Introduction
“omnis cellula e cellula”, in 1858, an important dogma in cell biology was born, when Rudolf Virchow established that every cell must derives from a pre-existing cell. And indeed cell division is the only way for life to expend, it is also the way for immortalization, and unfortunately when uncontrolled also the way for cancer. But unrevealing mechanisms leading to cell division took quite a while. How does a mother cell divide to give two daughters? This is known as the cell cycle, which describes a series of events that insures faithfully transition of the genetic information from one cell generation to the next. These dividing mechanisms have been conserved throughout evolution; they underlie growth and development in all living organisms and are central to their heredity and evolution.
In eukaryotic cells, the cell cycle was first described as two distinct phases: interphase and mitosis that just precedes cell division. The interphase was later on divided into three phases, S-phase standing for DNA synthesis surrounded by two G-phases G1 and G2 standing for Gap-phases. Fully described by Walter Flemming in 1882, mitosis remains the most spectacular and sophisticated part of the cell cycle. In less than an hour the mother cell organizes a complex machine aim to separate its genetic information and all its subcellular components into two identical sets that will be inherited by the two daughter cells. If mitosis proceeds without any error it eventually ends up with cytokinesis corresponding to the physical separation of the two daughter cells. Theodor Boveri predicted errors during mitosis to be at the origin of cancer in 1902. Hundred years later the scientific community is still debating on whether or not this might be true. The coordination of progression through mitosis is mainly orchestrated by protein phosphorylation insured by several serine/threonine kinases. In this short review we will focus on the four main mitotic kinase families: the cyclin-dependent kinase: Cdks, the polo-like kinases: Plks, the Aurora kinases and the NIMA-related kinases: Nerks.
“Cyclin Dependant Kinases” Cdks that must associate to a cyclin to become active kinases are key regulators of cell cycle progression. There are now about twelve Cdks; the fisrt one Cdk1 (or cdc2) has long been considered as THE cell cycle master kinase, thought to be responsible for all cell cycle transitions (1). This is true in yeast where Cdk1 kinase activity is required for the G1/S and the G2/M transition (2). In mammalian cell however, Cdk1 activity is only required for the G2/M transition (3). Cdk1 binds to cyclin A, cyclin B or Ringo to become an active kinase (4, 5).
The “Polo-Like Kinases” Plks form a family of four different proteins that regulates many aspects of the cell cycle progression. They all share small conserved domains named polo-box required fort protein localization. Only Plk1 that is the most extensively studied, is a true mitotic kinase homolog to the Drosophila polo kinase (6). Plk2, Plk3 and Plk4 are more likely involved only in interphase. However, Plk4 activity is required for centriole duplication, an event that must be achieved before entering mitosis, and necessary to assemble the bipolar mitotic spindle (7).
Aurora kinases were first identified in S.cerevisae and Drosophila (8, 9). Yeast cells possess only one Aurora-related kinase, invertebrates such Drosophila and C. elegans have two (A and B type) and mammals have three, named Aurora A, B and C (10). From an evolution point of view the A and B types have evolved from a common ancestor while C type have evolved from the B type (11). Consequently, Aurora A has distinct functions while Aurora B and C share same functions, though all three kinases are involved in the control of many processes required for mitosis.
“NIMA related kinases” Nrks belong to a very large family of protein kinases with 13 different Nek proteins in human, from Nek1 to Nek11 (Nek2A and Nek2B and Nek11L and Nek11S) (12). The belonging to the Nrk family is defined by the sequence homology with the kinase NIMA (Nerver In Mitosis A), a true Aspergillus nidulans mitotic kinase (13). However not all of the Nek kinases are involved in mitosis (14). Nek2 is the most studied of all; its activity is required for centrosome behavior and for cytokinesis (15, 16).
Mitosis (figure 1)
Figure 1. The different phases of the mitosis.
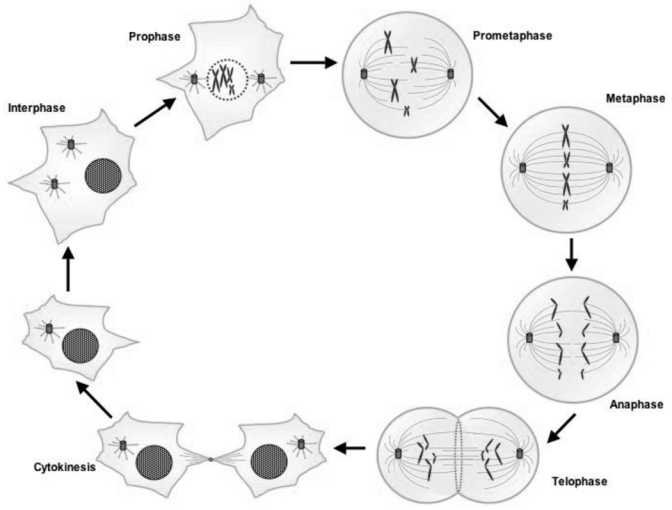
During the interphase the cell’s nucleus is well defined, with two pairs of centrioles adjacent to the nucleus. At the end of the interphase, the genome has been duplicated but the chromosomes are not distinguishable. When prophase starts, the nucleoli disappear and the chromatin starts to coil and fold into observable chromosomes, the spindle forms and the centrosomes move apart. During prometaphase, the nuclear membrane breaks down and some of spindle microtubules attach to sister chromatids at the kinetochores. The microtubules start to deplace the chromatid pairs to form a metaphase plate. At the metaphase the chromosomes have moved to the center of the dividing cell along the metaphase plate. Identical chromatids are attached to kinetochore fibers radiating from opposite ends of the parent cell. The sister chromatids begin to separate at the anaphase when the spindle microtubules pull separating chromosomes to opposite poles. During telophase, daughter nuclei begin to assemble with nuclear envelopes appearing around chromosomes. Nucleoli reappear and chromosomes decondense. The last step of mitosis is the cytokinesis step. It occurs when a contractile ring of actin and myosin filaments constricts the plasma membrane at the equator, triggering the physical division of the two daughter cells.
To get ready for mitosis
Mitosis comprises many complex events that must be accomplished in less than an hour. The length of a human full cell cycle is approximately 24h during which a dividing cell is preparing itself to enter mitosis. First of all the cell must have replicated its DNA (S phase) and possess two full copies of its genome (G2 phase). Secondary the cell must also have duplicated its centrosome and possess two centrosomes (four centrioles). These centrosomes then need to go through a maturation process, meaning that proteins involved in mitotic microtubule nucleation, such as γ-tubulin for instance, must have been recruited to the centrosome before cells may enter mitosis.
Prophase: leaving the starting blocks
During the prophase stage, the chromatin start to condense to form well-defined chromosomes, each chromosome consists of two sister chromatids connected at the level of their centromeres. While centrosome maturation is continuing during prophase, duplicated centrosomes must have separated and started to migrate around the nucleus to reach opposite position (the two centrosomes are now separated by the nucleus). By the end of prophase the nuclear membrane starts to breakdown.
Prometaphase: a cell without nucleus
At this the stage, the nuclear membrane has been dissolved, the chromosomes have become thicker. Centrosomes nucleate asters of microtubule that search for chromosomes to attach to. Other microtubules nucleated by the chromosomes will help to assemble the bipolar spindle. The chromosome centromeres where the kinetochores are assembled are an important attachment point for the microtubules. This attachment is controlled by the metaphase spindle checkpoint..
Metaphase: being under surveillance
The chromosomes have reached their maximum condensation state. One pair of sister chromatids linked together by cohesins forms each chromosome. Each pair of chromatid kinetochores must have one kinetochore attached to microtubules nucleated by a centrosome and the opposite kinetochore attached to microtubules emanating from the opposite centrosome. During all this process the spindle formation is controlled by the dynamic instability of the microtubules. At the end of metaphase, the spindle must be under tension with all the chromosome kinetochores attached to both centrosomes and aligned at the metaphase plate. Cell will remain in metaphase until all the above conditions are fulfilled leading to the spindle checkpoint switch off.
Anaphase: chromosome segregation
This stage is triggered once the cell has controlled the spindle is under tension and all the kinetochores have been captured by microtubules. When the spindle seems the most stable, the cohesins that maintain the sister chromatids are degraded and each sister chromatid is pulled towards each centrosome forming two identical set of chromosomes.
Telophase: get ready for cell division
During anaphase while chromosomes are moving, many kinetochore proteins detached from chromosomes to remain at the center of the cell where a central spindle is assembled. A contractile actin ring forms under the surface of the plasma membrane, around the central spindle. All these events lead to a contraction of the plasma membrane at the middle of the cell that will form two cells attached by the midbody.
Cytokinesis and abscission: daughter cells separation
This is the less understood event of mitosis: the two daughter cells must separate. To do so, the midbody must be broken and one of the cells will inherit a flemming body (remaining of the midbody). But more importantly the cell must repair the plasma membrane to avoid leaking of cell contain. This is achieved by recruiting membrane vesicles from the previously dissolved Golgi. These vesicles also carry proteins required for cytokinesis. The very last step of cytokenesis called abscission is the physical separation of the two daughter cells.
Control of mitosis by phosphorylation
The protein kinases described here are all involved in the regulation of multiple events during mitotic progression. Analyzing the function of a mitotic kinase is not easy since knock down of the protein expression by RNA interference usually generates a phenotype that corresponds to the first event controlled by the enzyme. For instance, eliminating CDK1 leads to a cell cycle arrest in G2 phase. The cell doesn’t enter mitosis because CDK1 is required for the G2/M transition. But CDK1 is also required for progression through mitosis.
The function of each kinase is also tightly linked to their localizations during progression through mitosis, “being at the right place at the right time” (figure 2). One can for instance rescue Aurora B knock down by an Aurora A kinase chimera containing Aurora B localization sequences (17).
Figure 2. Localization of the major mitotic kinase through the mitotic phase.
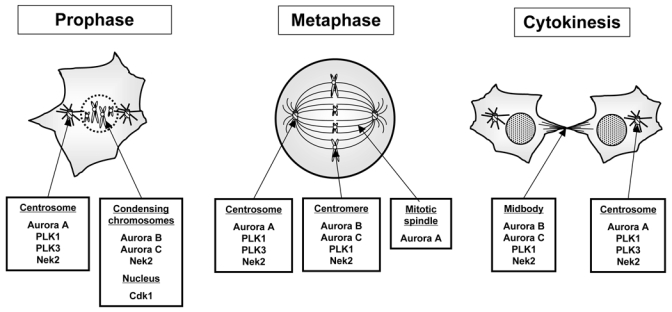
One of the clue to succeed in mitosis for mitotic kinases, is to be “at the right place at the right moment”. The short summary of where the kinases have been found gives an idea of the complexity of the controls insured by mitotic protein kinases.
CDK1/cyclinB activity delimits mitosis
Cdk1/cyclin B activity appears in late G2 and peaks at metaphase (the middle of M phase) and is inactivated upon exit from mitosis by cyclin B destruction, degraded first on the spindle at the chromosome level together with cohesins (18). Cdk1 kinase plays important roles in early stages that contribute to the G2/M transition. Cdk1 phosphorylates motor proteins involved in centrosomes separation required for bipolar spindle assembly (19). Cdk1 phosphorylates lamina inducing a destabilization of the nuclear structure leading to nuclear envelope breaks down (20). It also phosphorylates condensin contributing to chromosome condensation (21). When Cdk1 activity is maximum, it participates to the activation of the APC/C that insure the ubiquitination of the proteins targeted to be degraded at the metaphase/anaphase transition, including cyclin B and securin (22).
Plk1: a very busy kinase
Plk1 kinase activity peaks in mitosis. The kinase is composed of a catalytic domain and a PBD (polo Box Domain) that must bind to a docking protein previously phosphorylated by a priming kinase to allow Plk1 activation (23). Also, Plk1 is activated by phosphorylation of its T-loop by an activated kinase (24). Plk1 localizes to the centrosomes, the kinetochores and the midbody during mitosis. The kinase plays multiple roles during mitosis; it participates to the G2/M transition, its inhibition delays entry in mitosis. Among the Plk1 substrates one finds all the major players involved in the G2/M transition, CDC25, Myt1 and cyclin B1 (25–27). Plk1 would be involved in the feed back loop that controls the activation of Cdk1/cyclin B.
Plk1 activity is also required for centrosome maturation by recruiting protein necessary to nucleate the microtubules that will participate to bipolar spindle assembly; the kinase also interacts with and phosphorylates many proteins involved in microtubules dynamic (28, 29). In addition to be localized and active at the centrosome level, Plk1 also localizes to the chromosome kinetochores (30) where its activity participates to the localization of spindle checkpoint proteins. The exact function of Plk1 at the kinetochores and its participation to the spindle checkpoint remains to be clarified.
Plk1 is also required to activate the E3 Ubiquitine ligase APC/C required to trigger mitotic protein degradation. But although Plk1 directly phosphorylates APC/C subunits the effect of this phosphorylation on APC/C activity is minor (31) compare to the phosphorylation by Cdk1/cyclin Bl (32). However Plk1 contributes indirectly to APC/C activation by phosphorylating the APC/C-cdc20 inhibitor Emil in somatic cells. Phosphorylation of Emil by Plk1 triggers Emil degradation and APC/C-cdc20 activation (33, 34). This contributes to metaphase-anaphase transition controlled by APC/C-cdc20 and M/G1 transition controlled by APC/C-cdh1.
Finally evidence for a function of Plk1 in cytokinesis has been found in different organisms. Septum formation is impaired in the fission yeast kinase defective mutants while ectopic septum are formed when the kinase is overexpressed (35, 36). In Drosophila polo kinase mutant also shows cytokinesis defects at various stages of spermatogenesis (37). In vertebrate cells the kinase localizes at the midbody (38). Plk1 also interact with and phophorylates kinesin proteins required in cytokinesis such as MKLP1 (39).
Aurora A: a centrosome protein
Aurora A is activated by binding to some of its substrates like TPX2, a mechanism that insures a local activation of the kinase (40). Aurora A is restricted to the centrosome area where it phosphorylates CDC25B contributing to G2/M transition (41). But unlike Cdk1, Aurora A is dispensable; its absence only delays entry into mitosis (42). Aurora A activity is required for centrosomes separation and maturation that consists in recruiting proteins involved in microtubule nucleation. The kinase phosphorylates motor proteins (43) and proteins required for astral microtubule nucleation (44). Aurora A might also be involved later in mitosis because its overexpression induced a bypass of the Taxol induced mitotic checkpoint (45). The kinase is involved in cytokinesis since its overexpression induced polyploidy aggravated in the absence of p53 (46). However, these two last points need to be investigated further, in particular the relationship between Aurora A and p53. Upon exit from mitosis Aurora A is degraded by the proteasome in a CDH1 dependant manner (47, 48)
Aurora B: a chromosome passenger protein
Aurora B participates to at least two protein complexes with INCENP, and with INCENP/survivin/Borealin (49). Those proteins form the chromosome passenger protein family, they localize to the kinetochores until the metaphase-anaphase transition occurs then they relocalize to the midody (50). Like Aurora A, Aurora B is activated by binding to some of its substrates. Aurora B clearly fulfills three distinct functions during mitosis. Aurora B is a histone kinase, it phosphorylates serines 10 and 28 on histone H3 and the serine 7 in the centromere histone variant CENP-A (51–53). The function of these phosphorylations is still debated: chromosome condensation? Loading of mitotic proteins on chromosome? Signaling mitosis? (54)
Aurora B also phosphorylates MCAK (Mitotic Centromere-Associated Kinesin) that results in the inactivation of its microtubule depolymerase catalytic activity and its targeting to the kinetochores. MCAK is involved in the spindle checkpoint by correcting the non-amphitelic attachments of microtubules to the kinetochores (55).
Aurora B RNA interference or inhibition mainly induces the formation of polyploid cells indicating that Aurora B activity is required for cytokinesis. And indeed, the kinase phosphorylates vimentin, the kinesin ZEN-4/MKLP1 and MgcRacGAP, a GTPase Activating Protein (GAP) all required for cytokinesis (56–58).
Aurora C: an Aurora B substitute?
Aurora C is expressed only in testis (59). However overexpression of Aurora C has been observed in number of cancer cell lines and tumors (60, 61). Aurora C was first described as an anaphase centrosome protein (60). However it turn out that Aurora C when overexpressed behaved just like Aurora A in interphase and like Aurora B in mitosis (62, 63). Aurora C like other Aurora is activated by some of its substrates, in particular by Aurora B substrate such as INCENP (63). Not only Aurora C mimics Aurora B in mitosis but also it rescues Aurora B depleted cells (63). Strikingly, nobody has yet localized the endogenous protein nor analyzed whether the kinase is expressed in normal cells and what would be its function.
NIMA: a kinase with many relatives
NIMA (Never In Mitosis A) is an Aspergillus nidulans protein kinase. Mutations that inactivate the kinase led to a late G2 arrest with cells harboring duplicated but unseparated centrosomes (64). Among the 13 mammalian Nek, Nek2 is the closest NIMA relatives, its activity is absolutely required for mitosis. Nek2 phosphorylates C-Nap1 (Centrosomal Nek2-Associated Protein 1). Its phosphorylation is required for centrosome separation that is a prerequisite to bipolar spindle assembly (65). Nek2, at least in Drosophila, might also have a role late in mitosis since its overexpression leads to cytokinesis defects (16). These functions again are related to the localization of the kinase: the centrosomes and the kinetochores. Human cells express two isoforms of Nek2, Nek2A and Nek2B. Nek2A is degraded by the APC/C upon entry into mitosis whereas Nek2B remains stable during mitosis (15, 66). Nek2 is activated by trans-autophophorylation and inhibited by dephosphorylation by the phosphatase PP1 (67).
Other members of the Nrk kinase family play roles during mitosis. Nek6 that is highly expressed during mitosis is required for mitosis progression because its inhibition provokes a metaphase arrest (68, 69). Nek9 that is a mitotic centrosome kinase, phosphorylates and activates Nek6 (68). Inhibition of Nek9 impairs bipolar spindle assembly (70).
Mitotic kinases and cancer
In 1914, Theodore Bovary proposed aneuploidy (abnormal chromosome number) arising from mitotic defects as a mechanism that might lead to oncogenesis. Abnormal mitosis can indeed generate cells with multiple centrosomes and abnormal number of chromosomes frequently observed in cancer cells (71).
Theodor Beoveri was right; chromosome instability and aneuploidy generate genetic defects that are hallmarks of tumorigenesis. They arise through defects during mitosis when chromosomes are unequally segregated between the two daughter cells. Neoplastic development is a multisteps mechanism due to an accumulation of genetic defects that breaks the balance between growth-inhibitory signal and division-promoting signal. Tumors then would derive from one cell in which the growth-inhibitory signal is down regulated (loss of tumor suppressor genes) and the division-promoting signal is elevated (gain of proto-oncogenes). One of the best examples is the transformation of human cell line achieved by co-expression of the SV40 large-T oncoprotein, the H-ras oncogene and the telomerase catalytic subunit (72).
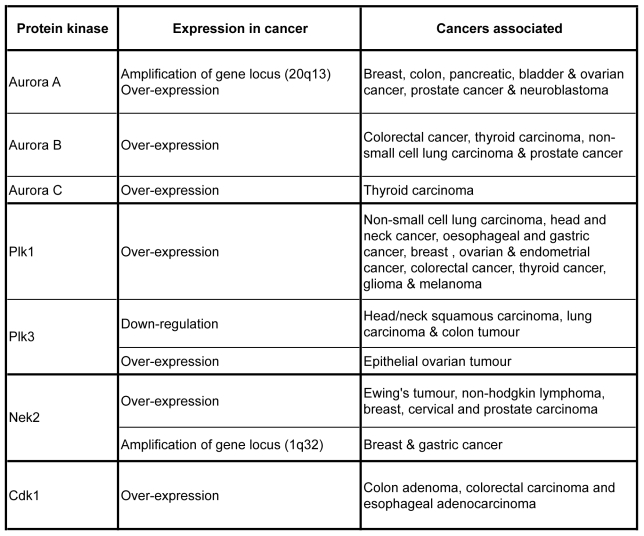
Chromosome segregation is a finely regulated process insured by the mitotic spindle that is a highly dynamic microtubule-based structure. The mitotic spindle is composed of two centrosomes connected by microtubules to the chromosomes aligned at the metaphase plate in the centre of the structure. In every pair of sister chromatid, each chromatid is connected to opposite centrosomes forming amphitelic attachment. During segregation each sister chromatid migrates to one pole of the cell leading to the formation of two identical groups of chromosome. This bipolarity is necessary to form two daughter cells with the same DNA content during cytokinesis. In mammalian cells, each spindle pole is organized around a centrosome. Remarkably, in cancer cells the number, the structure and the function of centrosomes are often abnormal and correlate with aneuploidy and chromosome instability. So, because the mitotic spindle plays a central role in chromosome segregation, many proteins involved in its establishment and in its regulation are often mis-regulated in cancers. This is the case for the mitotic protein kinases Cdk1, Aurora A, B and C, Plk1, and Nek2 protein kinases (figure 3).
Figure 3. An overview of relationship between mitotic kinase expression and cancer.
As mitosis regulators, the expression levels of the major mitotic kinases are crucial for cell division. In many cancers, up and down regulation of their expression have been observed, underlying the importance to follow the expression level of mitotic kinases for developing new targeted therapy.
CDKs
Alterations of Cdks have rarely been observed in cancer, overexpression of Cdk1 and Cdk2 has been reported in colon adenomas (73, 74). However alterations of proteins that regulate Cdks such as cyclins, Cdk-activating enzymes and CKI, are frequently observed (75, 76). For instance cyclin A is overexpressed in lung carcinoma and elevated expression correlated with shorter survival (77). But the best proves that Cdk hyperactivities are involved in cancer is the fact that drugs inhibiting Cdk s are particularly effective to inhibit tumor progression. A large variety of compounds are actually on the market and their successes are due to their effects, inhibition of cell proliferation, activation of apoptosis and in some cases they can even trigger differentiation (78–80).
Aurora A
The gene encoding Aurora A is located on chromosome 20q13. This chromosome region is frequently found amplified in human cancers, and the amplification is associated with over-expression of the protein kinase (81). This Aurora A amplification/over-expression is detected in cancers like breast, colon, pancreatic, bladder, ovarian, prostate cancer and neuroblastoma (82–85). Furthermore the Aurora A gene copy number correlates with chromosomal instability and aneuploidy in human bladder tumour. Overexpression of the kinase also correlates with clinical aggressiveness of the tumour (84, 86). Aurora A has also been found mutated in several cancers, the mutation that is proposed to be due to polymorphism designs Aurora A as a candidate susceptibility gene (87).
In vitro Aurora A kinase over-expression induces aneuploidy and abnormal centrosome numbers leading to cell tumorigenic transformation (82, 83). Long term overexpression of Aurora A is also sufficient to induce tumour formation in mice, after a long period of genomic instability (88). However it is not clear how the kinase induces tumorigenesis, it has been proposed that Aurora A overexpression would be sufficient to escape negative regulation by tumour suppressor pathway. Aurora A kinase interacts with and phosphorylates the tumour suppressor protein p53. Phosphorylation of p53 induced its degradation through mdm2 (89) and reduced it transactivation activity (90). Because p53 plays a major role in carcinogenesis, its interaction with Aurora A might be important in the kinase oncogenic activity. Moreover Aurora A kinase is a RasGap Src homology 3 domain binding protein and forms a complex with RasGap and Survivin proteins. This interaction inhibits Aurora A activity (91). Because RasGap is also a negative regulator of Ras pathway, it has been suggested that in cell overexpressing Ras, there would not be enough RasGap to inactivate both Ras and Aurora A leading to Aurora A hyperactivity participating to oncogenesis.
Aurora B
Aurora B overexpression has been found in many cancers like colorectal cancer (92, 93) or thyroid carcinoma (94). In colorectal and prostate cancer, Aurora B over-expression increases in correlation with the tumour malignancy (92, 95). However unlike Aurora A, Aurora B gene has never been found amplified and the origin of Aurora B over-expression is actually unknown. Also unlike Aurora A, Aurora B is not an oncogene, but its over-expression induces metastasis. Aurora B over-expression results in hyper-phosphorylation of histone H3 on serine 10 (96). This increase in serine10 phosphorylation is observed on lagging chromosomes during mitosis (96). Does hyperphosphorylation of histone H3 induce chromosome instability and aneuploidy? It seems so since, lagging chromosomes have been observed in cells transfected with a Ser10 phospho-mimetic form of histone H3 (96). Whether hyperphosphorylation of H3 participates to segregation defect is obvious, whether it participates to metastases apparition is not clear. Although Aurora B over-expression in cancer cells correlates with genetic instability (97) how Aurora B expression is linked to cancer remains to be determined. However inhibition of Aurora kinases (especially with anti-Aurora B drugs) efficiently reduced tumour growth in mice (98).
Aurora C
Little is know about this third member of Aurora kinase family. In normal physiological conditions Aurora C is expressed only in testis, (59). However cancer cell lines expressed the kinase (60). Aurora C is highly expressed in human thyroid carcinoma cell lines and tissues, where its expression correlates with the aggressiveness of the tumour (61). Over-expression of Aurora C gives rise to polyploid cells. Like for the other Aurora kinases, the phenotype is aggravated in the absence of p53 (62). Because Aurora C is very close to Aurora B one would expect over-expression of Aurora C to have the same consequences than over-expression of Aurora B.
Plk1
Over-expression of Plk1 in rodent cells is sufficient to confer a transform phenotype indicating that Plk1 is a potential oncogene (99). In agreement with this data, Plk1 has been found over-expressed in a large variety of cancers (100 for review). And high level of Plk1 is a sign of bad prognosis in several cancers (101–105). Beside over-expression, mutation in Plk1 has been observed in cancers; some of the mutations inhibit the interaction of Plk1 with Hsp90 and stabilize the kinase leading to a hyperactivity of Plk1 (106). Like for Aurora kinases, overexpression of Plk1 generates genomic instability by triggering the formation of polyploid cells (107) frequently observed in cancer cells (108). Taking together these data designed Plk1 as a good target for inhibitors used as anti-cancer drugs. And indeed inhibition of Plk1 was reported to have different effects in cancer cells versus normal cells (28). Inhibition of Plk1 arrests tumour cells in culture as well as it reduces tumour growth in mice indicating that the kinase is absolutely required for cells that highly proliferate (109–110).
Nek2
Ewing’s tumour cell line derived (a paediatric osteosarcoma) and non-Hodgkin lymphoma show elevated level of Nek2 mRNA, and the transcript level increase correlates with aggressiveness (111). Nek2 is also over-expressed in cervical and prostate carcinoma as well as in gastric and breast in which the chromosomal region 1q32 corresponding to the human Nek2 gene locus is amplified (six times in breast cancer) (112, 113). Nek2 is not a proto-oncogene, however its over-expression provokes defects in centrosome organization and function. In HBL100 cells over-expression of human Nek2 induces the formation of aneuploid cells containing abnormal numbers of centrosomes a hallmark of cancer cells (71).
Conclusion
The short review only describes the most known mitotic protein kinases, we voluntarily omitted checkpoint kinases and others to make this review comprehensive for people that are not use to protein kinase world. One sure thing is that many other mitotic protein kinases remain to be discovered and studied as proved by some screen recently performed to search for novels kinases (114).
Acknowledgments
I express my apologies to all the authors who have published work related to this review but have not been cited here owing to space constraints. Research in the Claude Prigent laboratory is financed by the CNRS, the INCa and the « Canceropole grand Quest ». Patrick Salaun is a fellow of the LNCC and Yoann Rannou a fellow of the French Minister.
References
- 1.Fisher DL, Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15(4):850–60. [PMC free article] [PubMed] [Google Scholar]
- 2.Durkacz B, Carr A, Nurse P. Transcription of the cdc2 cell cycle control gene of the fission yeast Schizosaccharomyces pombe. EMBO J. 1986;5(2):369–373. doi: 10.1002/j.1460-2075.1986.tb04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- 4.Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56(5):829–38. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- 5.Karaiskou A, Perez LH, Ferby I, Ozon R, Jessus C, Nebreda AR. Differential regulation of Cdc2 and Cdk2 by RINGO and cyclins. J Biol Chem. 2001;276(38):36028–34. doi: 10.1074/jbc.M104722200. [DOI] [PubMed] [Google Scholar]
- 6.Nigg EA. Polo-like kinases:positive regulators of cell division from start to finish. Curr Opinion in Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- 7.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7(11):1140–6. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 8.Chan CS, Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135(3):677–91. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81(1):95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 10.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2(1):21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 11.Brown JR, Koretke KK, Birkeland ML, Sanseau P, Patrick DR. Evolutionary relationships of Aurora kinases: implications for model organism studies and the development of anti-cancer drugs. BMC Evol Biol. 2004;4(1):39. doi: 10.1186/1471-2148-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13(5):221–8. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 13.Osmani SA, May GS, Morris NR. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J Cell Biol. 1987;104(6):1495–504. doi: 10.1083/jcb.104.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, Avruch JA. Mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem. 2003;278(37):34897–909. doi: 10.1074/jbc.M303663200. [DOI] [PubMed] [Google Scholar]
- 15.Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184–6194. doi: 10.1038/sj.onc.1205711. [DOI] [PubMed] [Google Scholar]
- 16.Prigent C, Glover DM, Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure. Exp Cell Res. 2005;303(1):1–13. doi: 10.1016/j.yexcr.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Scrittori L, Skoufias DA, Hans F, Gerson V, Sassone-Corsi P, Dimitrov S, Margolis RL. A small C-terminal sequence of Aurora B is responsible for localization and function. Mol Biol Cell. 2005;16(1):292–305. doi: 10.1091/mbc.E04-06-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clute P, Pines J. Temporal and spatial control of cyclin Bl destruction in metaphase. Nat Cell Biol. 1999;1(2):82–7. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 19.Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83(7):1159–69. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 20.Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Hirano M, Kobayashi R, Hirano T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science. 1998;282(5388):487–90. doi: 10.1126/science.282.5388.487. [DOI] [PubMed] [Google Scholar]
- 22.Rudner AD, Murray AW. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J Cell Biol. 2000;149(7):1377–90. doi: 10.1083/jcb.149.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003;115(1):83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 24.Qian YW, Erikson E, Mailer JL. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998;282(5394):1701–4. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Mytl as a Plk1 substrate. J Biol Chem. 2003;278(28):25277–80. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin Bl and targets it to the nucleus during prophase. Nature. 2001;410(6825):215–20. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- 28.Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.do Carmo Avides M, Tavares A, Glover DM. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nature Cell Biol. 2001;3:421–24. doi: 10.1038/35070110. [DOI] [PubMed] [Google Scholar]
- 30.Arnaud L, Pines J, Nigg EA. GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma. 1998;107(6–7):424–9. doi: 10.1007/s004120050326. [DOI] [PubMed] [Google Scholar]
- 31.Golan A, Yudkovsky Y, Hershko A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J Biol Chem. 2002;277(18):15552–7. doi: 10.1074/jbc.M111476200. [DOI] [PubMed] [Google Scholar]
- 32.Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22(24):6598–609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen DV, Loktev AV, Ban KH, Jackson PK. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC Inhibitor Emi1. Mol Biol Cell. 2004;(12):5623–34. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moshe Y, Boulaire J, Pagano M, Hershko A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci U S A. 2004;101(21):7937–42. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9(9):1059–73. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 36.Bahler J, Steever AB, Wheatley S, Wang Y, Pringle JR, Gould KL, McCollum D. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J Cell Biol. 1998;143(6):1603–16. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmena M, Riparbelli MG, Minestrini G, Tavares AM, Adams R, Callaini G, Glover DM. Drosophila polo kinase is required for cytokinesis. J Cell Biol. 1998;143(3):659–71. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;(12):8625–32. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neef R, Preisinger C, Stucliffe J, Kopajtich R, Nigg EA, Mayer TU, Barr FA. J Cell Biol. 2003;162:863–875. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5(3):242–8. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- 41.Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, Mirey G, Bouche JP, Theis-Febvre N, Schmitt E, Monsarrat B, Prigent C, Ducommun B. Phosphorylation of CDC25B by Aurora A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117(Pt 12):2523–31. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- 42.Marumoto T, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, Sasayama T, Kuninaka S, Mimori T, Tamaki N, Kimura M, Okano Y, Saya H. Roles of Aurora A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7(11):1173–82. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 43.Giet R, Uzbekov R, Cubizolles F, Le Guellec K, Prigent C. The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEgS. J Biol Chem. 1999;274(21):15005–13. doi: 10.1074/jbc.274.21.15005. [DOI] [PubMed] [Google Scholar]
- 44.Giet R, McLean D, Descamps S, Lee MJ, Raff JW, Prigent C, Glover DM. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J Cell Biol. 2002;156(3):437–51. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003 2003 Jan;3(1):51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 46.Meraldi P, Honda R, Nigg EA. Aurora A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21(4):483–92. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro A, Arlot-Bonnemains Y, Vigneron S, Labbe JC, Prigent C, Lorca T. APC/Fizzy-Related targets Aurora A kinase for proteolysis. EMBO Rep. 2002a;3(5):457–62. doi: 10.1093/embo-reports/kvf095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro A, Vigneron S, Bernis C, Labbe JC, Prigent C, Lorca T. The D-Box-activating domain (DAD) is a new proteolysis signal that stimulates the silent D-Box sequence of Aurora A. EMBO Rep. 2002b;3(12):1209–14. doi: 10.1093/embo-reports/kvf241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends in Cell Biol. 2006;15(5):241–50. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10(17):1075–8. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- 51.Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, Lin R, Smith MM, Allis CD. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102(3):279–91. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 52.Giet R, Glover DM. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152(4):669–82. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155(7):1147–57. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116(Pt 18):3677–85. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 55.Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell. 2004;15(6):2895–906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goto H, Yasui Y, Kawajiri A, Nigg EA, Terada Y, Tatsuka M, Nagata K, Inagaki M. Aurora B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278(10):8526–30. doi: 10.1074/jbc.M210892200. [DOI] [PubMed] [Google Scholar]
- 57.Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, Bao YC, Deng X, Tatsuka M, Narumiya S, May WS, Jr, Nosaka T, Semba K, Inoue T, Satoh T, Inagaki M, Kitamura T. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4(4):549–60. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 58.Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15(8):778–86. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 59.Hu HM, Chuang CK, Lee MJ, Tseng TC, Tang TK. Genomic organization, expression, and chromosome localization of a third aurora-related kinase gene, Aie1. DNA Cell Biol. 2000;19(11):679–88. doi: 10.1089/10445490050199063. [DOI] [PubMed] [Google Scholar]
- 60.Kimura M, Matsuda Y, Yoshioka T, Okano Y. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J Biol Chem. 1999;274(11):7334–40. doi: 10.1074/jbc.274.11.7334. [DOI] [PubMed] [Google Scholar]
- 61.Ulisse S, Delcros JG, Baldini E, Toller M, Curcio F, Giacomelli L, Prigent C, Ambesi-Impiombato FS, D’Armiento M, Arlot-Bonnemains Y. Expression of Aurora kinases in human thyroid carcinoma cell lines and tissues. Int J Cancer. 2006;119(2):275–82. doi: 10.1002/ijc.21842. [DOI] [PubMed] [Google Scholar]
- 62.Dutertre S, Hamard-Peron E, Cremet JY, Thomas Y, Prigent C. The absence of p53 aggravates polyploidy and centrosome number abnormality induced by Aurora C overexpression. Cell Cycle. 2005;4(12):1783–7. doi: 10.4161/cc.4.12.2172. [DOI] [PubMed] [Google Scholar]
- 63.Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M, Suzuki F, Nigg EA, Earnshaw WC, Brinkley WR, Sen S. Aurora C kinase is a novel chromosomal passenger protein that can complement Aurora B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59(4):249–63. doi: 10.1002/cm.20039. [DOI] [PubMed] [Google Scholar]
- 64.Osmani AH, McGuire SL, Osmani SA. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans Cell. 1991;67(2):283–91. doi: 10.1016/0092-8674(91)90180-7. [DOI] [PubMed] [Google Scholar]
- 65.Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol. 1998;141(7):1563–74. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fry AM, et al. Activity of the human centrosomal kinase, Nek2, depends on an unusual leucine zipper dimerization motif. J Biol Chem. 1999;274:16304–16310. doi: 10.1074/jbc.274.23.16304. [DOI] [PubMed] [Google Scholar]
- 67.Helps NR, Luo X, Barker HM, Cohen PT. NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem J. 2000;349(Pt 2):509–18. doi: 10.1042/0264-6021:3490509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, Avruch J. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem. 2003;278:34897–34909. doi: 10.1074/jbc.M303663200. [DOI] [PubMed] [Google Scholar]
- 69.Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem. 2003;278:52454–52460. doi: 10.1074/jbc.M308080200. [DOI] [PubMed] [Google Scholar]
- 70.Roig J, Groen A, Caldwell J, Avruch J. Active Nercc1 protein kinase concentrates at centrosomes early in mitosis, and is necessary for proper spindle assembly. Mol Biol Cell. 2005;16:4827–4840. doi: 10.1091/mbc.E05-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lingle WL, Barrett SL, Negron VC, D’Assoro AB, Boeneman K, Liu W, Whitehead CM, Reynolds C, Salisbury JL. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci USA. 2002;99(4):1978–83. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400(6743):464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto H, Monden T, Miyoshi H, Izawa H, Ikeda K, Tsujie M, Ohnishi T, Sekimoto M, Tomita N, Monden M. Cdk2/cdc2 expression in colon carcinogenesis and effects of cdk2/cdc2 inhibitor in colon cancer cells. Int J Oncol. 1998;13(2):233–9. doi: 10.3892/ijo.13.2.233. [DOI] [PubMed] [Google Scholar]
- 74.Kim JH, Kang MJ, Park CU, Kwak HJ, Hwang Y, Koh GY. Amplified CDK2 and cdc2 activities in primary colorectal carcinoma. Cancer. 1999;85(3):546–53. [PubMed] [Google Scholar]
- 75.Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 76.McDonald ER, 3rd, El-Deiry WS. Cell cycle control as a basis for cancer drug development. Int J Oncol. 2000;16(5):871–86. [PubMed] [Google Scholar]
- 77.Dobashi Y, Shoji M, Jiang SX, Kobayashi M, Kawakubo Y, Kameya T. Active cyclin A-CDK2 complex, a possible critical factor for cell proliferation in human primary lung carcinomas. Am J Pathol. 1998;153(3):963–72. doi: 10.1016/S0002-9440(10)65638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matushansky I, et al. Reprogramming leukemic cells to terminal differentiation by inhibiting specific cyclin-dependent kinases in G1. Proc Natl Acad Sci U S A. 2000;97:14317–14322. doi: 10.1073/pnas.250488697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Damiens E, et al. Anti-mitotic properties of indirubin-3-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene. 2001;20:3786–3797. doi: 10.1038/sj.onc.1204503. [DOI] [PubMed] [Google Scholar]
- 80.Edamatsu H, et al. Cdk inhibitors, roscovitine and olomoucine, synergize with farnesyl transferase inhibitor (FTI) to induce efficient apoptosis of human cancer cell lines. Oncogene. 2000;19:3059–3068. doi: 10.1038/sj.onc.1203625. [DOI] [PubMed] [Google Scholar]
- 81.Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC, Katz RL, Brinkley W, Czerniak B. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–1329. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 82.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka T, Kimura M, Matsunaga K, Fukada D, Mori H, Okano Y. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 1999;59:2041–2044. [PubMed] [Google Scholar]
- 85.Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- 86.Miyoshi Y, Iwao K, Egawa C, Noguchi S. Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int J Cancer. 2001;92:370–373. doi: 10.1002/ijc.1200. [DOI] [PubMed] [Google Scholar]
- 87.Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, MacCarthy-Morrogh L, Ponder BA, Nagase H, Burn J, Ball S, Almeida M, Linardopoulos S, Balmain A. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34(4):403–12. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- 88.Wang X, Zhou YX, Qiao W, Tominaga Y, Ouchi M, Ouchi T, Deng CX. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006 2006 May 22; doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 89.Katayama H, Sasai K, Kawai H, Yuan Z-M, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nature Genetics. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 90.Liu Q, KaNeko S, Yang I, Feldman RI, Nicosia SV, Chen J, Cheng JQ. Aurora A Abrogation of p53 DNA Binding and Transactivation Activity by Phosphorylation of Serine 215. J Biol Chem. 2004;279(50):52175–52182. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- 91.Gigoux V, L’Hoste S, Raynaud F, Camonis J, Garbay C. Identification of Aurora kinases as RasGAP Src homology 3 domain-binding proteins. J Biol Chem. 2002;277:23742–23746. doi: 10.1074/jbc.C200121200. [DOI] [PubMed] [Google Scholar]
- 92.Katayama H, Ota T, Jisaki F, Ueda Y, Tanaka T, Odashima S, Suzuki F, Terada Y, Tatsuka M. Mitotic kinase expression and colorectal cancer progression. J Natl Cancer Inst. 1999;91:1160–1162. doi: 10.1093/jnci/91.13.1160. [DOI] [PubMed] [Google Scholar]
- 93.Takahashi T, Futamura M, Yoshimi N, Sano J, Katada M, Takagi Y, Kimura M, Yoshioka T, Okano Y, Saji S. Centrosomal kinases, HsAIRK1 and HsAIRK3, are overexpressed in primary colorectal cancers. Jpn J Cancer Res. 2000;91:1007–1014. doi: 10.1111/j.1349-7006.2000.tb00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adams RR, Eckley DM, Vagnarelli P, Wheatley SP, Gerloff DL, Mackay AM, Svingen PA, Kaufmann SH, Earnshaw WC. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma. 2001;110:65–74. doi: 10.1007/s004120100130. [DOI] [PubMed] [Google Scholar]
- 95.Chieffi P, Cozzolino L, Kisslinger A, Libertini S, Staibano S, Mansueto G, De Rosa G, Villacci A, Vitale M, Linardopoulos S, Portella G, Tramontano D. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate. 2006;66(3):326–33. doi: 10.1002/pros.20345. [DOI] [PubMed] [Google Scholar]
- 96.Ota T, Suto S, Katayama H, Han ZB, Suzuki F, Maeda M, Tanino M, Terada Y, Tatsuka M. Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora-B overexpression contributes to chromosome number instability. Cancer Res. 2002;62:5168–5177. [PubMed] [Google Scholar]
- 97.Smith SL, Bowers NL, Betticher DC, Gautschi O, Ratschiller D, Hoban PR, Booton R, Santibanez-Koref MF, Heighway J. Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer. 2005;93(6):719–29. doi: 10.1038/sj.bjc.6602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, Miller KM. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 99.Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, Ferris DK. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234:397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 100.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24(2):267–76. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 101.Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Kobel M, Dietel M, Hauptmann S. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004;90:815–821. doi: 10.1038/sj.bjc.6601610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687–692. doi: 10.3892/ijo.15.4.687. [DOI] [PubMed] [Google Scholar]
- 103.Knecht R, Oberhauser C, Strebhardt K. PLK (polo-like kinase), a new prognostic marker for oropharyngeal carcinomas. Int J Cancer. 2000;89:535–536. [PubMed] [Google Scholar]
- 104.Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002;29(6):354–8. doi: 10.1034/j.1600-0560.2002.290605.x. [DOI] [PubMed] [Google Scholar]
- 105.Yamada S, Ohira M, Horie H, Ando K, Takayasu H, Suzuki Y, Sugano S, Hirata T, Goto T, Matsunaga T, Hiyama E, Hayashi Y, Ando H, Suita S, KaNeko M, Sasaki F, Hashizume K, Ohnuma N, Nakagawara A. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23(35):5901–11. doi: 10.1038/sj.onc.1207782. [DOI] [PubMed] [Google Scholar]
- 106.Simizu S, Osada H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nat Cell Biol. 2000;2:852–854. doi: 10.1038/35041102. [DOI] [PubMed] [Google Scholar]
- 107.Mundt KE, Golsteyn RM, Lane HA, Nigg EA. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Commun. 1997;239(2):377–85. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 108.Yamamoto Y, Matsuyama H, Kawauchi S, Matsumoto H, Nagao K, Ohmi C, Sakano S, Furuya T, Oga A, Naito K, Sasaki K. Overexpression of polo-like kinase 1 (PLK1) and chromosomal instability in bladder cancer. Oncology. 2006;70(3):231–7. doi: 10.1159/000094416. [DOI] [PubMed] [Google Scholar]
- 109.Spankuch-Schmitt B, Wolf G, Solbach C, Loibl S, Knecht R, Stegmuller M, von Minckwitz G, Kaufmann M, Strebhardt K. Downregulation of human polo-like kinase activity by antisense oligonucleotides induces growth inhibition in cancer cells. Oncogene. 2002;21(20):3162–71. doi: 10.1038/sj.onc.1205412. [DOI] [PubMed] [Google Scholar]
- 110.Spankuch B, Matthess Y, Knecht R, Zimmer B, Kaufmann M, Strebhardt K. Cancer inhibition in nude mice after systemic application of U6 promoter-driven short hairpin RNAs against PLK1. J Natl Cancer Inst. 2004 2004 Jun 2;96(11):862–72. doi: 10.1093/jnci/djh146. [DOI] [PubMed] [Google Scholar]
- 111.Weiss MM, Kuipers EJ, Postma C, Snijders AM, Pinkel D, Meuwissen SG, Albertson D, Meijer GA. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell Oncol. 2004;26(5–6):307–17. doi: 10.1155/2004/454238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schultz SJ, Fry AM, Sutterlin C, Ried T, Nigg EA. Cell cycle-dependent expression of Nek2, a novel human protein kinase related to the NIMA mitotic regulator of Aspergillus nidulans. Cell Growth Differ. 1994;5(6):625–35. [PubMed] [Google Scholar]
- 113.Loo LW, Grove DI, Williams EM, Neal CL, Cousens LA, Schubert EL, Holcomb IN, Massa HF, Glogovac J, Li CI, Malone KE, Daling JR, Delrow JJ, Trask BJ, Hsu L, Porter PL. Array comparative genomic hybridization analysis of genomic alterations in breast cancer subtypes. Cancer Res. 2004;64(23):8541–9. doi: 10.1158/0008-5472.CAN-04-1992. [DOI] [PubMed] [Google Scholar]
- 114.Bettencourt-Dias M, Giet R, Sinka RA, Mazumdar A, Lock WG, Balloux F, Zafiropoulos PJ, Yamaguchi S, Winter S, Carthew RW, Cooper M, Jones D, Frenz L, Glover DM. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432:23–30. doi: 10.1038/nature03160. [DOI] [PubMed] [Google Scholar]