Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) is a member of the PPAR family of transcription factors. Synthetic PPARγ agonists are used as oral anti-hyperglycemic drugs for the treatment of non-insulin-dependent diabetes. However, emerging evidence indicates that PPARγ activators can also prevent or attenuate neurodegeneration. Given these previous findings, the focus of this report is on the potential neuroprotective role of PPARγ activation in preventing the loss of mitochondrial function in Huntington disease (HD). For these studies we used striatal cells that express wild-type (STHdhQ7/Q7) or mutant (STHdhQ111/Q111) huntingtin protein at physiological levels. Treatment of mutant cells with thapsigargin resulted in a significant decrease in mitochondrial calcium uptake, an increase in reactive oxygen species production, and a significant decrease in mitochondrial membrane potential. PPARγ activation by rosiglitazone prevented the mitochondrial dysfunction and oxidative stress that occurred when mutant striatal cells were challenged with pathological increases in calcium. The beneficial effects of rosiglitazone were likely mediated by activation of PPARγ, as all protective effects were prevented by the PPARγ antagonist GW9662. Additionally, the PPARγ signaling pathway was significantly impaired in the mutant striatal cells with decreases in PPARγ expression and reduced PPARγ transcriptional activity. Treatment with rosiglitazone increased mitochondrial mass levels, suggesting a role for the PPARγ pathway in mitochondrial function in striatal cells. Altogether, this evidence indicates that PPARγ activation by rosiglitazone attenuates mitochondrial dysfunction in mutant huntingtin-expressing striatal cells, and this could be an important therapeutic avenue to ameliorate the mitochondrial dysfunction that occurs in HD.
Huntington disease (HD)2 is a neurodegenerative disease that is inherited in an autosomal dominant manner, and is caused by the pathological elongation of the CAG repeats in exon one of the huntingtin gene (1). The pathogenesis of HD is manifested by dysfunction and severe loss of striatal neurons in the initial stages, and subsequently involves the cortex and other brain regions in the later stages of the disease (2). Transcriptional deregulation (3) and proteasome dysfunction (4) have been suggested to be significant contributors to the pathogenic processes in HD. Additionally, calcium homeostasis deregulation (5) and mitochondrial dysfunction (5, 6) also have been strongly implicated in the pathogenesis of HD.
Abnormalities in mitochondrial function have been observed in postmortem HD brains (7-9). More recent findings have provided compelling evidence that mitochondrial dysfunction is central to the pathogenesis of HD. Lymphoblasts derived from HD patients exhibit alterations in mitochondrial membrane potential in response to mitochondrial toxins and lower calcium loads in comparison to control lymphoblasts (5, 10). In addition, mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin (6).
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor family of ligand-activated transcription factors (11). To date, three mammalian PPAR subtypes have been isolated and termed PPARα, PPARβ, and PPARγ. PPARα is highly expressed in several tissues and PPARβ is an APC-regulated target of non-steroidal anti-inflammatory drugs (12). PPARγ is a ligand-activated nuclear receptor implicated in several significant human pathologies, including cancer, atherosclerosis, and inflammation (13). PPARγ is the target of the insulin-sensitizing thiazolidinediones (TZDs) drugs, used to treat type II diabetes. Recent studies suggest that treatment of insulin resistance with a PPARγ agonist retards the development of Alzheimer disease (AD) (14, 15), and TZDs extend survival in a transgenic mouse model of amyotrophic lateral sclerosis (16). TZDs have been proposed as potential therapeutic agents for both AD and multiple sclerosis (17), and most of their neuroprotective effects are ascribed to either improved insulin sensitivity, or to their anti-inflammatory action through PPARγ activation in glial cells (18, 19). However, activation of PPARγ by three different TZDs protected rat hippocampal neurons against β-amyloid (Aβ)-induced damage (20), and the TZD rosiglitazone protects human neuroblastoma SH-SY5Y cells against acetaldehyde-induced cytotoxicity (21). In addition, PPARγ activation by rosiglitazone up-regulates the Bcl-2 protective pathway and prevents neuronal degeneration induced by both oxidative stress and treatment with Aβ fibrils, with a concomitant increase in mitochondrial viability (22). Recent studies have also provided evidence that the expression of PGC-1α, a potent co-activator of PPARγ, is repressed by mutant huntingtin expression, and when PGC-1α knock-out (KO) mice are crossed with HD knockin mice, this resulted in increased neurodegeneration of striatal neurons and motor abnormalities in the HD mice (2). At the same time, there is evidence suggesting that PPARγ agonists are neuroprotective and increase mitochondrial function (23, 24). It was also demonstrated that oral treatment with rosiglitazone induced mitochondrial biogenesis in mouse brain (25). Therefore, in this study, we explored the possibility of using PPARγ activation to ameliorate mutant huntingtin-induced mitochondrial dysfunction (5, 6, 26). Our results indicate that there are significant defects in the PPARγ signaling pathway in mutant huntingtin-expressing cells in comparison with cells that express wild-type huntingtin protein. In addition, pretreatment of mutant huntingtin-expressing cells with the PPARγ agonist rosiglitazone prevented the loss of mitochondrial potential, mitochondrial calcium deregulation, and oxidative stress overproduction in response to intracellular calcium overload. These findings suggest that activation of the PPARγ signaling pathway could ameliorate the mitochondrial function deficits that occur in HD.
EXPERIMENTAL PROCEDURES
Reagents—Chemicals, culture media, and serum were obtained from Sigma-Aldrich, Roche Applied Sciences, Alexis Biochemical, and Invitrogen. Fluo-3 AM, Rhod-2 AM, 4-BrA23187, Calsein AM, Mitotracker Green™ (MitoGreen), tetramethyl rhodamine methyl ester (TMRM), Mitotracker Red® CM-H2XRos (MitoRed), 4′,6-diamidino-2-phenylindole (DAPI), and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (2,7-DCF) were obtained from Molecular Probes (Eugene, OR).
Cell Culture—In this study, conditionally immortalized striatal progenitor cell lines STHdhQ7/Q7 (referred to in the text as wild-type cells) expressing endogenous wild-type huntingtin, and STHdhQ111/Q111 (referred to in the text as mutant cells) expressing comparable levels of mutant huntingtin with 111 glutamines were used (27). These cell lines were a generous gift from Dr. M. E. MacDonald and were prepared from wild-type mice and homozygous HdhQ111/Q111 knockin mice as described previously (27). Culturing conditions were the same as described in our previous studies (28).
Intracellular ROS Measurements—Clonal striatal cells were grow on polylysine-coated coverslips (30,000 cells/coverslip) and treated with 1 μm thapsigargin, 20 μm rosiglitazone, and/or 40 μm GW9662, as indicated. After treatment, the cells were incubated with the fluorescent probe 2,7-DCF at 10 μm for 30 min in Krebs-Ringer-Hepes (KRH) buffer supplemented with 5 mm glucose (29). The coverslips were washed two times with PBS and fixed with 4% p-formaldehyde for 5 min. Cells were photographed using a Nikon fluorescence microscope integrated with a Spot digital camera (Diagnostic Instruments). All photographs were taken using the same exposure time and gain to minimize the photobleaching of 2,7-DCF. Images were quantified using Image-Pro Plus 6 software. Results in intensity units were expressed as average of fluorescence signal (F) minus background fluorescence (F0) in every image (29, 30).
Mitochondrial Potential Determination in Live Cells—Mitochondria membrane potential was determined using Mitotracker® Red CM-H2XRos (MitoRed) or TMRM (22, 28, 30). Striatal cells were grown on poly-l-lysine-coated plates and cultured for 4 days. The cells were then loaded for 30 min with MitoRed or TMRM (100 nm) in KRH buffer supplemented with 5 mm glucose and containing 0.02% pluronic acid, then washed, and allowed to equilibrate for 20 min. Cells cultured in 35-mm dishes were then mounted on the stage of a confocal laser scanning microscope (Leica SP2, Germany), and the fluorescence changes were determined using a 40× water immersion objective. MitoRed and TMRM fluorescence were detected exciting with a 563-nm He-Ne laser very heavily attenuated (30% laser power), and the emission was collected at >570 nm for every dye per separate measure. Signal from control cells and cells treated with different stimuli were compared using identical settings for laser power, and detector sensitivity for each separate experiment. The images were analyzed with LCS Leica confocal software and recorded as mean MitoRed or TMRM fluorescence signal per live cell. Estimation of fluorescence intensities were presented as the pseudoratio (ΔF/Fo), which was calculated using the following formula: ΔF/Fo = (F - Fbase)/(Fbase - B), where F is the measured fluorescence intensity of the indicator, Fbase is the fluorescence intensity before the stimulation, and B is the background signal determined from the average of areas adjacent to the cells (22, 28, 30).
Cytosolic and Mitochondrial Calcium Measurements—Cells grown on poly-l-lysine-coated 35-mm dishes were loaded for 30 min (37 °C) with 5 μm Fluo-3 AM, and 10 μm Rhod-2 AM in KRH-glucose containing 0.02% pluronic acid. The fluorescence changes determined by Fluo-3 represent the cytoplasmic calcium changes (30, 31), and Rhod-2 fluorescence indicate calcium changes in the mitochondria (32, 33, 34). To estimate Rhod-2 fluorescence pattern in live mitochondria, we used Mitotracker Green™ dye (MitoGreen) (33). MitoGreen accumulates in the lipophilic environment of live mitochondria, and the signal is independent of the mitochondrial potential (33, 35). Cells were washed three times and left in KRH-glucose for 10 min until cell fluorescence had equilibrated. Fluorescence was imaged with a confocal laser scanning microscope (Leica TCS SP2) using a 40× water immersion lens, as described previously (28). Images were acquired using a 488-nm Argon laser to excite Fluo-3 fluorescence and a 563 nm He-Ne laser to excite Rhod-2 fluorescence. The signals were collected at 505-530 nm (Fluo-3) and at 590 nm (Rhod-2). The fluorescence background signal was subtracted from cell fluorescence measurements in every experiment. The fluorescence intensity variation was recorded from 10-20 cells on average per experiment. Estimation of fluorescence intensities of Fluo-3 and Rhod-2 were presented as a pseudoratio (ΔF/Fo), as described previously (30, 31).
Immunofluorescence Staining—Striatal cells plated on polylysine-coated coverslips (25,000 cells/coverslip) were double immunostained using the rabbit polyclonal anti-PPARγ antibody (Cell Signaling, Boston, MA) (1:500), and a mouse monoclonal anti-actin antibody (Sigma). The secondary antibodies used were 488 Alexa anti-mouse (Molecular Probes), for detection of actin and 593 Alexa anti-rabbit (Molecular Probes) for PPARγ. Coverslips were mounted and analyzed using a Zeiss fluorescence microscope (Carl Zeiss, Thornwood, NY) integrated with an Axiocam CCD camera (Carl Zeiss).
Reverse Transcription and Real-time PCR—Total RNA was extracted from wild-type or mutant striatal cells using TRIzol (Invitrogen) as described in the manufacturer's protocol. Extracted total RNA was treated with RNase free-DNase I, Amplification Grade, (Invitrogen) to remove contaminating DNA, heat-treated to inactivate DNase I, and precipitated with ethanol to clean up the reaction. 2 μg of total RNA was subjected to reverse transcription using SuperScriptIII reverse transcriptase (Invitrogen) following the manufacturer's protocol. The real-time PCR reaction was performed in triplicate in a real-time PCR system (Bio-Rad) using SYBR GreenER qPCR SuperMix (Invitrogen) in a final volume of 25 μl. Amplification conditions consisted of an initial hot start at 95 °C for 10 min followed by amplification of 45 cycles (95 °C for 15 s, 60 °C for 20 s, and 72 °C for 40 s). Melting curve analysis was performed immediately after amplification from 55 to 95 °C. The threshold cycle (CT) of PPARγ was normalized to the CT value of TBP, and the relative amounts of mRNA are shown.
Western Blotting—Cells were washed with ice-cold PBS and lysed in a modified radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Triton X-100, 0.4% SDS, 0.2% sodium deoxycholate, 5% glycerol, 1 mm EDTA, 20 mm NaF, 2 mm Na3VO4) containing protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin). The lysates were sonicated, cleared from cellular debris by centrifugation, and assayed to determine protein concentration using the BCA assay (Pierce). Proteins (10-100 μg) were separated by 10% SDS-PAGE and transferred to the nitrocellulose membrane. The membrane was blocked with 5% skim milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) and incubated with the primary rabbit antibody against PPARγ (1:500 dilution, Cell Signaling) in TBST containing 2% bovine serum albumin at 4 °C overnight. After washing three times, horseradish peroxidase-conjugated secondary antibody against rabbit (1:3000 dilution) in TBST containing 5% skim milk was added, followed by incubation, rinsing, and detection of the immunoreactive bands, by chemiluminescence.
Luciferase Assays—4 × 104 wild-type striatal cells or 8 × 104 mutant striatal cells were plated into each well of a 24-well plate. The following day, a reporter plasmid ((PPRE) X3-TK-Luc) (Addgene, Cambridge, MA)) (36) was transiently cotransfected with the other constructs as indicated (such as mPPARγ1 and/or dominant negative (DN)-mPPARγ1 (22)), using Lipofectamine 2000 (Invitrogen). phRL-TK (Promega, Madison, WI) was cotransfected to normalize the transfection efficiency for all experiments. 12-16 h after transfection, cells were given fresh medium and treated as indicated. 24 h after treatment, cells were washed with cold PBS, lysed with Passive lysis buffer (Promega), and collected. Cell lysates were put through one cycle of freeze-thaw and centrifuged. Luciferase activity was then measured using the Dual-Luciferase Reporter Assay System (Promega).
Statistical Analysis—Results were expressed as mean ± S.E., and were analyzed using Student's t test, an unpaired Student's t test, or one-way ANOVA followed by Student-Newman-Keuls multiple comparisons test as indicated. Differences were considered significant when p < 0.05.
RESULTS
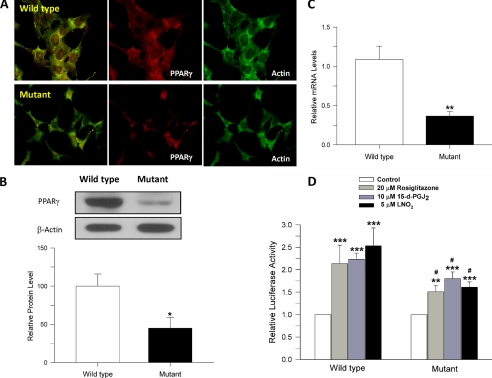
Mutant Huntingtin Expression Significantly Affects the PPARγ Signaling Pathway in Striatal Cells—We previously have shown that PPARγ activation protects against Aβ and oxidative stress damage in hippocampal neurons (20, 22). Additionally, one of the most interesting things that we observed was that the PPARγ-induced protection correlated with improved mitochondrial function (22). PPARγ activation has been proposed to increase mitochondrial biogenesis in vivo (25), and treatment with PPARγ agonists has been proven successful in ameliorating neurodegenerative damage in ischemia and ALS (16, 37). Therefore, our aim was to determine if PPARγ activation could ameliorate the mutant huntingtin-induced mitochondrial dysfunction observed by our group (6, 28) and others (5, 26, 38). Under basal conditions, mutant cells present with low levels of PPARγ expression, in comparison with wild-type cells (Fig. 1). Immunofluorescence studies (Fig. 1A), Western blotting (Fig. 1B), and RNA analysis (Fig. 1C) demonstrated that expression of PPARγ was significantly reduced in mutant cells. The transcriptional activity of PPARγ was also evaluated in the striatal cells using a PPRE luciferase reporter assay. Fig. 1D shows PPRE activity in striatal cells (wild type and mutant) treated with rosiglitazone, a well known PPARγ agonist (20, 22, 37), 15-d-PGJ2 a physiological PPARγ activator (19), or nitrolinoleic acid (LNO2) (a kind gift from Dr. Paul Brookes), which also activates the PPARγ signaling pathway (39). Rosiglitazone, 15-d-PGJ2, and LNO2 increased PPARγ-dependent activity in both cell types, but in mutant cells this increase was significantly lower in comparison with wild-type cells (Fig. 1D). These results suggest that PPARγ pathway is impaired in mutant huntingtin-expressing cells; however, treatment with PPARγ activators can still result in increased transcriptional activity in the mutant cells.
FIGURE 1.
PPARγ signaling pathway is severely compromised in mutant huntingtin-expressing cells. A, untreated striatal cells were double-labeled with an anti-actin antibody (green), and an anti-PPARγ antibody (red), and fluorescence images were captured. Representative images indicate that PPARγ expression is significantly decreased in mutant huntingtin-expressing cells in comparison with wild-type cells. B, top panel, representation Western blots for PPARγ and β-actin as a loading control; bottom panel, quantified data showing that the protein expression of PPARγ is significantly decreased in mutant huntingtin-expressing cells (45.1 ± 14.1, n = 9) compared with wild-type cells. C, PPARγ mRNA levels in mutant cells are significantly decreased compared with wild-type cells (0.367 ± 0.058, n = 6). D, mutant cells show significantly reduced PPARγ transcriptional activity in response to the PPARγ agonists rosiglitazone, 15-d-PGJ2, and LNO2. PPARγ activity was measured using a luciferase assay with a PPRE-Luc reporter (n = 6-8). All three agonists induced greater activation of PPARγ in wild-type cells (RSG, 2.04 ± 0.25; 15-d-PGJ2, 2.162 ± 0.13; LNO2, 2.46 ± 0.34) than mutant cells (RSG, 1.412 ± 0.10; 15-d-PGJ2, 1.70 ± 0.16; LNO2, 1.56 ± 0.11). Data are given as means ± S.E. *, #p < 0.05; **, p < 0.01; ***, p < 0.001; p values with asterisk versus control condition in each cell type. #p is comparison between wild-type and mutant cells treated with the same agonist. Statistical significance was determined by Student's t test (A and B) and one-way ANOVA followed by Student-Newman-Keuls multiple comparisons test (C).
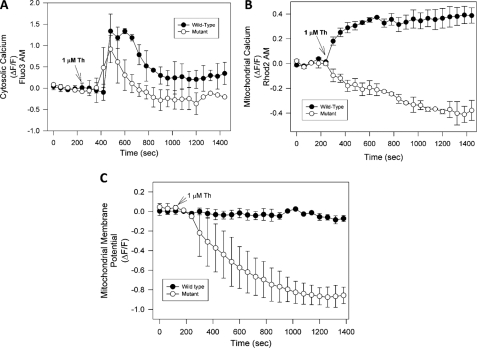
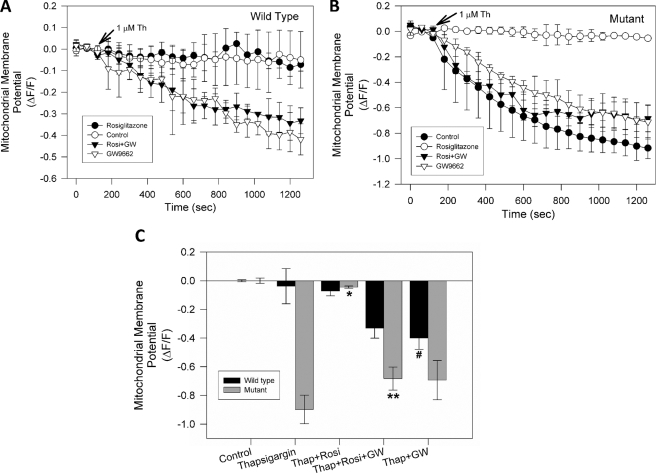
Pathological Calcium Increases Induce a Severe Mitochondrial Potential Loss in Mutant Huntingtin-expressing Cells—It has been suggested that mutant huntingtin expression results in calcium deregulation in different models (40). Therefore, we evaluated mitochondrial calcium changes in striatal cells treated with thapsigargin. Thapsigargin inhibits calcium uptake by endoplasmic reticulum and thus results in pathogenic increases in intracellular calcium levels (33). Fig. 2A shows that cytosolic calcium in wild-type and mutant striatal cells exhibit similar profiles and levels in response to treatment with 1 μm thapsigargin over 30 min. However, thapsigargin treatment in the mutant cells resulted in a significant decrease in mitochondrial calcium uptake, in contrast with wild-type cells that show an increase in mitochondrial calcium levels (Fig. 2B). Recently, Lim et al. (38) reported that mitochondria from mutant striatal cells are unable to manage large calcium loads, and this effect appears to be due to increased sensitivity to calcium-induced permeability transition pore (PTP) opening. Therefore, we next measured mitochondrial membrane potential changes in striatal cells exposed to thapsigargin (Fig. 2C). As in our previous studies, mitochondrial potential changes were evaluated using MitoRed and TMRM dyes (28). These two dyes show the same mitochondrial potential changes in striatal cells depolarized with 10 μm FCCP and in response to calcium overload in the presence of 1 nm 4-BrA23187 (28). Fig. 2C shows a representative graph from three independent experiments from striatal cells loaded with TMRM and exposed to 1 μm thapsigargin. Treatment of mutant cells with thapsigargin results in a loss of mitochondrial membrane potential, in comparison with wild-type cells (Fig. 2C). Identical results were obtained with MitoRed (data not shown). These results suggest that uncontrolled calcium increases induce mitochondrial dysfunction in mutant huntingtin-expressing cells.
FIGURE 2.
Pathological increases in intracellular calcium levels induce mitochondrial dysfunction in mutant huntingtin-expressing cells. A, striatal cells were loaded with Fluo3-AM, and cytosolic calcium levels were determined in both cell types exposed to 1 μm thapsigargin for 30 min. Fluorescence changes were recorded at 1-min intervals and presented as the ratio ΔF/F. Thapsigargin induced a robust and similar cytosolic calcium increase in both cell types. B, cells were loaded with Rhod2 AM to determine mitochondrial calcium levels in wild-type and mutant cells exposed to 1 μm thapsigargin for 30 min. Thapsigargin treatment increased mitochondrial calcium levels in wild-type cells, but in mutant cells, thapsigargin treatment resulted in a decrease in mitochondrial calcium uptake. C, time course of mitochondrial membrane potential in cells exposed to 1 μm thapsigargin for 30 min (black circles: wild-type cells; white circles: mutant cells). The graph represents quantification of mitochondrial potential fluorescence intensities as relative units, which shows that treatment of mutant cells treated with thapsigargin results in a loss of mitochondrial potential. Data are mean ± S.E. (bars) from three separate experiments.
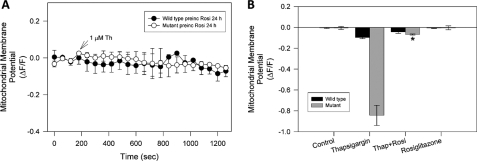
PPARγ Activation Prevents Mitochondrial Dysfunction Induced by Pathological Calcium Increases in Mutant Striatal Cells—To determine if PPARγ activation prevents mitochondrial dysfunction induced by thapsigargin in mutant cells, the cells were pretreated for 24 h with 20 μm rosiglitazone, before being exposed to 1 μm thapsigargin for a 30-min period. Wild-type and mutant cells were loaded with MitoRed, to determine mitochondrial potential changes using confocal microscopy. Treatment with 1 μm thapsigargin did not result in any significant changes in the mitochondrial membrane potential in wild-type cells (Figs. 2C and 3B) and treatment with rosiglitazone was without effect (Fig. 3A). Thapsigargin treatment of mutant cells resulted in a significant loss of mitochondrial membrane potential (Fig. 2C), which was inhibited by pretreatment with rosiglitazone (Fig. 3A). Additionally, short time incubations with rosiglitazone (2, 4, and 8 h), did not protect mutant cells against thapsigargin-induced mitochondrial potential loss (data not shown). Rosiglitazone pretreatment significantly attenuated the mitochondrial potential loss that occurred in response to thapsigargin treatment in mutant cells (Fig. 3B). These results indicate that PPARγ activation protects against the thapsigargin-induced mitochondrial potential loss in mutant huntingtin-expressing cells, indicating a potential role of this pathway in preventing the mitochondrial dysfunction that occurs in HD.
FIGURE 3.
PPARγ activation prevents mitochondrial dysfunction induced by cytosolic calcium deregulation in striatal cells. Striatal cells were treated with 1 μm thapsigargin after a 5-min control period at the beginning of each experiment. A, wild-type and mutant cells were preincubated with 20 μm rosiglitazone for 24 h, and subsequently treated with 1 μm thapsigargin and mitochondrial potential changes were measured. These experiments show that rosiglitazone treatment inhibited mitochondrial potential loss induced by thapsigargin in mutant cells (see Fig. 2). B, representative graph of mitochondrial potential levels after 30 min of exposure to 1 μm thapsigargin from three independent experiments. Data are mean ± S.E. (bars), and values are from three separate experiments. *, p < 0.01 compared with mutant thapsigargin-treated cells. p < 0.01 by unpaired Student t test.
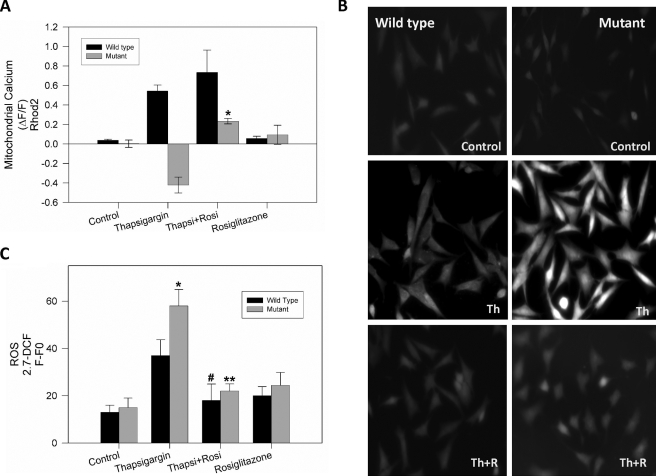
PPARγ Activation Restores Mitochondrial Calcium Transport and Intracellular ROS Levels Altered by Thapsigargin in Mutant Striatal Cells—To determine if activation of PPARγ could protect against the thapsigargin-induced mitochondrial calcium deregulation, wild-type and mutant striatal cells were preincubated with 20 μm rosiglitazone, for 24 h prior to treatment with 1 μm thapsigargin and mitochondrial calcium uptake measurements. Fig. 4A shows quantification of three independent experiments for mitochondrial calcium levels determination in striatal cells exposed to thapsigargin for 30 min. These data clearly demonstrate that pretreatment with rosiglitazone reestablishes mitochondrial calcium uptake in response to thapsigargin, which is impaired in mutant cells (Fig. 2B). This evidence indicates that activation of the PPARγ pathway results in significant improvement of mitochondrial function, with an attenuation of mitochondrial membrane potential loss and a recovery mitochondrial calcium uptake function in mutant cells exposed to calcium stress. In addition, to determine if increased PPARγ activation can attenuate thapsigargin-induced ROS production, striatal cells were incubated in the absence or presence of 20 μm rosiglitazone for 24 h prior to treatment with 1 μm thapsigargin and measurement ROS levels. Intracellular ROS production was measured using the fluorescent probe 2,7-DCF (29). Fig. 4B shows representative images of intracellular ROS levels in striatal cells exposed to the indicated conditions. Fluorescence images taken from cells were analyzed with Image Pro 6 software, and results were expressed as F-F0, were F0 indicates the background value in each image, and where F corresponds to cellular fluorescence levels. Thapsigargin induced an increase in ROS production in both cell types (Fig. 4, B and C). However, thapsigargin treatment resulted in a significantly greater increase in ROS levels in mutant cells in comparison with wild-type cells (Fig. 4, B and C). PPARγ activation by rosiglitazone significantly attenuated intracellular ROS production induced by thapsigargin treatment in both cell types (Fig. 4C). These results suggest that PPARγ activation by rosiglitazone reduces ROS production in mutant huntingtin-expressing cells exposed to a pathological calcium overload.
FIGURE 4.
Rosiglitazone treatment recovers mitochondrial calcium uptake capacity and reduces oxidative stress production in mutant cells exposed to calcium overload. A, striatal cells were loaded with Rhod2 AM for 40 min to measure changes in mitochondrial calcium using confocal microscopy in situ. Mitochondrial calcium levels after 30 min of thapsigargin treatment from three separate experiments are shown. *, p < 0.01 mutant cells treated with thapsigargin plus rosiglitazone compared with mutant cells treated with thapsigargin. B, wild-type and mutant cells were treated with 20 μm rosiglitazone (R) or vehicle for 24 h prior to incubation with 1 μm thapsigargin (Th) for 30 min and ROS production measurements using 2.7-DCF. PPARγ activation by rosiglitazone decreased intracellular ROS production trigger by thapsigargin in both cell types. C, quantified ROS data from four independent experiments. Data are mean ± S.E. (bars), and values are from four separate experiments. *, p < 0.05 mutant cells treated with thapsigargin compared with wild-type cells treated with thapsigargin; **, p < 0.01 mutant cells treated with thapsigargin plus rosiglitazone compared with thapsigargin treatment, and #, p < 0.05 wild-type cells treated with thapsigargin plus rosiglitazone compared with wild-type cells treated only with thapsigargin.
GW9662 Prevents Mitochondrial Function Improvement Induced by PPARγ Activation in Mutant Huntingtin-expressing Cells—Chronic treatment with rosiglitazone clearly improves mitochondrial function in mutant huntingtin-expressing cells challenged with intracellular calcium overload (see Figs. 3 and 4). Rosiglitazone acts predominantly through the PPARγ pathway (22); however, non-PPARγ related effects in response to rosiglitazone treatment have been reported (19, 22, 41). Therefore, to determine if rosiglitazone is eliciting its protective effects on mitochondrial function in this model through PPARγ activation, we measured mitochondrial potential in striatal cells preincubated for 24 h with rosiglitazone, in the presence or absence of GW9662 (Fig. 5). GW9662 is a PPARγ antagonist that blocks the neuroprotective effect of rosiglitazone in hippocampal neurons exposed to Aβ and oxidative stress (20, 22). Fig. 5, A and B shows mitochondrial potential determinations from three independent experiments in striatal cells loaded with MitoRed and exposed to 1 μm thapsigargin. As previously observed, wild-type cells did not show alterations in mitochondrial potential in response to thapsigargin treatment, in control and rosiglitazone-pretreated conditions (Fig. 5A). However, in wild-type cells pretreated for 24 h with rosiglitazone, in the presence of GW9662, thapsigargin induced a partial mitochondrial potential loss in comparison with untreated wild-type cells (Fig. 5A). Interestingly, short term treatments with GW9662 alone were without effect on mitochondrial potential levels in wild-type and mutant cells (data not shown). Additionally, mutant cells preincubated for 24 h with rosiglitazone in the presence of GW9662, showed a pronounced mitochondrial potential loss in response to thapsigargin treatment (Fig. 5B). This indicates that GW9662 blocked the mitochondrial protective effect produced by rosiglitazone treatment in mutant cells exposed to thapsigargin, and suggests that rosiglitazone is acting mainly through activation of the PPARγ pathway (Fig. 5C).
FIGURE 5.
GW9662 prevents mitochondrial functional improvement induced by rosiglitazone in mutant huntingtin-expressing cells. A, wild-type cells were treated with 1 μm thapsigargin added after a 5-min control period at the beginning of each experiment. Thapsigargin treatment of wild-type cells did not result in any significant mitochondrial membrane potential changes, in comparison with wild-type cells preincubated with rosiglitazone. Wild-type cells preincubated with 40 μm GW9662, in the absence or presence of rosiglitazone, showed significant mitochondrial potential loss when they were treated with 1 μm thapsigargin for 30 min. B, mutant cells treated with thapsigargin exhibited mitochondrial membrane potential loss. Mutant cells were preincubated with 20 μm rosiglitazone for 24 h, and then were treated with 1 μm thapsigargin, and mitochondrial potential changes were measured. Rosiglitazone treatment prevented the mitochondrial potential loss induced by thapsigargin in mutant cells In contrast, mutant cells preincubated with 40 μm GW9662, in the absence or presence of rosiglitazone, showed significant mitochondrial membrane potential loss when they were treated with 1 μm thapsigargin for 30 min. C, quantification of mitochondrial potential levels obtained from striatal cells exposed to thapsigargin for 30 min. Data are mean ± S.E. (bars), and values are from three separate experiments. *, p < 0.01 compared with mutant thapsigargin-treated cells; **, p < 0.05 compared with mutant thapsigargin + rosiglitazone-treated cells, and #, p < 0.05 wild-type cells treated with thapsigargin plus GW9662 compared with wild-type cells treated only with thapsigargin. *, p < 0.01 and **, p < 0.05 by unpaired Student's t test.
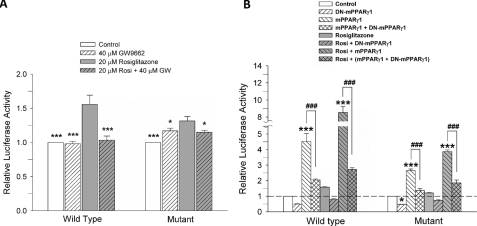
PPARγ Transcriptional Activity Is Compromised in Mutant Huntingtin-expressing Cells—Treatment with rosiglitazone attenuated the mitochondrial dysfunction that occurs in mutant cells in response to thapsigargin treatment, and the PPARγ antagonist GW9962 prevented this protective effect. These findings indicate that rosiglitazone is acting through the PPARγ pathway. Therefore, we next evaluated the transcriptional activity of the PPARγ receptor in striatal cells exposed to rosiglitazone and GW9962 using a luciferase activity assay. These studies showed that 24 h of rosiglitazone treatment increases PPARγ transcriptional activity in wild-type and mutant cells (Fig. 6A). However, in mutant cells the PPARγ transcriptional activity increase was significantly less compared with wild-type cells, suggesting that the PPARγ transcriptional response is altered in the mutant cells. Co-treatment with rosiglitazone and GW9662 prevented the increases in PPARγ transcriptional activity (Fig. 6A). The effect of PPARγ expression on the PPRE-dependent transcriptional activity in wild-type and mutant cells was also examined. Mouse PPARγ (mPPARγ1) or its dominant negative form (DN-mPPARγ1) (22) were transiently expressed in the cells. PPARγ transcriptional activity using PPARγ agonist rosiglitazone was then measured with the PPRE luciferase reporter (Fig. 6B). Rosiglitazone treatment induced PPARγ transcriptional activity in both cell types, although this effect was significantly attenuated in the mutant cells (Fig. 6B). Overexpression of PPARγ (mPPARγ1) resulted in an increase in PPARγ transcriptional activity, which was 3-fold (mutant cells) and 5-fold higher (wild-type cells) than the response observed in cells expressing the vector alone (control cells). Treatment with 20 μm rosiglitazone significantly increased PPARγ transcriptional activity stimulated by transient transfection of mPPARγ1 in both cell types (Fig. 6B). Striatal cells expressing the dominant negative form of PPARγ (DN-mPPARγ1 cells) showed almost no PPRE activity in wild-type and mutant cells, and prevented PPARγ activity increased by mPPARγ1 transfection in both cell types.
FIGURE 6.
Rosiglitazone treatment induced specific PPARγ activation in striatal cells. A, effect of GW9662, an antagonist of PPARγ, on PPRE luciferase activity. Rosiglitazone treatment increased PPRE luciferase activity in wild-type cells to 1.56 ± 0.13, and in mutant cells to 1.31 ± 0.06, respectively. GW9662 blocked the induction of PPRE luciferase activity in response to rosiglitazone in wild-type cells (1.03 ± 0.06) and in mutant cells (1.15 ± 0.03) (n = 5). The p values were determined by the comparison to the rosiglitazone-treated condition in each cell type. B, PPRE-luciferase reporter was cotransfected with mPPARγ1, DN-PPARγ1, or both. Exogenous mPPARγ1 expression increased the activity of PPRE luciferase in wild-type cells (4.52 ± 0.51) to a greater extent than in mutant cells (2.64 ± 0.11). Rosiglitazone treatment further increased the activity of PPRE luciferase in both cell types (wild-type cells: 8.53 ± 0.04, mutant cells: 3.87 ± 0.10). DN-PPARγ1 significantly attenuated the activity of PPRE luciferase both in the presence or the absence of rosiglitazone in both cell types without or with cotransfection of PPARγ1 (n = 4). Data are given as means ± S.E. *, #p < 0.05; **, p < 0.01; ***, ###p < 0.001; p values with asterisk versus control condition in each cell type. Statistical significance was determined by one-way ANOVA followed by Student-Newman-Keuls multiple comparisons test.
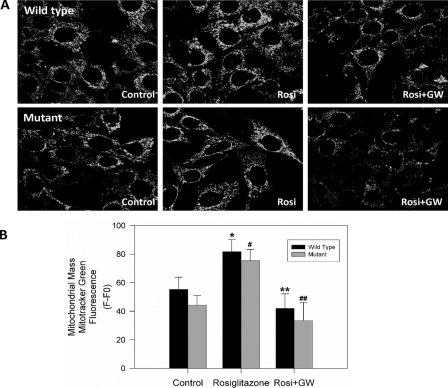
Rosiglitazone Treatment Results in an Increase in Mitochondrial Mass Levels in Striatal Cells—It has been reported in mice that rosiglitazone treatment increases mitochondrial biogenesis in the brain and others tissues (25, 42, 43). Therefore, we carried our preliminary studies to determine if PPARγ activation in striatal cells may increase mitochondrial biogenesis (Fig. 7). For these studies, striatal cells were incubated with 20 μm rosiglitazone for 24 h, in the absence or presence of 40 μm GW9662 prior to being loaded with MitoGreen, which is used for mitochondrial mass determinations (42, 44, 45). Confocal images were taken from striatal cells treated with the indicated conditions (Fig. 7A). The fluorescence intensity changes were recorded from 7-13 cells on average per each image, and analyzed using Image Pro 6 software. Rosiglitazone treatment induced a significant increase in mitochondrial mass levels in wild-type and mutant cells, as compared with controls (Fig. 7, A and B, see bars). Additionally, GW9662 blocked the mitochondrial mass increase induced by rosiglitazone, indicating a specific PPARγ-dependent effect. These findings indicate that PPARγ activation can increase mitochondrial biogenesis in striatal cells and this could contribute in part to the protective effect of rosiglitazone against thapsigargin-induced mitochondrial dysfunction in mutant huntingtin-expressing cells.
FIGURE 7.
PPARγ activation increases mitochondrial mass in clonal striatal cells. To determine mitochondrial mass, striatal cells were loaded with MitoGreen. A, cells loaded with MitoGreen were photographed with a confocal microscope adjusted for the same exposure time, thickness, and gain. The fluorescence intensity variation value was recorded from 7-13 cells on average per each image. B, rosiglitazone treatment (24 h) induced an increase in mitochondrial mass in wild-type and mutant cells when compared with controls (see bars). Co-incubation with GW9662 (40 μm) blocked the mitochondrial mass increase induced by rosiglitazone in both cell types. The graph shows quantified data from four separate experiments. *, p < 0.01 compared with mutant-untreated cells; #, p < 0.05 compared with wild-type-untreated cells; **, p < 0.05 compared with mutant rosiglitazone-treated cells; and ##, p < 0.05 wild-type cells treated with rosiglitazone plus GW9662 compared with wild-type cells treated with rosiglitazone. *, p < 0.01 and **, p < 0.05 by unpaired Student's t test.
DISCUSSION
In this report, we present evidence that treatment with rosiglitazone, a well known PPARγ agonist, attenuates mitochondrial dysfunction in mutant huntingtin-expressing cells challenged with a calcium overload. Specifically, treatment with rosiglitazone decreased the mitochondrial potential loss, oxidative stress, and mitochondrial calcium deregulation in the thapsigargin-treated mutant cells. These beneficial effects were blocked by GW9662, an antagonist of PPARγ activation. PPARγ expression was significantly lower in mutant huntingtin-expressing cells with a concomitant decrease in PPARγ transcriptional activity, confirming that PPARγ pathway is compromised in mutant cells. PPARγ activators, especially TZDs, have been shown to have neuroprotective effects in several different models (19). It has been observed that PPARγ activation protects from ischemia-induced damage in mouse models (46). Additionally, PPARγ activation ameliorated Aβ and oxidative stress toxicity in hippocampal neurons (20, 22). This protection was mediated in part by rosiglitazone-induced increases in Bcl-2 expression (22). This is intriguing because it has been suggested that the anti-apoptotic function of Bcl-2 is related to its ability to attenuate mitochondrial dysfunction induced by different stressors (47). In the model system used in this study, PPARγ activation ameliorated the thapsigargin-induced mitochondrial dysfunction in mutant cells. Given that mitochondrial dysfunction is likely to play a central role in the pathogenesis of HD (5, 28, 38), these findings indicate that the PPARγ pathway may be a rational therapeutic target in the treatment of this disease. Recently, Lim et al. (38) demonstrated that mutant huntingtin expression (using clonal striatal cells) induces mitochondrial dysfunction through disruption of mitochondrial calcium homeostasis. These aberrant changes in the handling of calcium by the mitochondria in mutant huntingtin-expressing cells occurred by a mechanism that involved PTP opening (38). In this present study, we present evidence that pathological increases in intracellular calcium levels significantly compromise mitochondria calcium regulation, mitochondrial membrane potential, and ROS production in mutant striatal cells. The observations presented in this report are in complete agreement with our previous studies that suggest that mutant huntingtin expression induced mitochondrial calcium handling defects that subsequently result in respiratory deficits in isolated mitochondria preparations (6, 28). In fact, analysis of mitochondria isolated from mutant huntingtin-expressing cells, and mitochondria in intact mutant cells revealed similar results with similar sensitivity to pathological calcium concentrations (28). Taken together, these data show that mutant huntingtin expression in striatal cells results in mitochondrial dysfunction, which likely contributes to the pathogenesis in HD.
In our studies, we observed that treatment with rosiglitazone induced an increase in mitochondrial mass levels in striatal cells (Fig. 7). This effect of rosiglitazone was prevented by co-incubation for 24 h with GW9662, indicating that rosiglitazone-induced increase in mitochondrial mass is due to activation of the PPARγ receptor. Also, the protective effects of rosiglitazone against thapsigargin-induced mitochondrial dysfunction were only apparent after 24 or 48 h of treatment. Short time incubations did not result in any beneficial response of rosiglitazone on the mitochondrial function (data not shown), evidence that supports a PPARγ-dependent effect. PPARγ activation induces mitochondrial biogenesis in different cell types (42, 43), and mitochondrial biogenesis has been reported in brain cells from mice treated with rosiglitazone (25). Further, in this study we clearly demonstrate that rosiglitazone not only increases mitochondrial mass, but improves mitochondrial function. Thus PPARγ likely plays a role in regulating mitochondrial function, and activation of this pathway could result in protection against different stressors. It is tantalizing to speculate that the PPARγ-induced increase in mitochondrial biogenesis in combination with the up-regulation of key mitochondrial and anti-apoptotic proteins (e.g. Bcl-2), increases the defense mechanisms against oxidative stress, and mitochondrial damage. In fact, treatment with rosiglitazone increased neuronal glucose uptake, and restored brain ATP levels in stressed rats (48). Additionally, in our studies we observed that PPARγ activity is important for the normal function of the mitochondria. These observations are supported by our findings that treatment of wild-type cells with GW9662 for 24 h resulted in a partial mitochondrial potential loss in response to thapsigargin treatment (see Fig. 5A). These observations are in agreement with previous findings, which showed that expression of DN-mPPARγ1 in PC12 cells compromised normal mitochondrial potential levels and increased intracellular ROS production in untreated and H2O2-treated cells (22).
Agonists of PPARγ have been shown to ameliorate AD-related pathology in animal models and improve cognition (49, 50). Preliminary evidence indicates that PPARγ agonists (rosiglitazone) may improve cognition and memory in AD patients (15). The evidence presented in this report indicates that activation of PPARγ receptors can ameliorate mitochondrial dysfunction in mutant huntingtin-expressing cells, which plays an important role in the pathogenesis of HD (5, 26). Thus, PPARγ agonists could represent a potential tool for the treatment of neurodegenerative disorders, including HD.
This work was supported, in whole or in part, by National Institutes of Health Grant NS041744. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HD, Huntington disease; PPAR, peroxisome proliferator-activated receptor; TZDs, thiazolidinediones; KRH, Krebs-Ringer-Hepes; TMRM, tetramethyl rhodamine methyl ester; Aβ, β-amyloid; ROS, reactive oxygen species; PTP, permeability transition pore; ANOVA, analysis of variance; 2,7-DCF, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester; PBS, phosphate-buffered saline; DN, dominant negative.
References
- 1.The Huntington Disease Col Res Group (1993) Cell 72 971-983 [DOI] [PubMed] [Google Scholar]
- 2.Brandt, J., Bylsma, F. W., Gross, R., Stine, O. C., Ranen, N., and Ross, C. A. (1996) Neurology 46 527-531 [DOI] [PubMed] [Google Scholar]
- 3.Cui, L., Jeong, H., Borovecki, F., Parkhurst, C. N., Tanese, N., and Krainc, D. (2006) Cell 127 59-69 [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Hernandez, M., Hernandez, F., Martin-Aparicio, E., Gomez-Ramos, P., Moran, M. A., Castano, J. G., Ferrer, I., Avila, J., and Lucas, J. J. (2003) J. Neurosci. 23 11653-11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panov, A. V., Gutekunst, C. A., Leavitt, B. R., Hayden, M. R., Burke, J. R., Strittmatter, W. J., and Greenamyre, J. T. (2002) Nat. Neurosci. 5 731-736 [DOI] [PubMed] [Google Scholar]
- 6.Milakovic, T., and Johnson, G. V. (2005) J. Biol. Chem. 280 30773-30782 [DOI] [PubMed] [Google Scholar]
- 7.Stahl, W. L., and Swanson, P. D. (1974) Neurology 24 813-819 [DOI] [PubMed] [Google Scholar]
- 8.Mann, V. M., Cooper, J. M., Javoy-Agid, F., Agid, Y., Jenner, P., and Schapira, A. H. (1990) Lancet 336 749. [DOI] [PubMed] [Google Scholar]
- 9.Browne, S. E., Bowling, A. C., MacGarvey, U., Baik, M. J., Berger, S. C., Muqit, M. M., Bird, E. D., and Beal, M. F. (1997) Ann. Neurol. 41 646-653 [DOI] [PubMed] [Google Scholar]
- 10.Sawa, A., Wiegand, G. W., Cooper, J., Margolis, R. L., Sharp, A. H., Lawler, J. F., Jr., Greenamyre, J. T., Snyder, S. H., and Ross, C. A. (1999) Nat. Med. 5 1194-1198 [DOI] [PubMed] [Google Scholar]
- 11.Rosen, E. D., and Spiegelman, B. M. (2001) J. Biol. Chem. 276 37731-37734 [DOI] [PubMed] [Google Scholar]
- 12.He, T. C., Chan, T. A., Vogelstein, B., and Kinzler, K. W. (1999) Cell 99 335-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger, J., and Moller, D. E. (2002) Annu. Rev. Med. 53 409-435 [DOI] [PubMed] [Google Scholar]
- 14.Watson, G. S., and Craft, S. (2003) CNS Drugs 17 27-45 [DOI] [PubMed] [Google Scholar]
- 15.Watson, G. S., Cholerton, B. A., Reger, M. A., Baker, L. D., Plymate, S. R., Asthana, S., Fishel, M. A., Kulstad, J. J., Green, P. S., Cook, D. G., Kahn, S. E., Keeling, M. L., and Craft, S. (2005) Am. J. Geriatr. Psychiatry 13 950-958 [DOI] [PubMed] [Google Scholar]
- 16.Kiaei, M., Kipiani, K., Chen, J., Calingasan, N. Y., and Beal, M. F. (2005) Exp. Neurol. 191 331-336 [DOI] [PubMed] [Google Scholar]
- 17.Watson, G. S., and Craft, S. (2006) J. Neurol. Sci. 245 21-33 [DOI] [PubMed] [Google Scholar]
- 18.Bernardo, A., and Minghetti, L. (2006) Curr. Pharm. Des. 12 93-109 [DOI] [PubMed] [Google Scholar]
- 19.Heneka, M. T., and Landreth, G. E. (2007) Biochem. Biophys. Acta 1771 1031-1045 [DOI] [PubMed] [Google Scholar]
- 20.Inestrosa, N. C., Godoy, J. A., Quintanilla, R. A., Koenig, C. S., and Bronfman, M. (2005) Exp. Cell Res. 304 91-104 [DOI] [PubMed] [Google Scholar]
- 21.Jung, T. W., Lee, J. Y., Shim, W. S., Kang, E. S., Kim, S. K., Ahn, C. W., Lee, H. C., and Cha, B. S. (2006) Biochem. Biophys. Res. Commun. 340 221-227 [DOI] [PubMed] [Google Scholar]
- 22.Fuenzalida, K., Quintanilla, R. A., Ramos, P., Piderit, D., Fuentealba, R. A., Martinez, G., Inestrosa, N. C., and Bronfman, M. (2007) J. Biol. Chem. 282 37006-37015 [DOI] [PubMed] [Google Scholar]
- 23.Schutz, B., Reimann, J., Dumitrescu-Ozimek, L., Kappes-Horn, K., Landreth, G. E., Schurmann, B., Zimmer, A., and Heneka, M. T. (2005) J. Neurosci. 25 7805-7812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter, R. L., Dragicevic, N., Seifert, K., Choi, D. Y., Liu, M., Kim, H. C., Cass, W. A., Sullivan, P. G., and Bing, G. (2007) J. Neurochem. 100 1375-1386 [DOI] [PubMed] [Google Scholar]
- 25.Strum, J. C., Shehee, R., Virley, D., Richardson, J., Mattie, M., Selley, P., Ghosh, S., Nock, C., Saunders, A., and Roses, A. (2007) J. Alzheimers Dis. 11 45-51 [DOI] [PubMed] [Google Scholar]
- 26.Oliveira, J. M., Chen, S., Almeida, S., Riley, R., Goncalves, J., Oliveira, C. R., Hayden, M. R., Nicholls, D. G., Ellerby, L. M., and Rego, A. C. (2006) J. Neurosci. 26 11174-11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trettel, F., Rigamonti, D., Hilditch-Maguire, P., Wheeler, V. C., Sharp, A. H., Persichetti, F., Cattaneo, E., and MacDonald, M. E. (2000) Hum. Mol. Genet. 9 2799-2809 [DOI] [PubMed] [Google Scholar]
- 28.Milakovic, T., Quintanilla, R. A., and Johnson, G. V. (2006) J. Biol. Chem. 281 34785-34795 [DOI] [PubMed] [Google Scholar]
- 29.Ruan, Q., Quintanilla, R. A., and Johnson, G. V. (2007) J. Neurochem. 102 25-36 [DOI] [PubMed] [Google Scholar]
- 30.Santos, M. J., Quintanilla, R. A., Toro, A., Grandy, R., Dinamarca, M. C., Godoy, J. A., and Inestrosa, N. C. (2005) J. Biol. Chem. 280 41057-41068 [DOI] [PubMed] [Google Scholar]
- 31.Quintanilla, R. A., Munoz, F. J., Metcalfe, M. J., Hitschfeld, M., Olivares, G., Godoy, J. A., and Inestrosa, N. C. (2005) J. Biol. Chem. 280 11615-11625 [DOI] [PubMed] [Google Scholar]
- 32.Zhu, L. P., Yu, X. D., Ling, S., Brown, R. A., and Kuo, T. H. (2000) Cell Calcium 28 107-117 [DOI] [PubMed] [Google Scholar]
- 33.Collins, T. J., Lipp, P., Berridge, M. J., and Bootman, M. D. (2001) J. Biol. Chem. 276 26411-26420 [DOI] [PubMed] [Google Scholar]
- 34.Darios, F., Muriel, M. P., Khondiker, M. E., Brice, A., and Ruberg, A. (2005) J. Neurosci. 25 4159-4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mironov, S. L., Ivannikov, M. V., and Johansson, M. (2005) J. Biol. Chem. 280 715-721 [DOI] [PubMed] [Google Scholar]
- 36.Kim, J. B., Wright, H. M., Wright, M., and Spiegelman, B. M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 4333-4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collino, M., Aragno, M., Mastrocola, R., Gallicchio, M., Rosa, A. C., Dianzani, C., Danni, O., Thiemermann, C., and Fantozi, R. (2006) Eur. J. Pharmacol. 530 70-76 [DOI] [PubMed] [Google Scholar]
- 38.Lim, D., Fedrizzi, L., Tartari, M., Zuccato, C., Cattaneo, E., Brini, M., and Carafoli, E. (2008) J. Biol. Chem. 283 5780-5789 [DOI] [PubMed] [Google Scholar]
- 39.Schopfer, F. J., Lin, Y., Baker, P. R., Cui, T., Garcia-Barrio, M., Zhang, J., Chen, K., Chen, Y. E., and Freeman, B. A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2340-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan, M. M., and Raymond, L. A. (2007) Prog. Neurobiol. 81 272-293 [DOI] [PubMed] [Google Scholar]
- 41.Xing, B., Xin, T., Hunter, R. L., and Bing, G. (2008) J. Neuroinflammation 4 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson-Fritch, L., Burkart, A., Bell, G., Mendelson, K., Leszyk, J., Nicolodro, S., Czech, M., and Corvera, S. (2003) Mol. Cell Biol. 23 1085-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson-Fritch, L., Nicoloro, S., Chouinard, M., Lazar, M. A., Chui, P. C., Leszyk, J., Straubhaar, J., Czech, M., and Corvera, S. (2004) J. Clin. Investig. 114 1281-1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendergrass, W., Wolf, N., and Poot, M. (2004) Cytometry. A. 61 162-169 [DOI] [PubMed] [Google Scholar]
- 45.Kim, G. J., Fiskum, G. M., and Morgan, W. F. (2006) Cancer Res. 66 10377-10383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, K. J., Jang, Y. H., Lee, H., Yoo, H. S., and Lee, S. R. (2008) Eur. J. Neurosci. 27 334-342 [DOI] [PubMed] [Google Scholar]
- 47.Soane, L., and Fiskum, G. (2005) J. Bioenerg. Biomembr. 37 179-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Bueno, B., Caso, J. R., Perez-Nievas, B. G., Lorenzo, P., and Leza, J. C. (2007) Neuropsychopharmacology 32 1251-1260 [DOI] [PubMed] [Google Scholar]
- 49.Landreth, G. (2006) Exp. Neurol. 199 245-248 [DOI] [PubMed] [Google Scholar]
- 50.Pedersen, W. A., McMillan, P. J., Kulstad, J. J., Leverenz, J. B., Craft, S., and Haynatzki, G. R. (2006) Exp. Neurol. 199 265-273 [DOI] [PubMed] [Google Scholar]