Abstract
Background
Case reports suggest a link between methamphetamine abuse and acute myocardial infarction (AMI), but no epidemiologic studies have examined this link. Our objective was to test the hypothesis that young adults who abuse amphetamines are at higher risk for AMI.
Methods
In this study of 3,148,165 discharges from Texas hospitals in a quality indicators database during 2000 to 2003, among persons aged 18 to 44 years we identified 11,011 AMIs, defined according to the Agency for Healthcare Research and Quality’s AMI mortality inpatient quality indicator.
Results
In a multiple logistic regression analysis—while controlling for cocaine abuse, alcohol abuse, tobacco use, hypertension, diabetes mellitus, lipid disorders, obesity, congenital defects, and coagulation defects—amphetamine abuse was significantly associated with AMI (adjusted odds ratio = 1.61; 95% confidence interval = 1.24—2.04, p=0.0004). The rate of AMIs among amphetamine abusers increased significantly from 2000 to 2003. The population attributable risk suggests that amphetamine abuse is responsible for 0.2% of AMIs in the state of Texas. The geographical distribution of amphetamine abuse varied by region, with the prevalence being highest in the North Texas and Panhandle regions of Texas.
Conclusions
This modest, though statistically robust, association suggests that amphetamine abuse may play a role in AMI.
1. Introduction
Abuse of amphetamine-like stimulants is a global problem. The United Nations Office on Drugs and Crime estimates that 35 million people worldwide used amphetamine-type stimulants in 2004 (United Nations Office on Drugs and Crime, 2006). The National Survey on Drug Use and Health reported that in 2005 19.1 million Americans had used an illicit or prescription-type stimulant nonmedically at least once in their lifetime (SAMHSA, 2006). The January 2007 proceedings from the Community Epidemiology Work Group suggest increasing levels of methamphetamine abuse in some major metropolitan U.S. cities (e.g., Los Angeles, Phoenix, San Diego, and Seattle) and, in particular (as it relates to the current study), methamphetamine abuse is increasing in Texas and that this increase is disproportionate across various regions within the state of Texas (CEWG, 2007).
While cocaine has been widely recognized as a causative agent in acute myocardial infarction (AMI) (Minor, Jr. et al., 1991; Mittleman et al., 1999; Qureshi et al., 2001; Jones and Weir, 2006), amphetamines have not been as widely recognized. Previous studies on amphetamines have been limited to case series and case reports (Orzel, 1982; Furst et al., 1990; Packe et al., 1990; Ragland et al., 1993; Costa et al., 2001; Sztajnkrycer et al., 2002; Turnipseed et al., 2003; Gandhi et al., 2005). To our knowledge, no population-based studies have examined the potential association between amphetamine abuse and AMI.
Alcohol abuse, tobacco dependence, hypertension, diabetes, obesity, lipid disorders, and coagulation defects are well-established risk factors for AMI (Grundy et al., 1998). Cardiac congenital defects are a potential risk factor, though their associated risk is not as well established as the other risk factors mentioned above (Crump et al., 2000; Warnes, 2005; Rovner et al., 2006; Sastry et al., 2006).
In the current study, we assess the association between amphetamine abuse and AMI in all patients aged 18 to 44 years who were hospitalized from 2000 to 2003 in Texas hospitals covered by a state quality-of-care reporting law . Specifically, we hypothesized that hospitalized patients with a diagnosis of amphetamine abuse would be more likely to have an AMI than hospitalized patients without a diagnosis of amphetamine abuse. As a secondary aim of the study, we also examined whether the magnitude of association between amphetamine abuse and AMI differed by public health regions within the state of Texas.
2. Methods
2.1. Study Design and Patients
A cross-sectional design was used to evaluate the association between amphetamine abuse and AMI. Data were obtained from the Texas Health Care Information Council (THCIC) administrative database. Eligibility requirements for entrance into the cohort were (1) aged 18 to 44 years and (2) hospitalization from January 1, 2000, to December 31, 2003, regardless of diagnosis. The THCIC database comprises virtually all inpatients in the State of Texas. Texas statute requires that all hospitals except the VA, military, and small rural hospitals report to the THCIC. The Institutional Review Board of The University of Texas Southwestern Medical Center at Dallas granted a waiver of approval for this study of de-identified hospital discharge data.
2.2. Texas Statewide Hospital Database and Study Population
THCIC has provided a standardized International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM)-coded discharge database to the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project since 1999. A description of this database has been published (Westover et al., 2007). In 2003, an average of 434 hospitals in Texas reported to THCIC, which represents 86.6% of hospitals in the 2003 American Hospital Association Guide. The THCIC database contained 95% (2,439,043/2,565,861) of all admissions in 2003 in the State of Texas (excluding Department of Veterans Affairs, military hospitals, and small rural hospitals) (American Hospital Association, 2004; Texas Department of State Health Services, 2004). The THCIC record contains 9 discharge diagnosis fields and has a sensitivity (0.926) nearly equivalent to databases containing 25 discharge diagnosis fields (0.933) (Romano and Mark, 1994).
The study population was all discharges in the State of Texas from January 1, 2000, to December 31, 2003, for persons aged 18 to 44 years. There were 751,568 such discharges in 2000, 777,997 in 2001, 806,353 in 2002, and 812,247 in 2003 (Texas Department of State Health Services, 2007).
We employed mapping methods to explore the geographic distribution of AMI and amphetamine and cocaine abuse. We also compared the geographical distribution of amphetamine and cocaine abuse in the THCIC database to that from a completely different data source—treatment data from the Texas Commission on Alcohol and Drug Abuse (TCADA). Incidence rates of AMI, amphetamine- and cocaine-associated-AMI, as well as the prevalence rates of amphetamine and cocaine abuse, were calculated by summing all cases (using the principal discharge diagnosis for AMI and any discharge diagnosis for amphetamine abuse), by county, in persons aged 18 to 44 years in the THCIC cohort, over the four-year period 2000 to 2003, then dividing by four times the 2000 Census county population (Figure 1). The resulting rates were multiplied by 100,000 to obtain the units of “per 100,000 persons per year.” Only admissions where the county of origin was available (97.4%) were included in the maps. Data from TCADA was used to calculate prevalence rates of amphetamine- and cocaine-related treatment in 2002 (Figure 1) (Texas Department of State Health Services, 2003). Treatment is defined by TCADA as (1) inpatient, free-standing, or ambulatory detoxification, (2) residential hospital, long-term residential, short-term residential, intensive outpatient, or non-intensive outpatient rehabilitation, or (3) opioid detoxification or opioid replacement therapy (L. San Jose, personal communication, 2007). Counties with missing data or with fewer than 20 treatments were excluded. Three or fewer treatments for either amphetamines or cocaine within a county were masked and were counted as zeroes. Maps were generated using ArcMap Version 9.1 (ESRI, Inc., Redlands, CA).
Figure 1.
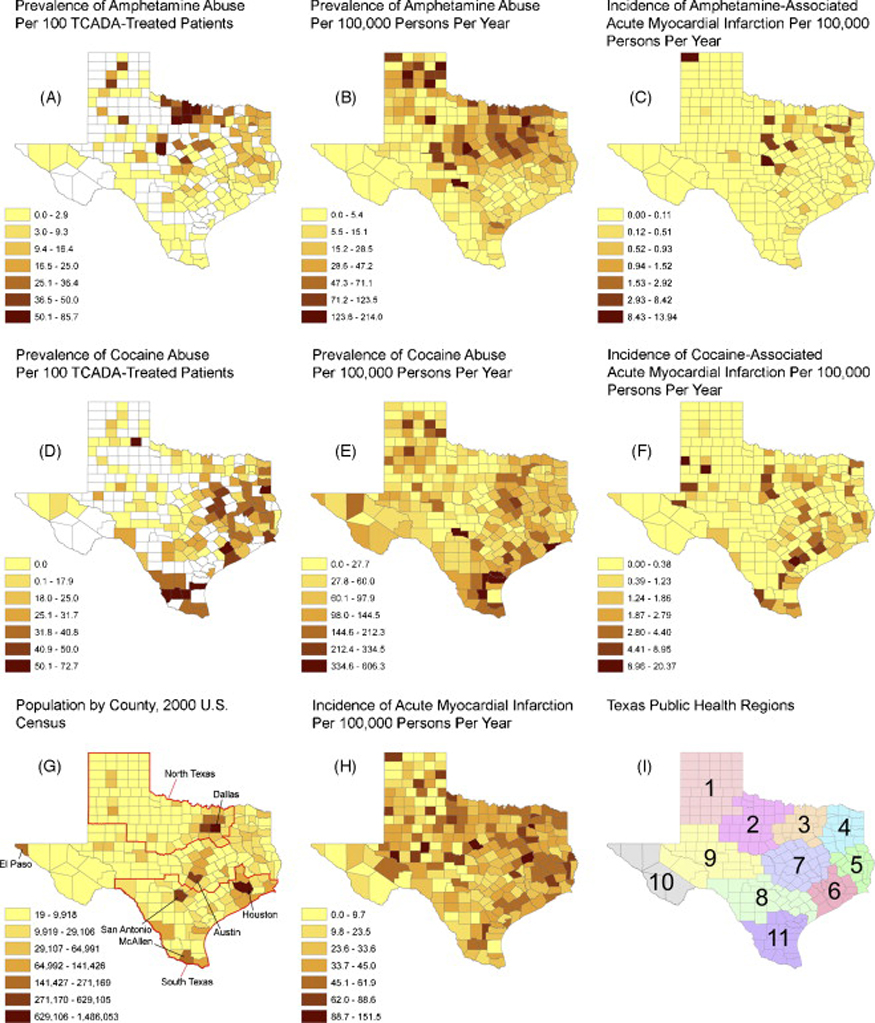
Geographical distribution of the rates of amphetamine and cocaine abuse and of amphetamine- or cocaine-associated acute myocardial infarction identified in healthcare settings in Texas, 2000–2003 (THCIC) and 2002 (TCADA).
Footnote:
The prevalence/incidence rates in B, C, E, F and H are the number of patients hospitalized in a given county having the indicated condition in 2000–2003 divided by the number of people 18–44 years of age in the county in the 2000 Census. In A and D, the white areas are counties with missing data or fewer than 20 cases of the indicated condition. In G, population is of people 18–44 years of age; North Texas refers to Public Health Regions 1, 2, and 3; South Texas refers to 5, 6, 8, and 11. TCADA =Texas Council on Alcohol and Drug Abuse.
2.3. Dependent Variable
The primary outcome measure was AMI, defined using the ICD-9-CM codes in the AHRQ’s Inpatient Quality Indicator 15 (Table 1) (DHHS, 2007). For the current study, AMI was a binomial variable operationally defined as the presence or absence of AMI based on the ICD-9-CM within each hospital discharge. To identify discharges where AMI was the principal reason for admission, we a priori searched in only the principal discharge diagnosis field to maximize specificity. To assess sensitivity, we tested a model where AMI was accepted from any discharge field. In the Women’s Health Initiative, the sensitivity of ICD-9-CM code 410 for detection of AMI was 80% (Heckbert et al., 2004). The positive predictive value of these codes (410.x1) in the principal or first secondary diagnosis fields was 94% (Kiyota et al., 2004). We identified 2,631 AMIs in 2000, 2,781 in 2001, 2,856 in 2002, and 2,743 in 2003.
Table 1.
Definition of Acute Myocardial Infarction, Substance Abuse, and Risk Factors from ICD-9-CM codes
| Condition | ICD-9 code |
|---|---|
| Acute Myocardial Infarction* | 410.01, 410.11, 410.21, 410.31, 410.41, 410.51, 410.61, 410.71, 410.81, 410.91 |
| Substance Abuse† | |
| Amphetamines | 304.40 to 304.42, 305.70 to 305.72 |
| Cocaine | 304.20 to 304.22, 305.60 to 305.62 |
| Alcohol | 303.00 to 303.02, 303.90 to 303.92, 305.00 to 305.02 |
| Risk Factors | |
| Tobacco | 305.1, 989.84 |
| Hypertension | 401.0 to 405.99 |
| Diabetes | 250.00 to 250.93 |
| Obesity | 278.00, 278.01 |
| Lipid Disorder | 272.0 to 272.9 |
| Coagulation Defects‡ | 286.0 to 286.9 |
| Congenital Defects§ | 745.0 to 747.49 |
Acute myocardial infarction was defined using the ICD-9-CM codes in the Agency for Healthcare Research and Quality's (AHRQ) Inpatient Quality Indicator (IQI) 15.
Codes indicating “in remission” were not used. Nonspecific codes of substance abuse or dependence not indicating the specific substance (304.8, 304.9,and 305.9) were not used.
Includes hemophilia A, B, and C, congenital deficieny of other clotting factors, von Willebrand's disease, disorders due to intrinsic circulating anticoagulants, defibrination syndromes, acquired coagulation factor deficiency (e.g. liver disease), thrombotic thrombocytopenic purpura; primary, secondary, and unspecified thrombocytopenia.
Includes common truncus, transposition of great vessels, tetralogy of Fallot, common ventricle, ventricular septal defect, ostium primum and secundum, endocardial cushion defects, cor biloculare, anomalies of pulmonary valve, congenital tricupid atresia and stenosis, Ebstein's anomaly, congenital stenosis and insufficiency of aortic valve, congenital mitral stenosis and insufficiency, hypoplastic left heart syndrome, subaortic stenosis, cor triatriatum, infundibular pulmonic stenosis, coronary artery anomaly, congenital heart block, malposition of heart and cardiac apex, congenital atresia and stenosis of pulmonary valve, patent ductus arteriosus, coarctation of aorta, atresia and stenosis of aorta, anomalies of pulmonary artery, and anomalies of great veins
2.4. Independent Variable and Covariates
We identified conditions and risk factors that might predispose a person to AMI (using the ICD-9-CM codes in all available discharge diagnosis fields): amphetamine abuse (primary independent variable), and covariates cocaine abuse, alcohol abuse, tobacco dependence, hypertension, diabetes, obesity, lipid disorders, coagulation defects, and cardiac congenital defects (see Table 1). Binomial variables were created for each of these conditions and were operationally defined as the presence or absence of each respective condition (based on the ICD-9-CM codes in all available diagnosis fields).
We defined “abuse” of amphetamines and the abuse of cocaine by the ICD-9-CM codes for either abuse or dependence (for purposes of this paper “abuse” refers to both formal constructs of ICD-9-CM abuse and dependence; see Table 1). ICD-9-CM diagnoses of amphetamine abuse and dependence, however, do not distinguish between methamphetamine and other types of amphetamines. Patients coded as “in remission” were not counted as substance abusers in our analysis, because they are unlikely to have a close temporal relationship between substance use and AMI. Nonspecific codes that do not specify the drug(s) of abuse (304.8x, 304.9x, and 305.9x) were also not counted as substance abusers, but were added to the omnibus model in a sensitivity analysis.
The THCIC database delineates age into five mutually exclusive categories for sensitive diagnoses, with patients aged 18 to 44 years comprising one of the five categories. Because the current study only included patients from this age category (18 to 44 years) into the cohort, we were unable to assess an age effect in our modeling of the risk of AMI. The THCIC database does not disclose gender, which thus prevented us from evaluating a gender effect in the risk of AMI. The THCIC database categorizes patients into five racial/ethnic categories: non-Hispanic Asian, non-Hispanic black, non-Hispanic white, Hispanic, or “other” (comprised of patients classified as American Indians/Eskimos/Aleutians, “other race,” or missing race data). Although “congenital heart defects” (Table 1) had the least supporting evidence in the existing literature as a risk factor of AMI, we, nonetheless, included it as a covariate, “cardiac anomalous arteries” (746.85), in a post-hoc analysis.
2.5. Data Analysis
2.5.1. Secular Trends
The prevalence of amphetamine and cocaine abuse, the incidence of AMI, and the incidence of amphetamine-associated and cocaine-associated AMI from 2000 to 2003 was each calculated by summing all respective cases and dividing by the total number of discharges (with the standard error of a proportion). The Cochrane-Armitage test for trend was used to test the significance of change over the four years.
2.5.2. Multiple Logistic Regression
Multiple logistic regression was used to estimate the odds of AMI with an amphetamine abuse diagnosis while controlling for known risk factors. As a point of reference, the unadjusted odds ratios were also estimated for each risk factor and AMI. Multiple logistic regression, using the same risk factors, was also used to examine the odds of amphetamine abuse and AMI within certain public health regions in Texas. The 95% likelihood ratio confidence intervals (CIs) were calculated, and the likelihood ratio Chi-Square statistic was used to test for a significant association between each risk factor and AMI. The level of significance for all tests was set at p < 0.05.
2.5.3. Population Attributable Risk
The multivariate population attributable risk percentage (PARP) of AMI accounted for by amphetamine abuse and cocaine abuse, respectively, for all patients in the state of Texas, for all patients in North Texas (Public Health Regions 1, 2, and 3; Figure 1G) and South Texas (public health regions 5, 6, 8, and 11; Figure 1G), and all patients in Public Health Regions 3 and 4 were calculated from risk factor prevalences and adjusted odds ratios using Bruzzi et al.’s method (Bruzzi et al., 1985). The PARP estimates the public health impact of individual risk factors in a given population under the causal assumption.
Population attributable risk is calculated by subtracting the rate of disease in the unexposed group from the rate of disease in the population. Simple population risk percentage is calculated by dividing the population attributable risk by the rate of disease in the population and multiplying by 100. Multivariate PARP, per the method by Bruzzi et al., which was used in this study, requires only “the value of the regression coefficient from the model, the coding presenting the levels of the risk factor used in fitting the model, and the number of cases at each level” (Bruzzi et al., 1985, p. 911). The Bruzzi et al. method allows us to interpret a multiple logistic regression in terms of the prevalences of the risk factors, and to estimate the magnitude of each risk factor’s contribution to AMI. Only those risk factors found to be significantly associated with AMI were used in the multivariate PARP calculation.
3. Results
3.1. Secular Trends
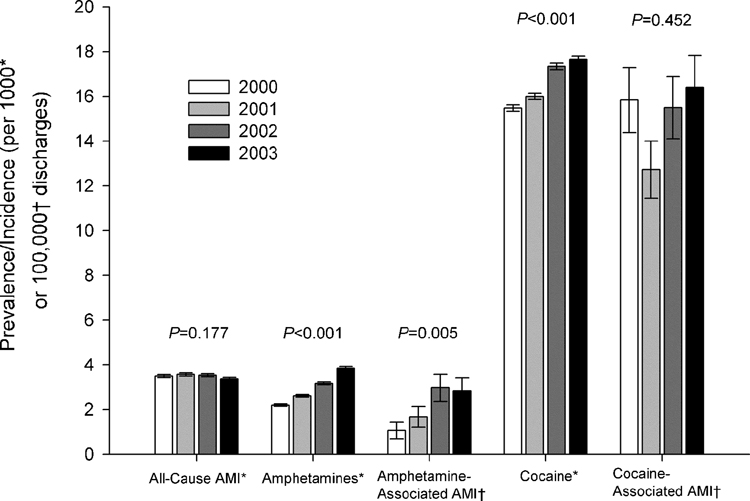
From 2000 to 2003, in patients aged 18 to 44 years, 11,011 (0.35%) had a principal discharge diagnosis of AMI. The rates of both amphetamine and cocaine abuse increased over the four years. The rate of increase from 2000 to 2003 was much higher for amphetamine-associated AMI (166%) than for cocaine-associated AMI (3.6%). This increase over the four years was significant for amphetamine-associated AMI, but not for cocaine-associated AMI and all-cause AMI (Figure 2).
Figure 2.
Trends in AMI, Amphetamine and Cocaine Abuse, and Amphetamine-Associated and Cocaine-Associated AMI
Footnote:
In discharges of persons 18 to 44 years of age from Texas hospitals, 2000 to 2003.
* Incidence of AMI, prevalence rate of amphetamine and cocaine abuse per 1000 discharges (±SE).
†Incidence of amphetamine-associated and cocaine-associated AMI per 100,000 discharges (±SE).
P values are from the Cochrane-Armitage test for trend (two-tailed).
The prevalence rate of amphetamine abuse is highest in the North Texas and Panhandle regions of Texas, whether calculated from TCADA (Figure 1A) or THCIC/Census data (Figure 1B). Likewise, the incidence rate of AMI associated with amphetamine abuse is largely confined to North Texas (Figure 1C). In contrast, the prevalence rate of cocaine abuse is highest in South Texas, regardless of the data source (Figures 1D and 1E), and cocaine-associated AMI showed the same north/south geographical difference (Figure 1F). Whereas the population of persons aged 18 to 44 years was concentrated in urban centers (Figure 1G), the incidence rate of AMI in this age group was distributed relatively homogeneously across geographic regions (Figure 1H).
3.2. Multiple Logistic Regression
In a multiple logistic regression model of inpatients aged 18 to 44 years in Texas hospitals from 2000 to 2003, amphetamine abuse was significantly associated with AMI (Table 2). In particular, when we examined nonspecific codes of substance abuse in the multiple logistic regression model, amphetamine abuse was significantly associated with AMI (adjusted odds ratio = 1.62; 95% CI = 1.26—2.06, p=0.0003). Further, when we examined AMI in any discharge diagnosis field, the multiple logistic regression found that the association between amphetamine abuse and AMI became stronger (adjusted odd ratio = 1.83; 95% CI = 1.48—2.24, p<0.0001). When a dichotomized race variable (coded non-Hispanic white vs. all other categories) and its interaction with amphetamine abuse were added to the model, neither was statistically significant (p=0.89 and p=0.31, respectively), and amphetamine abuse remained significantly associated with AMI (adjusted odds ratio = 1.92; 95% CI = 1.04—3.22, p=0.02), suggesting that the association between amphetamine abuse and AMI was not moderated by race.
Table 2.
Logistic Regression Models for Acute Myocardial Infarction For Patients Aged 18 to 44 Years, 2000 to 2003
| Acute Myocardial Infarction (N=11,011) | |||||
|---|---|---|---|---|---|
| Risk Factor | Unadjusted OR* | 95% CI for Unadjusted OR | Adjusted OR† | 95% CI for Adjusted OR | p for Adjusted OR |
| Amphetamine Abuse | 2.09 | (1.63—2.63) | 1.61 | (1.24—2.04) | 0.0004 |
| Cocaine Abuse | 2.69 | (2.45—2.94) | 2.14 | (1.94—2.36) | <0.0001 |
| Tobacco | 10.21 | (9.82—10.61) | 6.32 | (6.06—6.59) | <0.0001 |
| Alcohol Abuse | 1.04 | (0.94—1.15) | 0.55 | (0.50—0.61) | <0.0001 |
| Lipid Disorder | 29.90 | (28.72—31.14) | 11.61 | (11.06—12.19) | <0.0001 |
| Hypertension | 7.76 | (7.47—8.07) | 2.90 | (2.77—3.04) | <0.0001 |
| Diabetes | 4.26 | (4.06—4.46) | 1.30 | (1.23—1.37) | <0.0001 |
| Obesity | 3.29 | (3.09—3.50) | 1.05 | (0.98—1.12) | 0.179 |
| Congenital Defects | 3.70 | (2.88—4.67) | 2.53 | (1.94—3.24) | <0.0001 |
| Coagulation Defects | 1.18 | (1.01—1.37) | 1.27 | (1.08—1.49) | 0.004 |
Unadjusted odds ratios were estimated for each risk factor and AMI using simple logistic regression
Adjusted odds ratios were estimated for each risk factor and AMI, while controlling for the other risk factors, using multiple logistic regression
The adjusted odds ratio for the association between amphetamine abuse and AMI was higher in North Texas (adjusted odds ratio = 1.59; 95% CI = 1.12—2.19, p=0.012) than in South Texas (adjusted odds ratio = 1.29; 95% CI = 0.61—2.36, p=0.47). The omnibus multiple logistic regression using the STEPWISE function in SAS (slentry=0.05 and slstay=0.05) was performed separately in each Public Health Region (Figure 1H). Amphetamine abuse entered the predictive model of AMI only in Regions 3 and 4. In Region 4, along with amphetamine abuse, the covariates cocaine abuse, tobacco use, hypertension, and lipid disorders entered the model. With these covariates in the model, the adjusted odds ratio for amphetamine abuse in Region 4 was 2.64 (95% CI = 1.31—4.77, p=0.008). The Region 3 model also contained the covariates diabetes, alcohol, cardiac congenital defects, and coagulation defects in addition to those in the Region 4 model. The adjusted odds ratio for amphetamine abuse in Region 3, with these covariates in the model, was 1.68 (95% CI = 1.13—2.39, p=0.012). We retested the omnibus multiple logistic regression model (without STEPWISE) in Regions 3 and 4 combined and found an adjusted odds ratio for the occurrence of AMI with amphetamine abuse of 1.85 (95% CI = 1.32—2.51, p=0.0006). The adjusted odds ratio for amphetamine abuse in the omnibus multiple logistic regression of all Texas Public Health Regions combined, excluding Regions 3 and 4, was lower and not significant (adjusted odds ratio = 1.39; 95% CI = 0.92—2.01, p=0.112).
Post-hoc analysis of the variable “congenital heart defects” revealed that “cardiac anomalous arteries” primarily accounted for this variable’s significance in the omnibus multiple logistic regression model. If the cardiac anomalous arteries are removed from the variable “congenital heart defects” it becomes nonsignficant (adjusted odds ratio = 1.41; 95% CI = 0.96—2.00, p=0.081). Conversely, a variable indicating “cardiac anomalous arteries” in the omnibus model, excluding all other cardiac congenital defects, was highly significant (adjusted odds ratio = 9.08; 95% CI = 5.95—13.50, p<0.0001). In both of these models amphetamine abuse remained significantly associated with AMI (cardiac anomalous arteries removed, adjusted odds ratio = 1.61; 95% CI = 1.24—2.04, p=0.0004; exclusively cardiac anomalous arteries, adjusted odds ratio = 1.61; 95% CI = 1.25—2.05, p=0.0004).
3.3. Population Attributable Risk
In the state of Texas from 2000 to 2003 amphetamine abuse accounted for 0.2% of AMIs and cocaine abuse, for 1.9%. The percent of AMI accounted for by amphetamine abuse was ten times higher in North Texas (0.31%) than in South Texas (0.03%; Figure 1G). In Texas Public Health Regions 3 and 4 combined, the percent of AMI accounted for by amphetamine abuse was 0.46%.
4. Discussion
The current study examined the relationship between amphetamine abuse and AMI in all patients aged 18 to 44 years who were hospitalized in the state of Texas from 2000 to 2003. The findings indicate a modest, but significant, association between amphetamine abuse and AMI, which approximates the strength of the relationship between cocaine abuse and AMI in this Texas-based population. Our basic finding is consistent with the pharmacodynamic and pathophysiological mechanisms of amphetamine-associated AMI, including coronary artery vasospasm, catecholamine-induced platelet aggregation, and atherosclerotic plaque rupture as well as myocardial necrosis and increased myocardial oxygen demand (Bashour, 1994; Costa et al., 2001). Lending further plausibility to the pharmacodynamic and pathophysiological links are results from prior studies which found (1) that among medical examiner autopsies the rate of coronary artery disease was 3–4 times higher in deceased methamphetamine users than non-users (Karch et al., 1999), and (2) that methamphetamine use may cause cardiomyopathy in some users (Wijetunga et al., 2003; Yeo et al., 2007). Amphetamines cause the release of catecholamines from presynpatic nerve terminals—stimulating peripheral α- and β-adrenergic receptors—which commonly leads to tachycardia and hypertension (Mendelson et al., 2006).
The findings of the current study suggest that amphetamine abuse differs in geographical distribution across the state of Texas (which is different from that of cocaine abuse), which in turn corresponds with the geographical distribution of amphetamine-associated AMI in Texas, indicating that the need for medical surveillance may differ by Public Health Region in the state of Texas. In particular, we found that the association between amphetamine abuse and AMI is higher in North Texas than in South Texas (Figure 1G), with the highest rates found in Public Health Regions 3 and 4 (Figure 1I). One possible interpretation of this finding by geographical region in Texas is that areas with higher prevalence of amphetamine abuse (such as North Texas and Regions 3 and 4) might contain more “heavy” abusers, and that these “heavy” abusers are the ones most likely to experience AMI as a result of their abuse. Indeed, buttressing the supposition of regional variation of methamphetamine abuse within states, the January 2007 proceedings from the Community Epidemiology Work Group suggest that “in three States with large rural populations [Texas, New Mexico, and Maine], methamphetamine abuse indicators are increasing, especially in some areas of these states” (CEWG, 2007, p. 42). If this is plausible, geographical analysis by Public Health Region might prove useful as a further sentinel measure of the intensity of amphetamine abuse in other areas of the United States.
Among hospitalized patients aged 18 to 44 years in Texas from 2000 to 2003 the rate of amphetamine-associated AMI increased much faster than the rate of cocaine-associated AMI. This may be explained by the large relative increase in amphetamine abuse compared to cocaine abuse from 2000 to 2003 (75% versus 14%, respectively; Figure 2). Currently, while only a modest percentage of AMIs in young adults in Texas is related to amphetamine abuse, the population attributable risk is ten times higher in North Texas than in South Texas, and is highest in Texas Public Health Regions 3 and 4 (Figure 1I). In 2006, Texas ranked 27th among all U.S. states in methamphetamine use among adults aged 18 to 25 (SAMHSA OAS, 2006). While cocaine has been associated with AMI in more than 250 published cases (Hollander, 1996) and is believed to cause AMI through a variety of chronic and acute effects (Jones and Weir, 2006), to our knowledge, this is the first population-based epidemiologic study to examine the risk of AMI with amphetamine abuse. Further, while there have been case series and many case reports of anomalous coronary arteries associated with AMI and sudden death (Taylor et al., 1992; Taylor et al., 1997; Basso et al., 2000; Virmani et al., 2001; Kannam et al., 2005), we believe this is the first epidemiological study that supports this association.
The results of this study may be tempered by the nature of the study. The design and analysis, as in any quasi-experimental setting, cannot rule out the possibility that other risk factors besides amphetamine abuse either combined with or independently influenced the risk of AMI. The obvious risk factors, however, were evaluated and included in our multiple logistic regression analysis. Additionally, we note that the analysis on which the current study is based uses the ICD-9-CM diagnosis of amphetamine abuse and dependence, which is an umbrella diagnosis that includes methamphetamine, amphetamine, methylphenidate, and other stimulants. Thus, the association between methamphetamine abuse and AMI found in the current study must be interpreted within this context. Further, the exact temporal association between amphetamine abuse and AMI is not determinable in the THCIC database. Another concern is about the potential misclassification of diagnoses in an ICD-9-CM coded discharge database. Two validation studies, however, have shown that the hospital diagnosis of AMI is accurately captured by ICD-9-CM discharge diagnoses (Kiyota et al., 2004; Heckbert et al., 2004).
To our knowledge, no validation studies of cocaine and amphetamine abuse ICD-9-CM diagnoses have been conducted. The specificity of a drug abuse diagnosis, when recorded, is thought to be high due to the usual query on drug abuse in medical history taking and frequent use of toxicology screens. False negatives—those patients that have abused substances, but for whom no diagnosis was made—are missing information and may be distributed randomly or systematically with respect to the dependent variable, AMI. If distributed randomly, one expects to find a similar point estimate as compared to a database lacking missing information, but with widened confidence intervals. Two studies have demonstrated this: (1) in a comparison of hospital discharge data versus chart review among patients with percutaneous coronary interventions, hospital discharge data tended to underestimate comorbid conditions, but importantly, multivariate models of each source showed virtually identical adjusted risk ratios (Humphries et al., 2000), and (2) a more recent study, which compared hospital discharge data to chart review among heart failure patients, also found that multivariate odds ratios derived from the two data sources were similar. The authors concluded that missing comorbidity data are relatively random, resulting in non-differential misclassification and no appreciable information bias (Lee et al., 2005). While a prior risk factor analysis has used substance abuse diagnoses in administrative data (Bateman et al., 2006), it remains uncertain as to the extent their possible biases. Finally, broadly generalizing the results to populations beyond the state of Texas is beyond the scope of this study.
Despite these potential limitations, the current study has strengths. The current study population consists of all discharges in the State of Texas from January 1, 2000, to December 31, 2003, for persons aged 18 to 44 years. The large size of the THCIC database, thus, lends itself to strong external validity, and permitted us (through the large sample size) to attain ample statistical power to test the relationship between amphetamine abuse and AMI. While the THCIC database is subject to misclassification, like any other discharge database, the THCIC employs procedures to maximize the accuracy of data. These procedures include a standardized coding protocol, rigorous edit criteria required by law, and electronic auditing. Hospitals are mandated by state law to report to the THCIC, which operates under the auspices of the Texas Department of State Health Services. Importantly, the number of discharge diagnoses in the THCIC database does not limit the sensitivity of diagnosis reporting (Westover et al., 2007). Although the exact temporal link between drug use and AMI is not determinable in the THCIC database, by excluding drug abusers in remission, we were able to narrow the measure of drug abuse to active abusers.
5. Conclusions
To our knowledge, this is the first population-based epidemiologic study of hospital patients to examine the risk of AMI with amphetamine abuse. The findings indicate a modest, but significant, association between amphetamine abuse and AMI. The plausibility of the relationship between amphetamine abuse and AMI is bolstered by (a) numerous case reports (Orzel, 1982; Furst et al., 1990; Packe et al., 1990; Ragland et al., 1993; Costa et al., 2001; Sztajnkrycer et al., 2002; Turnipseed et al., 2003; Gandhi et al., 2005), (b) well-documented pathophysiological mechanisms, and (c) that cocaine, a drug with similar effects, is considered a cause of AMI. If rates of amphetamine abuse continue to increase, amphetamine-associated AMI may become more common in the acute care setting. Marked geographical variations in rates of amphetamine and cocaine abuse provide an opportunity for a geographical focus in surveillance and prevention efforts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Hospital Association. AHA guide to the health care field American Hospital Association. Chicago: 2004. [Google Scholar]
- Bashour TT. Acute myocardial infarction resulting from amphetamine abuse: a spasm-thrombus interplay? Am Heart J. 1994;128:1237–1239. doi: 10.1016/0002-8703(94)90757-9. [DOI] [PubMed] [Google Scholar]
- Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, Berman MF. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006;67:424–429. doi: 10.1212/01.wnl.0000228277.84760.a2. [DOI] [PubMed] [Google Scholar]
- Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- Community Epidemiology Work Group. [accessed December 14, 2007];National Institute on Drug Abuse; Epidemiologic Trends in Drug Abuse: Highlights and Executive Summary. 2007 http://www.drugabuse.gov/PDF/CEWG/Vol1_107.pdf.
- Costa GM, Pizzi C, Bresciani B, Tumscitz C, Gentile M, Bugiardini R. Acute myocardial infarction caused by amphetamines: a case report and review of the literature. Ital Heart J. 2001;2:478–480. [PubMed] [Google Scholar]
- Crump R, Shandling AH, Van NB, Ellestad M. Prevalence of patent foramen ovale in patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2000;85:1368–1370. doi: 10.1016/s0002-9149(00)00772-4. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Agency for Healthcare Research and Quality, 2007. [accessed November 2, 2007];Department of Health and Human Services Agency for Healthcare Research and Quality; Inpatient Quality Indicators Technical Specifications, Ver. 3.1. 2007 March; http://www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf.
- Furst SR, Fallon SP, Reznik GN, Shah PK. Myocardial infarction after inhalation of methamphetamine. N Engl J Med. 1990;323:1147–1148. doi: 10.1056/NEJM199010183231617. [DOI] [PubMed] [Google Scholar]
- Gandhi PJ, Ezeala GU, Luyen TT, Tu TC, Tran MT. Myocardial infarction in an adolescent taking Adderall. Am J Health Syst Pharm. 2005;62:1494–1497. doi: 10.2146/ajhp040220. [DOI] [PubMed] [Google Scholar]
- Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- Hollander JE. Cocaine-associated myocardial infarction. J R Soc Med. 1996;89:443–447. [PMC free article] [PubMed] [Google Scholar]
- Humphries KH, Rankin JM, Carere RG, Buller CE, Kiely FM, Spinelli JJ. Co-morbidity data in outcomes research: are clinical data derived from administrative databases a reliable alternative to chart review? J Clin Epidemiol. 2000;53:343–349. doi: 10.1016/s0895-4356(99)00188-2. [DOI] [PubMed] [Google Scholar]
- Jones JH, Weir WB. Cocaine-induced chest pain. Clin Lab Med. 2006;26:127–146. doi: 10.1016/j.cll.2006.01.010. viii. [DOI] [PubMed] [Google Scholar]
- Kannam HC, Satou G, Gandelman G, DeLuca AJ, Belkin R, Monsen C, Aronow WS, Peterson SJ, Krishnan U. Anomalous origin of the left main coronary artery from the right sinus of Valsalva with an intramural course identified by transesophageal echocardiography in a 14 year old with acute myocardial infarction. Cardiol Rev. 2005;13:219–222. doi: 10.1097/01.crd.0000131812.44224.2d. [DOI] [PubMed] [Google Scholar]
- Karch SB, Stephens BG, Ho CH. Methamphetamine-related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. J Forensic Sci. 1999;44:359–368. [PubMed] [Google Scholar]
- Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American Heart Journal. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P, Everhart ET, Jones RT. Human Pharmacology of the methamphetamine stereoisomers. Clin Pharmacol Ther. 2006;80:403–420. doi: 10.1016/j.clpt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Minor RL, Jr, Scott BD, Brown DD, Winniford MD. Cocaine-induced myocardial infarction in patients with normal coronary arteries. Ann Intern Med. 1991;115:797–806. doi: 10.7326/0003-4819-115-10-797. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Mintzer D, Maclure M, Tofler GH, Sherwood JB, Muller JE. Triggering of myocardial infarction by cocaine. Circulation. 1999;99:2737–2741. doi: 10.1161/01.cir.99.21.2737. [DOI] [PubMed] [Google Scholar]
- Orzel JA. Acute myocardial infarction complicated by chronic amphetamine use. Arch Intern Med. 1982;142:644. [PubMed] [Google Scholar]
- Packe GE, Garton MJ, Jennings K. Acute myocardial infarction caused by intravenous amphetamine abuse. Br Heart J. 1990;64:23–24. doi: 10.1136/hrt.64.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Guterman LR, Hopkins LN. Cocaine use and the likelihood of nonfatal myocardial infarction and stroke: data from the Third National Health and Nutrition Examination Survey. Circulation. 2001;103:502–506. doi: 10.1161/01.cir.103.4.502. [DOI] [PubMed] [Google Scholar]
- Ragland AS, Ismail Y, Arsura EL. Myocardial infarction after amphetamine use. Am Heart J. 1993;125:247–249. doi: 10.1016/0002-8703(93)90086-o. [DOI] [PubMed] [Google Scholar]
- Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32:81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Rovner A, Valika AA, Kovacs A, Kates AM. Possible paradoxical embolism as a rare cause for an acute myocardial infarction. Echocardiography. 2006;23:407–409. doi: 10.1111/j.1540-8175.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- Sastry S, Riding G, Morris J, Taberner D, Cherry N, Heagerty A, McCollum C. Young Adult Myocardial Infarction and Ischemic Stroke: the role of paradoxical embolism and thrombophilia (The YAMIS Study) J Am Coll Cardiol. 2006;48:686–691. doi: 10.1016/j.jacc.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Substance Abuse & Mental Health Services Administration. [accessed November 2, 2007];2005 National Survey on Drug Use & Health: National Results. 2006 http://oas.samhsa.gov/nsduh/2k5nsduh/AppG.htm.
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. [accessed November 2, 2007];State Estimates of Past Year Methamphetamine Use. 2006 http://www.oas.samhsa.gov/2k6/stateMeth/stateMeth.htm.
- Sztajnkrycer MD, Hariharan S, Bond GR. Cardiac irritability and myocardial infarction in a 13-year-old girl following recreational amphetamine overdose. Pediatr Emerg Care. 2002;18:E11–E15. doi: 10.1097/00006565-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: "high-risk" abnormalities in the initial coronary artery course and heterogeneous clinical outcomes. Am Heart J. 1997;133:428–435. doi: 10.1016/s0002-8703(97)70184-4. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20:640–647. doi: 10.1016/0735-1097(92)90019-j. [DOI] [PubMed] [Google Scholar]
- Texas Department of State Health Services. [accessed November 2, 2007];Texas Department of State Health Services; TCADA Treatment Assessment Database. 2003 http://www.tcada.state.tx.us/research/statistics/index.shtml.
- Texas Department of State Health Services. Center for Health Statistics. [accessed November 2, 2007];Texas Department Of State Health Services Center For Health Statistics; Texas Department Of State Health Services Center For Health Statistics–THCIC User Manual For Texas Hospital Inpatient Discharge Public Use Data File 2003. 2004 http://www.dshs.state.tx.us/THCIC/Hospitals/UserManual.pdf.
- Texas Department of State Health Services. Center for Health Statistics. [accessed November 2, 2007];Austin, Texas Department of State Health Services, Center for Health Statistics; Texas Hospital Inpatient Discharge Public Use Data File, calendar years 2000 to 2003. 2007 http://www.dshs.state.tx.us/thcic/Hospitals/HospitalData.shtm.
- Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med. 2003;24:369–373. doi: 10.1016/s0736-4679(03)00031-3. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. [accessed November 2, 2007];United Nations Office on Drugs and Crime; 2006 World Drug Report, Volume I: Analysis. 2006 http://www.unodc.org/pdf/WDR_2006/wdr2006_volume1.pdf.
- Virmani R, Burke AP, Farb A. Sudden cardiac death. Cardiovasc Pathol. 2001;10:211–218. doi: 10.1016/s1054-8807(01)00091-6. [DOI] [PubMed] [Google Scholar]
- Warnes CA. The Adult With Congenital Heart Disease: Born To Be Bad? Journal of the American College of Cardiology. 2005;46:1–8. doi: 10.1016/j.jacc.2005.02.083. [DOI] [PubMed] [Google Scholar]
- Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch Gen Psychiatry. 2007;64:495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, Alimineti K, Kimata C, Schatz IJ. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. doi: 10.1016/j.amjmed.2006.01.024. [DOI] [PubMed] [Google Scholar]