synthesis, as seen in Fig. 13. This requirement for additional inositol could explain the inability of these strains to grow at 27 °C when exogenous inositol is absent (Fig. 15).
Dephosphorylation of PA Diverted from Phospholipid Synthesis Drives Production of TAG—The fact that TAG accumulation increases in sec13-1 cells (Fig. 6) shifted to 37 °C indicates that the simultaneous decline in phospholipid synthesis (Fig. 2) is not because of an overall failure of lipid synthesis. Neither can it be due to a failure to synthesize PA, which is the precursor to both TAG and phospholipids (Fig. 1). Rather, as phospholipid synthesis decreases in sec13-1 cells, it appears that PA is dephosphorylated to produce DAG, which is subsequently converted to TAG. Importantly, no single TAG synthase appears to be responsible for the increase in TAG synthesis in sec13-1 cells at 37 °C. As the major genes encoding TAG synthases were systematically deleted in the sec13-1 genetic background, TAG synthesis decreased in a cumulative fashion (Fig. 12), in comparison with the sec13-1 parent. DAG levels were also substantially elevated in sec13-1lro1Δdga1Δ and sec13-1 lro1Δdga1Δare1Δ strains (Fig. 12). In the sec13-1 strain, levels of DAG were similar to wild type at 25 °C but had almost doubled after 180 min at 37 °C. Furthermore, the decline in TAG levels in these strains was offset by increasing phospholipid accumulation compared with the sec13-1 parent (Fig. 13). sec13-1 strains carrying mutations blocking TAG synthesis accumulated both PI and PC for at least 120 min and, in some cases, up to 180 min after the shift to 37 °C, whereas the level of these phospholipids plateaued in sec13-1 after the first 60 min at 37 °C (Fig. 13). Thus, we conclude that the cell possesses a mechanism for diverting PA from phospholipid synthesis into production of DAG and TAG, under conditions in which membrane trafficking from the ER is impeded. However, this metabolic switch is not absolute because the introduction of mutations blocking TAG synthesis in the sec13-1 background resulted in both increased DAG levels and a continuation of phospholipid synthesis after the shift to 37 °C (Figs. 12 and 13).
Each of the sec13-1 strains carrying mutations in TAG synthesis also showed increasing free sterol accumulation, again with the sec13-1lro1Δdga1Δare2Δ strain showing the greatest increase (Fig. 12). In a previous study (31), we observed a large increase in [14C]acetate labeling of free sterols in cells treated with cerulenin to block fatty acid synthesis. This result suggested that when actively grown cells are unable to make fatty acids, excess acetyl-CoA is shunted from fatty acid synthesis into the mevalonate pathway (Fig. 1). In the case of the sec13-1 strains carrying mutations in TAG synthases, fatty acid synthesis is clearly still occurring as shown by accumulation of [14C]acetate into phospholipids, DAG, and free fatty acids. However, synthesis of these metabolites may not suffice to absorb all of the cellular energy normally channeled through acetyl-CoA into fatty acids in the strains studied here, in which membrane trafficking from the ER is inhibited because of Sec13p inactivation and especially when the synthesis of TAG, a major “sink” for fatty acid storage, is blocked. Under these conditions, we propose that acetyl-CoA may be shunted into sterol synthesis.
Overall, the cell appears to employ a hierarchy of strategies for channeling the flow of carbon from acetyl-CoA into various lipids under conditions created by a block in membrane trafficking from the ER. We propose that these strategies serve to reduce the buildup of bioactive intermediates of lipid metabolism, such as PA, DAG, and acetyl-CoA under conditions of membrane stress. Previous studies have shown that as cells approach stationary phase, phospholipid synthesis is curtailed, and synthesis of TAG and sterol esters increases, leading to the production of lipid droplets (53, 54). Lipid droplet formation is also stimulated when growing cells are supplemented with oleic acid (41, 42). However, the conditions we employed here involved abrupt cessation of membrane trafficking during active growth, under conditions of ongoing metabolism, with no supplementation with exogenous fatty acids. Under these conditions, cells apparently continue to produce fatty acids, which normally would have gone into phospholipids to drive membrane biogenesis, but which, in the absence of membrane trafficking from the ER, are channeled via PA through DAG into TAG.
Under Restrictive Growth Conditions, sec13-1 Cells Accumulated Lipid Droplets and Glycogen—Depending on the nature of their defect, mutants with defects in membrane trafficking have been reported to accumulate abnormal intracellular membranes, both in quantity and structure, when they are shifted to their specific restrictive growth conditions (21, 39, 55-57). Genetic and biochemical studies have revealed that Sec13p is involved in vesicle formation in the ER and that Sec13p with the Sec23p/Sec24p complex and Sar1p together drive COPII vesicle formation (55, 58, 59). A pool of Sec13p is also required for the proper function of the nuclear pore complex, and several sec13 mutants exhibit aberrant nuclear morphology (60-62). However, the sec13-1 mutation affects neither the nuclear envelope nor nuclear pore complex morphology (60).
Fluorescence microscopy of sec13-1 cells following the shift to 37 °C revealed the accumulation of lipid droplets of abnormal morphology (Fig. 7). Fluorescence-activated cell sorting (Fig. 8) confirmed the increase in lipid droplets as did electron microscopy (Table 3). Many of the lipid droplets observed by electron microscopy in sec13-1 cells grown at 37 °C were associated with the vacuole (Fig. 10; Table 3). Interestingly, Szymanski et al. (45) detected abnormal lipid droplet morphology in a significant number of mutants having defects in endosomal vacuolar trafficking.
Large numbers of unusual electron lucent grape-like structures were also detected in sec13-1 cells shifted to 37 °C. Similar structures are present (but were not commented upon) in published electron micrographs of the sec16-2 mutant (21) and of an mga2Δspt23ts strain, defective in oleic acid synthesis (63). Later, Arvindekar and Patil (64) described these structures as accumulations of glycogen. Therefore, we speculated that the sec13-1 cells grown at the restrictive temperature might have similar glycogen deposits. Indeed, Lugol staining of sec13-1 cells confirmed the presence of heavily condensed stained patches of glycogen (Fig. 11B), in the sec13-1 strains, but not in wild type, after a shift to 37 °C for 120 min (Fig. 11A). The accumulation of such structures in cells with defects in lipid metabolism also may relate to regulation of cellular mechanisms for carbon and energy storage under conditions of changing or aberrant lipid metabolism.
Synthesis of TAG Is Essential under Conditions of ER Stress Produced by a Block in ER Vesicle Formation—The finding that an S. cerevisiae quadruple mutant strain lacking the ability to synthesize TAG and sterol esters and completely lacking lipid droplets is viable (42, 65) led to the conclusion that storage lipid synthesis is not essential for growth in the budding yeast. However, our results indicate that synthesis of TAG plays a functional role during secretory stress. This conclusion is supported by the partial synthetic lethality of sec13-1 in combination with deletion of the TAG synthases encoded by the DGA1 and LRO1 genes (Figs. 14 and 15). TAG represents a very efficient way to store energy in the form of fatty acids, and its synthesis provides a route for converting other bioactive molecules such as DAG and PA into relatively inactive storage compounds. Thus, the 3-fold elevated levels of DAG, and the increased load of free fatty acids, accumulated in the sec13-1lro1Δdga1Δ triple mutant, following the shift to 37 °C (Fig. 12), could be toxic. Similarly, TAG accumulation in nonadipose mammalian cultured cells is proposed to represent a cellular response to fatty acid-induced lipotoxicity (66). When cultured mammalian cells are overloaded with saturated fatty acids, the cellular capacity to store fatty acids as TAG or to use them for energy is over-whelmed, and the resulting overload of fatty acids facilitates the accumulation of reactive oxygen species, which subsequently induce ER stress and cell death (67).
To the best of our knowledge, this is the first report of an essential role for TAG synthesis during ER secretory stress. TAG synthesis appears to function as a metabolic sink to remove excess free fatty acids, PA, and/or DAG accumulated in the ER as a consequence of the slowing of phospholipid synthesis following a block in membrane trafficking from the ER.
Acknowledgments
We thank Dr. Andrew Yen and Dr. James Smith of the Biomedical Sciences Flow Cytometry Core Laboratory at the Cornell University College of Veterinary Medicine for providing assistance in the experiments involving flow cytometry. Dr. Yen also provided invaluable consultation and advice on methodology and analysis of the data acquired.
This work was supported, in whole or in part, by National Institutes of Health Grants GM019629 (to S. A. H.) and DK51596 (to W. J. B.) from the NIGMS and NIDDK, respectively. This work was also supported by SFB Lipotox P3005 of the Austrian Science Fund (to S. D. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; TAG, triacylglycerol; DAG, diacylglycerol; PA, phosphatidic acid; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; UPR, unfolded protein response; CDP-DAG, cytidine diphosphate-diacylglycerol; YPD, yeast extract/peptone/dextrose; TPL, total 32P associated with the sum of the following phospholipids: PA, PI, PS, PE, PC; FACS, fluorescence-activated cell sorter; PBS, phosphate-buffered saline; CPY, carboxypeptidase Y.
References
- 1.Gaspar, M. L., Aregullin, M. A., Jesch, S. A., Nunez, L. R., Villa-Garcia, M., and Henry, S. A. (2007) Biochim. Biophys. Acta 1771 241-254 [DOI] [PubMed] [Google Scholar]
- 2.Block-Alper, L., Webster, P., Zhou, X., Supekova, L., Wong, W. H., Schultz, P. G., and Meyer, D. I. (2002) Mol. Biol. Cell 13 40-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox, J. S., Chapman, R. E., and Walter, P. (1997) Mol. Biol. Cell 8 1805-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sriburi, R., Jackowski, S., Mori, K., and Brewer, J. W. (2004) J. Cell Biol. 167 35-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriburi, R., Bommiasamy, H., Buldak, G. L., Robbins, G. R., Frank, M., Jackowski, S., and Brewer, J. W. (2007) J. Biol. Chem. 282 7024-7034 [DOI] [PubMed] [Google Scholar]
- 6.Cox, J. S., Shamu, C. E., and Walter, P. (1993) Cell 73 1197-1206 [DOI] [PubMed] [Google Scholar]
- 7.Cox, J. S., and Walter, P. (1996) Cell 87 391-404 [DOI] [PubMed] [Google Scholar]
- 8.Mori, K., Ma, W., Gething, M. J., and Sambrook, J. (1993) Cell 74 743-756 [DOI] [PubMed] [Google Scholar]
- 9.Chang, H. J., Jesch, S. A., Gaspar, M. L., and Henry, S. A. (2004) Genetics 168 1899-1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leber, J. H., Bernales, S., and Walter, P. (2004) PLoS Biol. 2 E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M., and Walter, P. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18773-18784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou, J., Liu, C. Y., Back, S. H., Clark, R. L., Peisach, D., Xu, Z., and Kaufman, R. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 14343-14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travers, K. J., Patil, C. K., Wodicka, L., Lockhart, D. J., Weissman, J. S., and Walter, P. (2000) Cell 101 249-258 [DOI] [PubMed] [Google Scholar]
- 14.Bernales, S., McDonald, K. L., and Walter, P. (2006) PLoS Biol. 4 e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyde, M., Block-Alper, L., Felix, J., Webster, P., and Meyer, D. I. (2002) J. Cell Biol. 156 993-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson, L. L., Parrish, M. L., Koning, A. J., and Wright, R. L. (2002) Yeast 19 373-392 [DOI] [PubMed] [Google Scholar]
- 17.Chang, H. J., Jones, E. W., and Henry, S. A. (2002) Genetics 162 29-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jesch, S. A., Zhao, X., Wells, M. T., and Henry, S. A. (2005) J. Biol. Chem. 280 9106-9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jesch, S. A., Liu, P., Zhao, X., Wells, M. T., and Henry, S. A. (2006) J. Biol. Chem. 281 24070-24083 [DOI] [PubMed] [Google Scholar]
- 20.Carman, G. M., and Henry, S. A. (1999) Prog. Lipid Res. 38 361-399 [DOI] [PubMed] [Google Scholar]
- 21.Novick, P., Field, C., and Schekman, R. (1980) Cell 21 205-215 [DOI] [PubMed] [Google Scholar]
- 22.Salama, N. R., Yeung, T., and Schekman, R. W. (1993) EMBO J. 12 4073-4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryer, N. K., Salama, N. R., Schekman, R., and Kaiser, C. A. (1993) J. Cell Biol. 120 865-875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilstring, C. F., Melin-Larsson, M., and Ljungdahl, P. O. (1999) Mol. Biol. Cell 10 3549-3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhausen, T. (2007) Cell 129 1251-1252 [DOI] [PubMed] [Google Scholar]
- 26.Letts, V. A., and Dawes, I. W. (1983) J. Bacteriol. 156 212-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longtine, M. S., McKenzie, A., III, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998) Yeast 14 953-961 [DOI] [PubMed] [Google Scholar]
- 28.Atkinson, K., Fogel, S., and Henry, S. A. (1980) J. Biol. Chem. 255 6653-6661 [PubMed] [Google Scholar]
- 29.Burnett, W. V. (1997) BioTechniques 22 668-671 [DOI] [PubMed] [Google Scholar]
- 30.Hirsch, J. P., and Henry, S. A. (1986) Mol. Cell. Biol. 6 3320-3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaspar, M. L., Aregullin, M. A., Jesch, S. A., and Henry, S. A. (2006) J. Biol. Chem. 281 22773-22785 [DOI] [PubMed] [Google Scholar]
- 32.Wolinski, H., and Kohlwein, S. D. (2008) Methods Mol. Biol., (Vancura, A., ed) Humana Press, in press
- 33.Cahill, G., Walsh, P. K., and Donnelly, D. (2000) Biotechnol. Bioeng. 69 312-322 [DOI] [PubMed] [Google Scholar]
- 34.Wright, R. (2000) Microsc. Res. Tech. 51 496-510 [DOI] [PubMed] [Google Scholar]
- 35.Kelley, M. J., Bailis, A. M., Henry, S. A., and Carman, G. M. (1988) J. Biol. Chem. 263 18078-18085 [PubMed] [Google Scholar]
- 36.Fatal, N., Suntio, T., and Makarow, M. (2002) Mol. Biol. Cell 13 4130-4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens, T., Esmon, B., and Schekman, R. (1982) Cell 30 439-448 [DOI] [PubMed] [Google Scholar]
- 38.Ron, D., and Walter, P. (2007) Nat. Rev. Mol. Cell Biol. 8 519-529 [DOI] [PubMed] [Google Scholar]
- 39.Salama, N. R., Chuang, J. S., and Schekman, R. W. (1997) Mol. Biol. Cell 8 205-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.TerBush, D. R., Maurice, T., Roth, D., and Novick, P. (1996) EMBO J. 15 6483-6494 [PMC free article] [PubMed] [Google Scholar]
- 41.Casey, W. M., Rolph, C. E., Tomeo, M. E., and Parks, L. W. (1993) Biochem. Biophys. Res. Commun. 193 1297-1303 [DOI] [PubMed] [Google Scholar]
- 42.Oelkers, P., Cromley, D., Padamsee, M., Billheimer, J. T., and Sturley, S. L. (2002) J. Biol. Chem. 277 8877-8881 [DOI] [PubMed] [Google Scholar]
- 43.Kurat, C. F., Natter, K., Petschnigg, J., Wolinski, H., Scheuringer, K., Scholz, H., Zimmermann, R., Leber, R., Zechner, R., and Kohlwein, S. D. (2006) J. Biol. Chem. 281 491-500 [DOI] [PubMed] [Google Scholar]
- 44.Czabany, T., Athenstaedt, K., and Daum, G. (2007) Biochim. Biophys. Acta 1771 299-309 [DOI] [PubMed] [Google Scholar]
- 45.Szymanski, K. M., Binns, D., Bartz, R., Grishin, N. V., Li, W. P., Agarwal, A. K., Garg, A., Anderson, R. G., and Goodman, J. M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 20890-20895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry, S. A., Atkinson, K. D., Kolat, A. I., and Culbertson, M. R. (1977) J. Bacteriol. 130 472-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson, K. D., Kolat, A. I., and Henry, S. A. (1977) J. Bacteriol. 132 806-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez, R. M., Ishida-Schick, T., Krilowicz, B. L., Leish, B. A., and Atkinson, K. D. (1983) J. Bacteriol. 154 1276-1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitters, E. A., Cleves, A. E., McGee, T. P., Skinner, H. B., and Bankaitis, V. A. (1993) J. Cell Biol. 122 79-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikawa, J., Murakami, A., Esumi, E., and Hosaka, K. (1995) J. Biochem. (Tokyo) 118 39-45 [DOI] [PubMed] [Google Scholar]
- 51.Donahue, T. F., and Henry, S. A. (1981) J. Biol. Chem. 256 7077-7085 [PubMed] [Google Scholar]
- 52.Loewen, C. J., Gaspar, M. L., Jesch, S. A., Delon, C., Ktistakis, N. T., Henry, S. A., and Levine, T. P. (2004) Science 304 1644-1647 [DOI] [PubMed] [Google Scholar]
- 53.Taylor, F. R., and Parks, L. W. (1979) Biochim. Biophys. Acta 575 204-214 [DOI] [PubMed] [Google Scholar]
- 54.Hosaka, K., and Yamashita, S. (1984) Biochim. Biophys. Acta 796 110-117 [PubMed] [Google Scholar]
- 55.Kaiser, C. A., and Schekman, R. A. (1990) Cell 631 723-733 [DOI] [PubMed] [Google Scholar]
- 56.Semenza, J. C., Hardwick, K. G., Dean, N., and Pelham, H. R. (1990) Cell 61 1349-1357 [DOI] [PubMed] [Google Scholar]
- 57.Yamanushi, T., Hirata, A., Oka, T., and Nakano, A. (1996) J. Biochem. (Tokyo) 120 452-458 [DOI] [PubMed] [Google Scholar]
- 58.Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M. F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994) Cell 77 895-907 [DOI] [PubMed] [Google Scholar]
- 59.Matsuoka, K., Orci, L., Amherdt, M., Bednarek, S. Y., Hamamoto, S., Schekman, R., and Yeung, T. (1998) Cell 93 263-275 [DOI] [PubMed] [Google Scholar]
- 60.Siniossoglou, S., Wimmer, C., Rieger, M., Doye, V., Tekotte, H., Weise, C., Emig, S., Segref, A., and Hurt, E. C. (1996) Cell 84 265-275 [DOI] [PubMed] [Google Scholar]
- 61.Siniossoglou, S., Lutzmann, M., Santos-Rosa, H., Leonard, K., Mueller, S., Aebi, U., and Hurt, E. (2000) J. Cell Biol. 149 41-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan, K. J., and Wente, S. R. (2002) BMC Genet. 3 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, S., Skalsky, Y., and Garfinkel, D. J. (1999) Genetics 151 473-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arvindekar, A. U., and Patil, N. B. (2002) Yeast 19 131-139 [DOI] [PubMed] [Google Scholar]
- 65.Sandager, L., Gustavsson, M. H., Stahl, U., Dahlqvist, A., Wiberg, E., Banas, A., Lenman, M., Ronne, H., and Stymne, S. (2002) J. Biol. Chem. 277 6478-6482 [DOI] [PubMed] [Google Scholar]
- 66.Listenberger, L. L., Han, X., Lewis, S. E., Cases, S., Farese, R. V., Jr., Ory, D. S., and Schaffer, J. E. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 3077-3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borradaile, N. M., Han, X., Harp, J. D., Gale, S. E., Ory, D. S., and Schaffer, J. E. (2006) J. Lipid Res. 47 2726-2737 [DOI] [PubMed] [Google Scholar]