Abstract
Interleukin 13 (IL-13) is immunoregulatory in many diseases, including cancer. The protective or suppressive role of CD1-restricted natural killer T cells (NKT cells) in tumor immunosurveillance and immunity is well documented. Interleukin 12 (IL-12) can activate type I NKT cells to produce interferon-gamma (IFN-γ), whereas type II NKT cells may produce IL-13. The high-affinity chain of IL-13Rα2 may act as negative inhibitor, suppressing the action of IL-13 and helping to maintain tumor immunosurveillance. We constructed an mIL-13Rα2-Fc chimera in a eukaryotic expression vector and confirmed the identity of the recombinant protein by immunoblot analysis and binding to IL-13 in chemiluminescent ELISA. Such DNA vaccine was tested against syngeneic B16F10-Nex2 murine melanoma. In vivo experiments showed a protective effect mediated by high production of IFN-γ and down-regulation of anti-inflammatory interleukins mainly by NKT 1.1+ T cells. Biochemoterapy in vivo with plasmid encoding mIL-13Rα2-Fc in association with plasmid encoding IL-12 and the 7A cyclopalladated drug led to a significant reduction in the tumor evolution with 30% tumor-free mice. We conclude that IL-12 gene therapy, followed by continuous administration of IL-13Rα2-Fc gene along with 7A-drug has antitumor activity involving the high production of proinflammatory cytokines and low immune suppression, specifically by NK1.1+T cells producing IL-13 and IL-10.
Introduction
The challenge in the development of anticancer vaccines has been to elicit cellular immune responses that may effectively control tumor growth despite the negative regulatory mechanisms that are simultaneously induced. To increase the antitumor response, diverse modalities of gene therapy have been used, such as administration of genes encoding proinflammatory cytokines or inhibitors of immune suppressor components. Cells inducing immunosuppressive responses include CD4+CD25+ T-regulatory (T-reg) cells, conventional TH2 cells, CD1d-restricted natural killer T cells (NKT cells), myeloid suppressor cells, and M2 macrophages [1–5].
CD1d-restricted NKT cells have a dual role in tumor immunity, depending on different cell subsets [6]. They have been implicated in the down-regulation of immunosurveillance in tumor models [3,4,7] and in the promotion of antitumor immunity [8–10]. These opposing activities are explained by the recruitment of selected NKT subpopulations producing different cytokine profiles, depending on the stimulus or microenvironment. Dendritic cells (DCs) pulsed with α-galactosylceramide (α-Gal-Cer) inhibited metastasis in experimental melanoma [11]. The Vα14-Jα18 NKT cells were the first to be activated after administration of IL-12, displaying in vitro cytotoxicity in B16 tumor cells and in vivo protection against the subcutaneous growth and pulmonary colonization by B16 murine melanoma [12].
In contrast, Ahlers et al. [13] found that the suppression of a cytotoxic T lymphocytes-inducing vaccine apparently was due at least in part to NKT cells, because they were able to enhance vaccine efficacy by blockade of IL-13 or by using CD1-deficient mice that lack NKT cells. Terabe et al. [14] showed that, in the absence of both type I NKT cells and T-reg cells, type II (non-Vα14Jα18+) NKT cells were responsible for suppression of immunosurveillance. Therefore, NKT cells can regulate positively or negatively the immune response, and a strategy that would involve stimulation of NKT type I subpopulation (e.g., by administering IL-12) and suppression or control of NKT type II cells might render a more efficient antitumor immune response.
NKT type II cells regulate negatively the immune response probably through the production of IL-13. First described in 1993 [15], IL-13 is secreted preferentially by activated TH2 lymphocytes and NKT cells, but macrophages, DCs, NK cells, mast cells, and basophils can also produce it. This interleukin inhibits inflammatory cytokine and chemokine production, up-regulates MHC class II expression and CD23 on monocytes [15,16], increases the expression of VCAM-1 on endothelial cells [17], and promotes B-cell proliferation and IgE class switching [18,19]. It plays crucial roles in the pathophysiology of allergic asthma, helminthiasis, autoimmune disorders, and chronic diseases [20]. IL-13 signaling requires binding to the IL-13Rα1 receptor, which then forms heterodimers with the IL-4 receptor (IL-4Rα) [21–23]. IL-13, however, shows higher affinity binding to the α2 chain of the IL-13 receptor (IL-13Rα2), which may function as a decoy receptor and is important to downregulate a TH2-mediated immune response [24]. This chain is unable of signaling because it has a short cytoplasmic tail and does not activate the STAT6 pathway [25]. However, Fichtner-Feigl et al. [26] found that IL-13 binding to IL-13Rα2 may activate AP-1 transcriptional factor to induce secretion of transforming growth factor beta (TGF-β).
The role of IL-13 on tumor immunity seems to be complex and may depend on both the tumor type and the genetic background of the host. Previous studies have shown that IL-13 enhanced antitumor responses in some model systems [27] or did not affect tumor growth [28,29]. Conversely, mIL-13Rα2-Fc prevented IL-13-mediated suppression of tumor immunosurveillance [3,14]. In a 15-12RM fibrosarcoma model of tumor recurrence, the authors showed that CD8+ CTL-mediated tumor elimination was suppressed by IL-13 produced by CD1d-restricted T cells and activated IL-4Rα-STAT6 signaling pathway. IL-4αR knockout (KO) and STAT6 KO mice but not IL-4 KO mice were resistant to tumor recurrence. When these IL-4 KO mice were treated with soluble inhibitor of IL-13, they became resistant to tumor recurrence indicating that IL-13 was responsible for the suppression of tumor immunosurveillance in this model. Moreover, CD1d-KO mice were also resistant to tumor growth because they lack NKT cells hence did not produce IL-13. In a metastasis model of colon carcinoma, the same mechanism was observed where treatment with soluble protein IL-13Rα2-Fc diminished the number of metastasis [7]. An effector mechanism in this suppressive pathway was proposed by Terabe et al. [4], who showed that CD11b+ Gr-1+ myeloid cells produced TGF-β by a mechanism dependent on the presence in vivo of both IL-13 and CD1d-restricted T cells. Because T cells do not respond to IL-13 [16], other cells are stimulated to produce TGF-β that can be the cytokine responsible for the inhibition of CTL activity. Now, this hypothesis can be examined by studying IL-13Rα2 chain function in macrophages responding to IL-13 [26,30].
Therapy with soluble mIL-13Rα2-Fc leads to antitumor response in models where type II NKT cells inhibit natural tumor immunosurveillance by a mechanism involving IL-13, whereas IL-12 stimulates several antitumor pathways, including type I NKT activation. Here, we associated gene therapy with chemotherapy by using a cyclopalladated drug (7A) that has been shown to be protective in mice challenged subcutaneously with B16F10-NEX2 melanoma cells [31]. The combination of gene therapy and chemotherapy conferred increased protection against melanoma with 30% mice free of tumor at the end of experiment.
Materials and Methods
Cell Lines and Reagents
B16F10-Nex2 is a subline from B16F10 murine melanoma [32], isolated at the Experimental Oncology Unit (UNONEX). It is characterized by low immunogenicity and moderate virulence. The melanoma cells were maintained in culture in RPMI 1640 medium pH 7.2, supplemented with 10% heat-inactivated fetal calf serum, 10 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid), 24 mM NaHCO3, all from GIBCO (Minneapolis, MN), and 40 mg/ml gentamycin sulfate (Hipolabor Farmacêutica, Sabará, MG, Brazil). Monoclonal antibodies (mAbs) conjugated with phycoerythrin (PE) against mouse CD3, CD4, and CD8, mAbs conjugated with flourescein isothiocyanate (FITC) against mouse NK1.1 and F4/80, and mAbs biotinylated against cytokines were all purchased from PharMingen (San Diego, CA).
Mice
Inbred male 6- to 8-week-old C57BL/6 mice were purchased from Centro de Desenvolvimento de Modelos Experimentais at Federal University of São Paulo (UNIFESP). All animal experiments were approved by the Animal Experimental Ethics Committee of UNIFESP, protocol number 1340/2003. In all experiments, 10 mice were used per group.
Construction of the mIL-13Rα2-Fc DNA Vaccine
To construct the mIL-13Rα2-Fc (murine IL-13 receptor alpha-2/IgG2a Fc fusion protein) chimera, the sequence corresponding to the extracellular domain of the receptor (amino acids 1–332) was obtained from macrophage total RNA, whereas those from Fc regions (CH2–CH3) were amplified from hybridoma 17C [33], both using reverse transcription-polymerase chain reaction (RT-PCR). RNA extractions from mouse peritoneal macrophages pretreated with IL-13 and from hybridoma 17C were carried out using Trizol reagent (Invitrogen Brasil, São Paulo, Brazil), following the manufacturer's instructions with minor modifications. Reverse transcription-polymerase chain reaction was performed using “Thermo Script RT-PCR System” (Invitrogen) and oligo dT, according to themanufacturer's instructions. DNA was eliminated after treatment with DNase I (Rnase-free; Amersham GE Healthcare, Piscataway, NJ), and controls without reverse transcription were included in all reactions. The PCR mixture consisted of 1/10 of the RT reaction, Taq buffer (20 mM Tris-HCl pH8.4, 50mMKCl), 200 µM deoxynucleoside triphosphates (dNTP), 1.5 mMMgCl2, 2.5 U Taq DNA polymerase (Invitrogen), and 1 µM of each primer. The oligonucleotide primers used to amplify mIL-13Rα2 and Fc region were as follows: 5′ -d GTC GAC ATG GCT TTT GTG CAT ATC AGA TGC- 3′ (forward, P1); 5′ -d TCC GGA GCC CTT TGA GTC TGG CCC TGT GTA- 3′ (reverse, P2) and 5′ -d GGC TCC GGAM GCA CCT AAC CTC TTG GGT G- 3′ (forward, P3); 5′ -d TCTAGATCATTTACC CGG AGT CCG GGA- 3′ (reverse, P4). The mIL-13Rα2 and Fc coding sequences were amplified after 35 cycles at 94°C for 1 minute, 59°C (or 60°C for Fc) for 2 minutes, and 72°C for 1 minute. Each fragments was cloned into a pGEMT easy vector (Promega, Madison, WI), the inserts were automatically sequenced, and the sequences compared with those available in the GenBank (IL-13Rα2, gi = 6680404; CH2–CH3 IgG2a, gi = 51767065 and gi = 406252). Nucleotide sequencing was carried out in the facilities of the Center of Human Genome at São Paulo University (USP). To generate the final construct (1656 bp) encoding IL-13Rα2 fused in frame with the Fc region through the spacer Gly-Ser-Gly, we followed a modified PCR overlap technique [34] using both plasmids as template (10 ng in 25 µl of reaction mixture). The first round of PCR (35 cycles at 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 2 minutes) was run in the presence of 0.4 µM of each internal primer (P2, P3), 1 µM of each external primer (P1, P4), Taq buffer, 1.5 mM MgCl2, 200 µM of each dNTP, and 2.5 U of platinum Taq polymerase (Invitrogen). Reamplification of the product, used as template at 1:100, took only external primers, at an annealing temperature of 58°C. Polymerase chain reaction products were purified using “Bioclean for Purification of DNA Bands” (Biotools, Brazil) and cloned into a pGEM-T vector. The sequence of the final construct was confirmed by automatically sequencing the insert in both directions using sense T7 and antisense SP6 vector primers. The insert was then subcloned into a Sal I restriction site of a VR1012 vector (Vical Co., San Diego, CA), which was used to transform DH5α bacteria by heat shock. Plasmids from a selected clone were purified in CsCl gradient with ethidium bromide after ultracentrifugation at 500,000g for 16 hours (VTi 90 rotor; Beckman, Fullerton, CA), followed by washing with saturated butanol (to remove bromide). Plasmids were precipitated with ethanol 100% for 3 days, washed twice with ethanol 70%, and then resuspended in sterile phosphate-buffered saline (PBS).
Production and Analysis of the Recombinant Protein IL-13Rα2-Fc
B16F10-Nex2 tumor cells were transiently transfected with 5 µg of the expression plasmid with lipofectin (Invitrogen). Culture supernatants that contained secreted mIL-13Rα2-Fc were filtered through Millipore Millidisk (0.22 µm) and purified through protein G Sepharose affinity chromatography (Pharmacia, Uppsala, Sweden). The solid phase was equilibrated with PBS pH 7.4, washed with PBS, and eluted with 0.1 M glycine, pH 2.8. The eluate was neutralized by 1:5 volume of Tris-HCl 1 M, pH 9.0. The final product was examined by reducing SDS-PAGE stained with silver nitrate and its identity confirmed by immunoblot analysis. Briefly, the sample was reduced with DTT, separated in 8% SDS-PAGE, and then transferred onto a nitrocellulose membrane (0.2 µm; Amersham Bioscience, England) by electroblot analysis. The blot was blocked with PBS containing 5% dry skim milk and then incubated overnight at 4°C with biotinylated polyclonal goat antibody to murine IL-13Rα2 (0.2 µg/ml; R&D Systems, São Paulo, Brazil) diluted in PBS-1% BSA (bovine serum albumin). After three washes with 0.05% Tween 20 in PBS (PBST) and once with PBS, horseradish peroxidase-conjugated streptavidin (1:1000; Jackson Immuno-Research Laboratories, West Grove, PA) was added (1 hour at 37°C). The blot was developed with 5 mg of DAB (Sigma, São Paulo, Brazil) and 100 µl of H2O2 in PBS and blocked with distilled water. The biologic activity (high and specific interaction with IL-13) was assessed in a chemiluminescent enzyme-linked immunosorbent assay (ELISA). Briefly, white immulon-2 plates (Nunc, Roskilde, Denmark) were coated with recombinant murine IL-13 (2 µg/ml; Peprotech, Ribeirão, Brazil) in PBS overnight. Plates were washed with PBST and blocked with PBS- 1% BSA (3 hours at 37°C). Purified supernatant from transfected cells diluted in PBS-1% BSA was added and incubated for 3 hours at 37°C. Biotinylated goat antimouse IL-13Rα2 (1 µg/ml; R&D Systems) was added and incubated overnight at 4°C. Peroxidase-labeled streptavidin (1:1000; 1 hour at 37°C) was used to detect biotinylated Ab. Finally, 1:50 ECL (Enhanced Chemiluminescent detection kit; Amersham Pharmacia Biotech) was added, diluted in 0.5 M carbonate-bicarbonate buffer pH9.6. Readings (relative luminescent units) were recorded in a luminometer (Cambridge Technology, Auburn, CA). The concentration of mIL-13Rα2-Fc in the sample was determined from a serial-fold diluted standard mIL-13Rα2-hIgG1 (R&D Systems). Controls with empty vector were used in all experiments.
IL-12 Plasmid
The expression vector encoding IL-12 was provided by Alexander Rakhmilevich [35] from the University of Wisconsin and tested for in vitro activity before gene therapy. The plasmid (pWRG3169) contains sequences encoding the p35 and p40 subunits of murine IL-12, localized in opposite direction and each driven by its own cytomegalovirus (CMV) i/e promoter/enhancer, simian virus 40 sd/sa intron sequence, bovine growth hormone polyadenylation sequence, and ampicillin-resistant gene. A control vector containing luciferase cDNA under the CMV promoter was constructed as described by Cheng et al. [36].
To confirm the production of IL-12, this plasmid was used to transfect B16F10-Nex2 tumor cells as described above, followed by ELISA of the supernatant. The biologic activity of the recombinant IL-12 was tested by production of nitric oxide (NO) in a macrophage activation assay. Nitric oxide was quantified as nitrite by Griess reagent.
Cyclopalladated Drug
The cyclopalladated drug 7A was synthesized from N,N-dimethyl-1-phenethylamine, complexed to 1,2 ethanebis(diphenylphosphine) ligand, and was active in vitro and in vivo against B16F10-Nex2 murine melanoma cells as previously described [31].
In Vivo Experiments
C57Bl/6 male mice were injected subcutaneously in the right flank with 5 x 104 B16F10-Nex2 viable tumor cells, and on day 2, the animals were vaccinated with pIL-12 (100 µg per animal) or with the empty plasmid, intradermally at the tail base. The treatment with 10 µM of the 7A drug started 4 days later, three times a week by intraperitoneal route, until the tumor volume reached 2 cm3. On day 5 and subsequently at 5-day intervals, the animals received the pIL-13R vaccine (VR1012 vector + mIL-13Rα2-Fc) or the respective empty plasmid (100 µg/animal), i.d. at the tail base, until day 30. The therapeutic efficacy was evaluated by tumor growth and survival index. The tumor volume was measured every 3 days using a caliper according to the formula: V = 0.52 x D1 2 x D3, where D1 and D3 are the short and long diameters, respectively. All experiments included 10 mice per group, and the animals with maximum 3 cm3 tumor size were killed. Survivals of mice were scored and statistically compared (Kaplan-Meier log rank test).
Flow Cytometry
A possible correlation between the immune protection of treated animals and the leukocyte populations producing cytokines (IFN-γ, IL-12, IL-10, IL-6, IL-4, IL-13, IL-2, TNF-α, and TGF-β) was assessed by using fluorescence-activated cell sorting (FACS). All mice (control or vaccinated) were evaluated individually, and when the tumor diameter reached a maximum of 3 cm3, splenocytes were collected. T CD4+, T CD8+, F4/80+, andNK1.1+T cells (CD3+NK1.1+, CD4+NK1.1+ or CD8+NK1.1+) and their intracellular cytokine production were immediately analyzed ex vivo without restimulation. Briefly, splenocytes were harvested and erythrocytes were lysed by osmotic shock (0.1 M NH4Cl, pH 7.2). Cells were incubated with PBS-1% BSA for 10 minutes on ice, washed twice in 1% PBS, and the Fc receptors were blocked with inactivated mouse serum for 1 hour on ice.Monoclonal antibodies anti-CD3, CD4, CD8, F4/80 and NK1.1 were added, and the samples were incubated for 1 hour on ice in the dark. Cells were then washed and permeabilized with saponin buffer (0.5% saponin, 1% paraformaldehyde in PBS). After incubation with inactivated mouse serum for 1 hour on ice, the cells were incubated with biotinylated antibodies against cytokines, followed by incubation with FITC-conjugated streptavidin, PE-conjugated streptavidin or allophycocyanin (APC)-conjugated streptavidin (all from PharMingen). Controls with these reagents alone were run in all assays. Cells were then fixed with 2% paraformaldehyde in PBS (wt/vol) and surface and intracellular fluorescence was measured using a FACS Calibur flow cytometer (BD Biosciences, São Paulo, Brazil). Data were collected for 10,000 viable cells and analyzed using CellQuestPro software (Becton Dickinson, San Jose, CA).
Statistical Analysis
Significant differences were assessed using Student's t test. All experiments were conducted two or more times. Reproducible results were obtained, and representative data are shown. The survival plots were analyzed by Kaplan-Meier log rank test. In both tests, the differences were considered statistically significant when P < .05. Cytokine production relating treated and untreated animals was evaluated by ANOVA, nonparametric Dunn test.
Results
Construction of the mIL-13Rα2-Fc DNA Vaccine
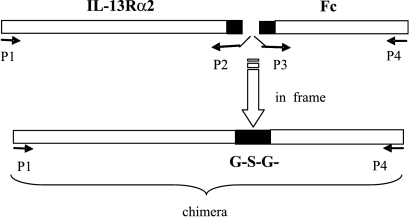
The coding sequence to the extracellular domain of the IL-13 receptor was cloned from total RNA of macrophages pretreated with IL-13 for 48 hours [37] and RT-PCR generated a fragment of 1002 bp, whereas the coding sequences to the Fc regions (CH2–CH3) were cloned from total RNA of hybridoma 17C [33]. Our PCR conditions could not detect the expression of the IL-13Rα2 chain in nonstimulated macrophages. A modified PCR overlap technique was used to generate the final construct, IL-13Rα2 fused in frame with Fc region and with a Gly-Ser-Gly spacer, as shown in Figure 1. The final construct was confirmed by sequencing, subcloned into the Sal I restriction site of a VR1012 expression vector, and was called pIL-13R. BamH I restriction showed the correct direction of the insert (not shown). Plasmids were purified through CsCl gradient with ethidium bromide and used for in vitro transfection and in vivo experiments.
Figure 1.
Scheme of DNA construction to produce the mIL-13Rα2-Fc chimera using overlap PCR. The complete sequences that encode the extracellular domain of the IL-13Rα2 receptor (1002 bp) and the Fc region (654 bp) were joined by a fragment encoding Gly-Ser-Gly. PCR overlap was done with primers P1 to P4, followed by a final PCR with external primers to increase the amount of the 1665-bp chimera.
Recombinant Protein IL-13Rα2-Fc Is Efficiently Secreted and Preserves Its Biologic Function After Transfection of Tumor Cells
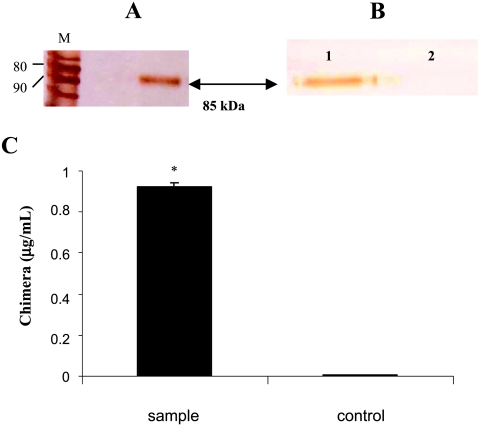
B16F10-Nex2 tumor cells were transiently transfectedwith pIL-13R, and culture supernatants that contained secreted mIL-13Rα2-Fc were filtered and purified through protein G Sepharose chromatography. The eluate was examined by reduced SDS-PAGE developed with silver nitrate, showing a recombinant protein of approximately 85 kDa (Figure 2A). Immunoblot analysis was carried out to confirmits identity (Figure 2B). The biologic activity (binding to IL-13) was evaluated by chemiluminescent ELISA test. The sensitivity of this assay was 250 ng/ml, and the concentration of IL-13Rα2-Fc in the sample was determined from serial dilution of a standard commercial mIL-13Rα2-hIgG1 (R&D Systems). The sample had 0.92 µg/ml of mIL-13Rα2-Fc (Figure 2C). Controls with supernatants obtained after transfection with the empty vector were used in all experiments.
Figure 2.
Production and analysis of recombinant mIL-13Rα2-Fc chimera. (A) SDS-PAGE in reducing conditions of recombinant protein of approximately 85 kDa (arrow). The protein was analyzed after purification in protein G Sepharose of the supernatant obtained from transfected B16F10-Nex2 tumor cells after 48 hours of gene expression. M, protein ladder from Invitrogen; the 80- and 90-kDa bands are indicated. (B) Immunoblot analysis showing recombinant protein (chimera IL-13Rα2-Fc) in lane 1 reacting with anti.IL-13Rα2 after staining with DAB reagent. Negative control in lane 2 (supernatant from transfected cells with empty plasmid). (C) Bioactivity (binding to IL-13) of the chimera obtained as described in (A) in chemiluminescent ELISA. The sample (black bar) had 0.92 µg/ml, according to standardization with commercial chimera (R&D Systems). The control is the supernatant obtained from transfected B16F10-Nex2 tumor cells with empty plasmid. *P < .0005, Student's t test.
IL-12 Plasmid
The expression vector encoding IL-12 (pIL-12) was initially tested in vitro in transfected B16F10-Nex2 tumor cells, followed by ELISA of the supernatant to evaluate IL-12 production. A high production of IL-12, ca. 7 ng/ml protein, was obtained. The secreted IL-12 was biologically active, as judged by its ability to induce NO production in macrophages from C57Bl/6 mice. Positive controls with lipopolysaccharide and IFN-γ were used in all experiments, and negative controls consisted of macrophages stimulated with supernatant from cells transfected with the empty vector or untreated. Macrophages stimulated with 30% of the supernatant obtained after transfection with pIL-12 showed a production of 57 µM NO, after 72 hours in culture (data not shown).
Gene Therapy and Association with Drug 7A Effectively Protected Mice Challenged with B16F10-Nex2 Cells Resulting in Delayed Tumor Growth and 30% Tumor-Free Animals
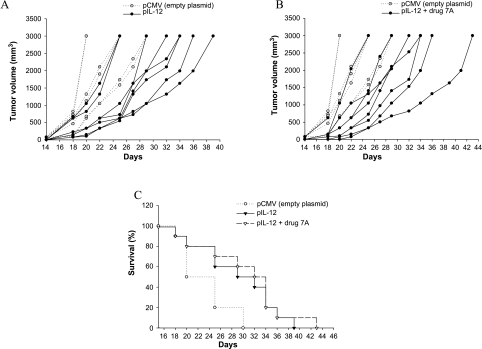
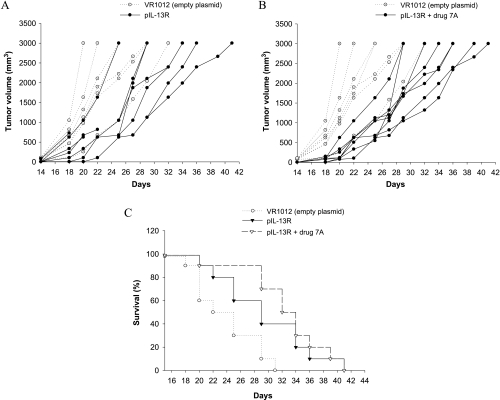
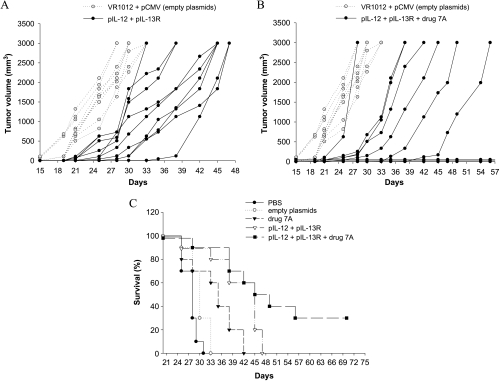
C57Bl/6 male mice were injected subcutaneously with 5 x 104 B16F10-Nex2 tumor cells and were vaccinated with pIL-12 alone, pIL-13R alone, together, and in association with the cyclopalladated drug 7A. Treatment in vivo with pIL-13R or pIL-12 alone protected mice significantly prolonging their survival (Figures 3 and 4). DNA vaccine with pIL-12 prolonged survival to day 39, and the association with drug 7A to day 43. DNA vaccine with pIL-13R was also protective, resulting in increased survival compared to the untreated control animals until day 41 when administered alone or associated with drug 7A. The combined therapy with pIL-12 or pIL-13R and drug 7A significantly delayed tumor growth and was more efficacious (P < .05) than the therapy with plasmid alone, controlled by vaccination with the empty plasmid. Mice injected with drug 7A alone were also partially protected and survived until day 42. By administering both DNA vaccines associated with drug 7A, the best results were obtained with protection of 30% of mice that remained tumorfree until the end of the experiment (Figure 5). In the group vaccinated with both plasmids, animals survived until the 47th day. In all experiments, mice of the control groups died before day 34. Four control groups were always used, which were vaccinated with both empty plasmids, with only one empty plasmid (pCMV or VR1012) and PBS. No significant differences were observed among these control groups.
Figure 3.
Therapeutic effect of pIL-12 against subcutaneous murine melanoma. (A) Tumor development in animals vaccinated with pIL-12. (B) Combined treatment with pIL-12 and drug 7A. (C) Increased survival of IL-12 and IL-12 + drug 7A-treated mice. C57Bl/6 mice were injected with 5 x 104 B16F10-Nex2 tumor cells, and on day 2, the animals were vaccinated with pIL-12 or plasmid with drug 7A. Drug 7A was administered i.p. three times a week, 10 µM per animal. Control groups were vaccinated with empty plasmid. For each group, n = 10; Kaplan-Meier test for the control and vaccinated groups with pIL-12 (P = .023) or pIL-12 + drug 7A (P = .009).
Figure 4.
Therapeutic effect of pIL-13R against subcutaneous murine melanoma. (A) Tumor development in animals vaccinated with pIL-13R. (B) Combined treatment with pIL-13R and drug 7A. (C) Increased survival of pIL-13R and pIL-13R plus drug 7A-treated mice. C57Bl/6 mice were injected with 5 x 104 B16F10-Nex2 tumor cells, and on days 5, 10, 15, 20, 25 and 30, the animals were vaccinated with pIL-13R (plasmid VR1012 containing the insert IL-13Rα2-Fc; (A). The same protocol was used in association with drug 7A (B). Drug 7A was administered i.p. three times a week, 10 µM per animal. Control groups were vaccinated with empty plasmid. For each group, n = 10. Kaplan-Meier test for the control and the vaccinated groups with pIL-13R (P = .0298) or with pIL-13R + drug 7A (P = .0005).
Figure 5.
Therapeutic effects of gene therapy with and without cyclopalladated drug 7A against subcutaneous murine melanoma. (A) Tumor development in animals vaccinated with pIL-12 plus pIL-13R or (B) both plasmids in association with drug 7A. (C) Survival curves with 30% tumor-free animals in the biochemotherapy protocol. C57Bl/6 mice were injected with 5 x 104 B16F10-Nex2 tumor cells; on day 2, the animals were vaccinated with pIL-12; and on days 5, 10, 15, 20, 25, and 30 with pIL-13R. Drug 7A was administered i.p., three times a week, 10 µM per animal. Control groups were vaccinated with the empty plasmid. For each group, n = 10. Kaplan-Meier test for the control group (empty vectors) and the vaccinated group with both plasmids (P = .0005) or the vaccinated group with both plasmids + drug 7A (P = .0001).
Gene Therapy Associated with Drug 7A Induced CD4+ and CD8+ T Cells as well as F4/80+ Cells Producing Mainly Type I Proinflammatory Cytokines
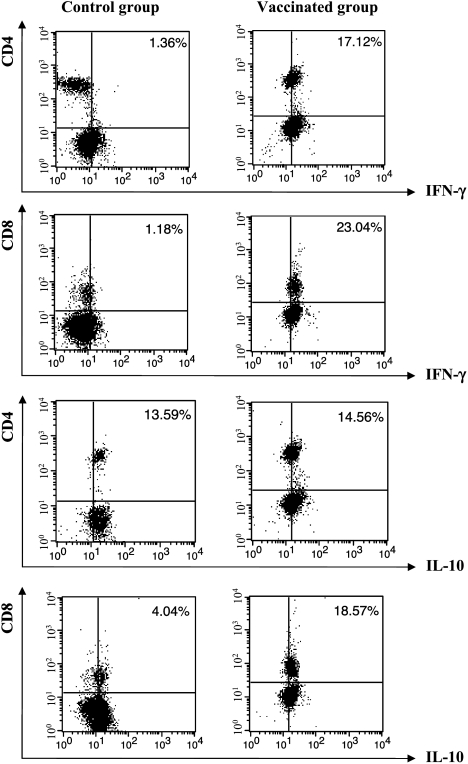
The immune response of mice to gene therapy was assessed by FACS of splenocytes to measure the expression of surface markers and intracellular interleukins. The results for T lymphocytes are shown in Figure 6. Untreated, tumor-bearing animals had 2.5% CD4+ and CD8+ T cells producing IFN-γ compared to 17.6% cells producing IL-10. Data are representative of three independent experiments with similar results.
Figure 6.
Analysis of T lymphocyte population using FACS from tumor-bearing animals untreated (control group) and vaccinated with both plasmids in association with drug 7A. Cells were labeled with PE-conjugated anti-CD4 mAb or PE-conjugated anti-CD8 mAb. After permeabilization, intracellular detection of the cytokines was carried out with anti-cytokine biotinylated antibody and labeling with FITCconjugated streptavidin. Data are shown as percentages of double-positive cells (CD4/CD8 and IFN-γ/IL-10) and are representative of three independent experiments with similar results.
Nonvaccinated tumor-bearing mice showed a mixed cytokine profile, with cells producing IFN-γ, IL-6, IL-2, and TNF-α fewer than cells producing IL-10, IL-13, TGF-β, and IL-4 showing a tendency toward immunosuppression. Mice challenged with tumor cells and submitted to biochemotherapy (gene therapy + drug 7A) showed an increased frequency of cells producing proinflammatory IFN-γ, IL-6, IL-2, TNF-α, and IL-12 (Table 1). Cells producing IFN-γ had a relative increase per 100 cells by 15.8-fold. Comparatively, the CD4+ and CD8+ T cells producing IFN-γ represented 40.9% compared to 33.6% of IL-10-producing cells (Figure 6). Other cytokines were also produced such as TNF-α (42.1%), IL-6 (41.0%), and IL-2 (39.5%) with a balance toward a proinflammatory type I response. As expected, treatment also increased the number of F4/80+ cells producing IL-12 (25.1%), more than eightfold compared to cells from untreated mice (3.0%) and sevenfold more IL-6 as well. Cells producing IL-4, TGF-β, and IL-13 were also represented with 12.1%, 29.5%, and 31.9%, respectively. Therefore, the combined gene therapy and drug treatment, although showing a marked proinflammatory immune response, also produced type II cytokines. The inflammation induced by DNA vaccines is then controlled by immunoregulatory cytokines. It seems that IFN-γ production is the most important cytokine for a protective effect against melanoma cells [38] and that the IFN-γ/IL-10 ratio must be >1 as in the vaccinated mice.
Table 1.
Increase with Biochemotherapy for Cytokine Expressing Cells Defining a Proinflammatory Response with Low Type II Interleukins.
| Cytokine Expression | Population Fold-Increase Relative to 100 Cells (95% Confidence Intervals)* |
| 1. CD4+ plus CD8+ cells | |
| IFN-γ† | 16.67 (2.938–30.408) |
| IL-2 | 3.0 (1.468–4.576) |
| IL-6† | 18.85 (2.418–27.297) |
| TNF-α | 2.49 (1.485–3.498) |
| IL-4‡ | 1.21 (0.938–1.473) |
| IL-13 | 3.0 (1.866–4.265) |
| TGF-β | 3.56 (1.086–6.049) |
| IL-10 | 1.64 (1.007–2.276) |
| 2. F4/80+ cells | |
| IL-12 | 5.35 (2.898–7.795) |
| IL-6 | 6.29 (3.092–9.502) |
| TNF-α | 2.96 (1.764–4.172) |
Cytokine rates from cells of 11 pairs of treated and control (untreated) animals.
Significant differences between IFN-γ and IL-4 and IL-10 (P < .001) or IL-6 and IL-4 and IL-10 (P < .001 and P < .01, respectively).
Only CD4+ cells.
NK1.1+ T Cells Increase IFN-γ Production in Mice Given the Combined Therapy
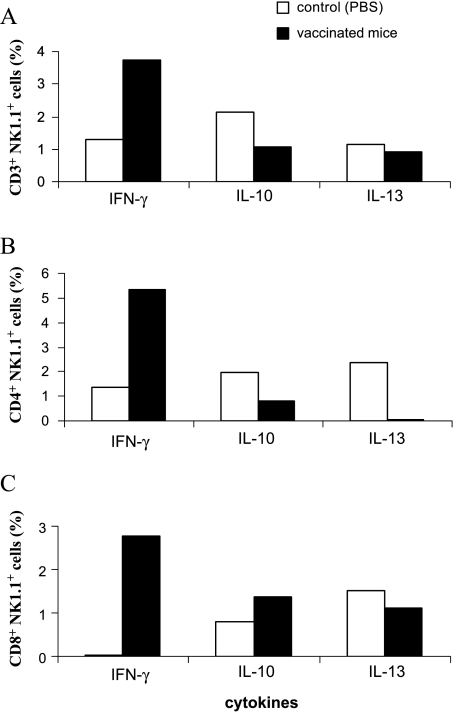
Among splenocytes, populations of NK1.1+ T cells (CD3+NK1.1+, CD4+NK1.1+, and CD8+NK1.1+ cells) were also evaluated for their ability to produce IFN-γ, IL-10, and IL-13. NK1.1+ T cells include classic NKT cells (type I cells), nonclassic NKT cells (type II cells), and NKT-like cells. CD3+NK1.1+ cells included CD4/CD8 double-negative cells (CD4-CD8-NK1.1+). Cells were labeled simultaneously with three fluorescent conjugates, and the results are summarized in Figure 7. The combined therapy used in the present work induced IFN-γ production and down-regulated IL-10 and IL-13. Accordingly, NKT cells from vaccinated mice produced much more IFN-γ than those from untreated tumor-bearing mice (Figure 7). Seemingly, a polarized immune response was induced. Type I NKT cells predominated in mice given biochemotherapy and were responsible for IFN-γ production, whereas untreated mice had more type II NKT cells that were responsible for IL-13 and IL-10 production. In these subpopulations, CD4+NK1.1+ cells were the main source of IL-13 in tumor-bearing mice, and after therapy, this production was abolished (Figure 7B). Cellswith both markers (CD4+NK1.1+) alsoproduced more IFN-γ than other subpopulations in vaccinated mice.
Figure 7.
Cytokine expression in NK1.1+ T cells by using FACS. The splenocytes were obtained from tumor-bearing animals vaccinated with both plasmids in association with drug 7A (black bars) or injected with PBS (white bars). (A) Cells were labeled with FITC-conjugated anti-NK1.1 monoclonal antibody (mAb) and PE-conjugated anti-CD3 mAb or (B) PE-conjugated anti-CD4 mAb or (C) PE-conjugated anti-CD8 mAb. Intracellular detection of cytokines in permeabilized cells was carried out with anti-cytokine biotinylated antibody and labeled with APC-conjugated streptavidin. Data are representative of three independent experiments with similar results and show the percentage of the positive labeled cells for each cytokine within a defined subpopulation.
Discussion
In the present work, we report on the protective effect in vivo of gene therapy with plasmids expressing IL-12 and IL-13Rα2-Fc in a murine melanoma model. A protocol of biochemotherapy was then introduced combining immunization with both plasmids and chemotherapy with a cyclopalladated drug (7A). Biochemotherapy is a term generally used for combined treatment of biologic agents such as cytokines, antigen-pulsed DCs or lymphocytes, and chemotherapeutic drugs.
Clinical trials focusing on malignant melanoma have tested IL-2, IFN-α, and lymphokine-activated killer cells stimulated with IL-2 [39,40]. These agents showed reproducible antitumor effects but with low efficacy. Perhaps, administration of inflammatory cytokines alone (and other immune activators) is not sufficient to cause tumor regression in the presence of suppressor elements that regulate the immune response leading to a poor in vivo CTL activity. The administration of proinflammatory cytokines and blockade of immunosuppressive components could be essential to the control of tumor development thus increasing the efficacy of antitumor vaccines [41]. Here, we used gene therapy with genes encoding IL-12 and IL-13-receptor associated with chemotherapy. This combined strategy induced a protective immune response and was successful in prolonging animal survival in the B16F10-Nex2 melanoma model.
Cytokine IL-12 included in the gene therapy protocol mediates effector mechanisms of both innate and adaptive immunity to render antitumor resistance. IFN-γ and many other secondary proinflammatory cytokines induced by IL-12 have a direct toxic effect on the tumor cells and may act as antiangiogenic elements [42,43]. Mechanisms responsible for IL-12-mediated tumor rejection in several experimental models have been investigated showing that IL-12 uses a variety of effector pathways involving NK, NKT, CD4+, and CD8+ T cells. IL-12 pretreatment enhanced immunotherapy with low doses of IFN-α, and this effect was dependent on endogenous IFN-γ production [44]. Gene gun therapy with plasmid encoding IL-12 resulted in complete tumor regression or suppression of tumor growth in six tumor models, including murine B16 melanoma [35]. The involvement of CD8+ T cells was confirmed after in vivo depletion of these cells. Moreover, IL-12 therapy led to the generation of tumor-specific immunologic memory in at least three tumor models. These data are in agreement with the findings of Brunda et al. [45] who reported that tumor regression caused by IL-12 is mediated by CD8+ T cells, whereas NK cells seemed less important for antitumor effects. Another study showed that plasmid delivery of IL-12 by in vivo electroporation was an efficacious strategy against B16F10 murine melanoma. Intratumor but not intramuscular treatment resulted in the cure of 47% of tumor-bearing mice, and tumor rejection was mediated by IFN-γ, tumor-infiltrating lymphocytes, and anti-angiogenic effects [46]. Natural killer T cells have also been implicated in IL-12-mediated tumor rejection [12,47]. After administration of IL-12, NKT cells were a primary functional target in vivo, and they were important for tumor rejection in at least three tumor models: B16 melanoma, Lewis lung carcinoma, and FBL3 erythroleukemia [12]. Park et al. [48] revealed other mechanisms leading to tumor rejection under IL-12 treatment, by which NK cells mediated the rejection of liver metastases, and lymphoid DCs were possibly responsible for rejection of skin tumors. Therapeutic doses of IL-12 were as effective in normal mice as in NKT-deficient mice (CD1-deficient), using one of the tumor types and the same IL-12 treatment regimen as Cui et al. [12]. Although their results failed to confirm an essential role of NKT cells in B16 melanoma, Park et al. [48] agreed that IL-12 can stimulate diverse mechanisms of resistance to tumors, depending on tumor cell type, tumor microenvironment, and mouse strain. In our B16F10-Nex2 system, it is clear that CD1d-dependent NKT cells are critical for antitumor response in untreated tumor-bearing mice (Dias et al., unpublished data). Type I NKT cells predominated in vaccinated mice and were responsible for IFN-γ production which is the key factor in antimelanoma immune response. Some other studies confirmed the critical role of NKT cells in antitumor response, in which NKT cells promote potent tumor rejection in response to exogenous factors such as IL-12 [49] and α-GalCer [11,50,51] as well as in the absence of exogenous stimuli [8,52]. Smyth et al. [49] demonstrated that, at high dose, IL-12 induced tumor immunity mediated preferentially by NK cells in a perforin-dependent mechanism. In B16F10 melanoma and RM-1 prostate carcinoma tumor models, a lower IL-12 dose or delayed administration of IL-12 revealed the role of NKT cells in tumor protection. Both NK and NKT cells thus seem to contribute to natural and IL-12-induced immunity against tumors, and the relative role of each population is tumor- and therapy-dependent.
Not all NK1.1+ T cells are classic NKT cells [53]. NK1.1+ T cells include type I and type II NKT cells (CD1d-dependent) and other NKT-like cells (CD1d-independent). When type I and type II NKT cells were stimulated simultaneously, type II NKT cells seemed to suppress the activation in vitro and the protective effect in vivo of type I NKT cells. Furthermore, when type I cells were absent, the suppressive effect of type II cells increased, suggesting that type I cells can control the suppressive effects of type II NKT cells in a new immunoregulatory axis [6,54].
Our strategy for antitumor immune protection through gene therapy aimed at stimulating IFN-γ production by type I NKT cells among other cells stimulated by IL-12 while suppressing the immunoregulatory properties of type II NKT cells. In fact, type II NKT cells were responsible for IL-13-mediated inhibition of tumor immunosurveillance [14]. Interleukin 13 has emerged as an important mediator of TH2 immune response with immunoregulatory activities in many experimental models such as allergic asthma [55,56], schistosomiasis [57,58], leishmaniasis [59], nematode parasitism [60], fibrosis [26], and cancer [3–5,7,61]. IL-13 produced by CD4+ T cells inhibited CD8+ CTL [3] by inducing the production of TGF-β by CD11b+Gr-1+ myeloid-derived cells [4]. Another mechanism proposed for IL-13-mediated immunosuppression is macrophage polarization toward the M2 phenotype, inhibiting the generation of tumoricidal M1 macrophages [5]. We, therefore, engineered a construct containing the cDNA encoding IL-13 receptor chain-2 fused to the Fc region of mIgG2a and obtained the IL-13Rα2-Fc chimera DNA vaccine. As also described by Zheng et al. [37], we observed that IL-13Rα2 is regulated by its own ligand, IL-13. Macrophages stimulated with IL-13 expressed the IL-13α2 receptor unlike the unstimulated macrophages. Recent studies showed that therapy with soluble IL-13Rα2-Fc protein effectively stimulated antitumor immunity, enhanced vaccine efficacy, and prevented some chronic diseases [3,13,57,61]. Therefore, IL-13Rα2 may act as a “decoy receptor,” suppressing the action of the IL-13, thus helping to maintain tumor immunosurveillance [3]. Presently, we show that treatment of C57Bl/6 mice with plasmid containing IL-13Rα2-Fc vaccine and/or plasmid encoding IL-12 significantly prolonged survival of mice challenged with tumor cells and that the combined gene therapy and chemotherapy conferred a significant level of protection against B16F10-Nex2. Such therapy led to a high production of proinflammatory cytokines by CD4+ and CD8+ cells, particularly IFN-γ. Antiinflammatory cytokines were produced in greater amounts in cells from unvaccinated tumor-bearing mice. Analysis of the NKT subsets showed a type I/type II balance, as in an immunoregulatory axis [6] with cytokines being produced depending on the stimulus. It has been shown that NKT cells can produce IFN-γ, IL-4, IL-10, and IL-13 [62–64]. Presently, we show that CD4+NK1.1+ cells from vaccinated mice produced threefold more IFN-γ than cells from unvaccinated mice, probably by a mechanism mediated by IL-12 DNA vaccine. In addition, CD4+NK1.1+ cells from tumor-bearing mice without any vaccine treatment or exogenous stimulus produced predominantly IL-13 but did not produce detectable IFN-γ, and this IL-13 production was abolished after combined therapy. After therapy with IL-12 and IL-13 receptor DNA vaccines associated with drug 7A, cells produced IFN-γ instead of IL-10 and IL-13. Recently, the existence of functionally distinct NKT cell subsets has been recognized [65]. The authors demonstrated that murine type I NKT cells that responded to α-GalCer-mediated antitumor activity are found only in the liver-derived CD4-CD8- subset but not in the CD4+ subset. Spleen- and thymus-derived NKT cells did not confer similar protective responses. CD4+ NKT cells could be the exclusive producers of interleukins IL-4 and IL-13 on primary stimulation, whereas double-negative NKT cells had a strict TH1 profile [63]. According to our results, CD4+ NKT cells can produce both TH1 and TH2 cytokines as also found by Gumperz et al. [66]. In conclusion, we observed a significant delay in tumor evolution and prolonged survival using a protocol of one dose of pIL-12 followed by six doses of pIL-13R and continuous treatment with cyclopalladated drug 7A, obtaining 30% of tumor-free mice. To elucidate the mechanisms that mediated the antitumor effects, we assessed the T cells producing cytokines in the tumor-bearing mice that had been vaccinated with the complete protocol. In vivo induction of proinflammatory cytokines was detected in mice given gene therapy plus chemotherapy, suggesting that it was sufficient to stimulate a strong immune response mediated by IFN-γ production. Likely IFN-γ increase was IL-12-mediated and IL-13 decreased on administration of IL-13Rα2-Fc vaccine, whereas drug 7A directly killed B16F10-Nex2 tumor cells. These results can also be explained by immunoregulatory NKT cells of different subsets producing functionally distinct cytokines.
Acknowledgments
The authors thank Alexander Rakhmilevich for the IL-12 plasmid and Wagner Batista for help with molecular techniques and helpful suggestions.
Footnotes
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil. E.G.R., R.P., and L.R.T. are recipients of research fellowships from the Brazilian National Research Council.
References
- 1.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 4.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 6.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKTregulatory cells and IL-13. Int J Cancer. 2005;114:80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 8.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart TJ, Smyth MJ, Fernando GJ, Frazer IH, Leggatt GR. Inhibition of early tumor growth requires J alpha 18-positive (natural killer T) cells. Cancer Res. 2003;63:3058–3060. [PubMed] [Google Scholar]
- 10.Zhou D. OX40 signaling directly triggers the antitumor effects of NKT cells. J Clin Invest. 2007;117:3169–3172. doi: 10.1172/JCI33976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J Immunol. 1999;163:2387–2391. [PubMed] [Google Scholar]
- 12.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 13.Ahlers JD, Belyakov IM, Terabe M, Koka R, Donaldson DD, Thomas EK, Berzofsky JA. A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L. Proc Natl Acad Sci USA. 2002;99:13020–13025. doi: 10.1073/pnas.192251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for downregulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 16.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 17.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 18.Punnonen J, de Vries JE. IL-13 induces proliferation, Ig isotype switching, and Ig synthesis by immature human fetal B cells. J Immunol. 1994;152:1094–1102. [PubMed] [Google Scholar]
- 19.Lai YH, Mosmann TR. Mouse IL-13 enhances antibody production in vivo and acts directly on B cells in vitro to increase survival and hence antibody production. J Immunol. 1999;162:78–87. [PubMed] [Google Scholar]
- 20.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 21.Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 22.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callard RE, Matthews DJ, Hibbert L. IL-4 and IL-13 receptors: are they one and the same? Immunol Today. 1996;17:108–110. doi: 10.1016/0167-5699(96)80600-1. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O'Hara RM, Jr, Beier DR, Turner KJ, Wood CR, et al. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 25.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–2679. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 26.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 27.Ma HL, Whitters MJ, Jacobson BA, Donaldson DD, Collins M, Dunussi-Joannopoulos K. Tumor cells secreting IL-13 but not IL-13Ralpha2 fusion protein have reduced tumorigenicity in vivo. Int Immunol. 2004;16:1009–1017. doi: 10.1093/intimm/dxh105. [DOI] [PubMed] [Google Scholar]
- 28.Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 29.Terabe M, Khanna C, Bose S, Melchionda F, Mendoza A, Mackall CL, Helman LJ, Berzofsky JA. CD1d-restricted natural killer T cells can downregulate tumor immunosurveillance independent of interleukin-4 receptor-signal transducer and activator of transcription 6 or transforming growth factor-β. Cancer Res. 2006;66:3869–3875. doi: 10.1158/0008-5472.CAN-05-3421. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald TT. Decoy receptor springs to life and eases fibrosis. Nat Med. 2006;12:13–14. doi: 10.1038/nm0106-13. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues EG, Silva LS, Fausto DM, Hayashi MS, Dreher S, Santos EL, Pesquero JB, Travassos LR, Caires AC. Cyclopalladated compounds as chemotherapeutic agents: antitumor activity against a murine melanoma cell line. Int J Cancer. 2003;107:498–504. doi: 10.1002/ijc.11434. [DOI] [PubMed] [Google Scholar]
- 32.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- 33.Gesztesi JL, Puccia R, Travassos LR, Vicentini AP, Franco MF, Lopes JD. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15:415–422. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 34.Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C, Wang P, Heitman J. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology. 2002;148:2607–2615. doi: 10.1099/00221287-148-8-2607. [DOI] [PubMed] [Google Scholar]
- 35.Rakhmilevich AL, Turner J, Ford MJ, McCabe D, Sun WH, Sondel PM, Grota K, Yang NS. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci USA. 1996;93:6291–6296. doi: 10.1073/pnas.93.13.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng L, Ziegelhoffer PR, Yang NS. In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci USA. 1993;90:4455–4459. doi: 10.1073/pnas.90.10.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng T, Zhu Z, Liu W, Lee CG, Chen Q, Homer RJ, Elias JA. Cytokine regulation of IL-13Ralpha2 and IL-13Ralpha1 in vivo and in vitro. J Allergy Clin Immunol. 2003;111:720–728. doi: 10.1067/mai.2003.1383. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues EG, Garofalo AS, Travassos LR. Endogenous accumulation of IFN-gamma in IFN-gamma-R(-/-) mice increases resistance to B16F10-Nex2 murine melanoma: a model for direct IFN-gamma anti-tumor cytotoxicity in vitro and in vivo. Cytokines Cell Mol Ther. 2002;7:107–116. doi: 10.1080/13684730310000121. Erratum in: Cytokines Cell Mol Ther 2006; 8, 188. [DOI] [PubMed] [Google Scholar]
- 39.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton DL, Essner R, Kirkwood JM, Wollman RC. The skin. In: Kufe DW, Bast RC Jr, Hait WN, Hong WK, Pollock RE, Weichselbaum RR, Holland JF, Frei E III, editors. Malignant Melanoma in Cancer Medicine 7. Hamilton, Ontario: BC Decker Inc.; 2006. pp. 1644–1662. [Google Scholar]
- 41.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 42.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 44.Lesinski GB, Badgwell B, Zimmerer J, Crespin T, Hu Y, Abood G, Carson WE., III IL-12 pretreatments enhance IFN-alpha-induced Janus kinase-STAT signaling and potentiate the antitumor effects of IFN-alpha in a murine model of malignant melanoma. J Immunol. 2004;172:7368–7376. doi: 10.4049/jimmunol.172.12.7368. [DOI] [PubMed] [Google Scholar]
- 45.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16-F10 melanoma. Mol Ther. 2002;5:668–675. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 47.Takeda K, Seki S, Ogasawara K, Anzai R, Hashimoto W, Sugiura K, Takahashi M, Satoh M, Kumagai K. Liver NK1.1+ CD4+ alpha beta T cells activated by IL-12 as a major effector in inhibition of experimental tumor metastasis. J Immunol. 1996;156:3366–3373. [PubMed] [Google Scholar]
- 48.Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003;170:1197–1201. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 49.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 52.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 54.Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 55.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 56.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for TH2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162:920–930. [PubMed] [Google Scholar]
- 58.Mentink-Kane MM, Cheever AW, Thompson RW, Hari DM, Kabatereine NB, Vennervald BJ, Ouma JH, Mwatha JK, Jones FM, Donaldson DD, et al. IL-13 receptor alpha 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci USA. 2004;101:586–590. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–1462. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 60.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 61.Park JM, Terabe M, Donaldson DD, Forni G, Berzofsky JA. Natural immunosurveillance against spontaneous, autochthonous breast cancers revealed and enhanced by blockade of IL-13-mediated negative regulation. Cancer Immunol Immunother. 2008;57:907–912. doi: 10.1007/s00262-007-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 63.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zlotnik A, Godfrey DI, Fischer M, Suda T. Cytokine production by mature and immature CD4-CD8- T cells. Alpha beta. T cell receptor+CD4-CD8- T cells produce IL-4. J Immunol. 1992;149:1211–1215. [PubMed] [Google Scholar]
- 65.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]