Abstract
Salivary duct carcinomas (SDCs) and adenoid cystic carcinomas (ACCs) are the most aggressive and the most frequent carcinomas of the salivary glands, respectively. Little is known about them in terms of molecular/biochemical characterization and conventional treatments are ineffective. On cryopreserved material, we analyzed the expression/activation status of TRK-A, HER-2/neu, and KIT receptors by means of immunoprecipitation and Western blot analysis experiments, and the presence of their cognate ligands by means of Western blot analysis and/or reverse transcription-polymerase chain reaction in 9 SDCs, 12 ACCs, and 8 normal glands. The amplification status of HER-2/neu was also investigated by means of fluorescent in situ hybridization analysis on fixed material. The receptor tyrosine kinase (RTK)-deregulated profile of the SDCs was characterized by the overexpression of activated TRK-A in the presence of its ligand, and the overexpression of HER-2/neu sustained by gene amplification. The RTK signature of the ACCs was represented by the overexpression of activated KIT and TRK-A and their cognate ligands, and the overexpression of activated HER-2/neu, in the absence of gene amplification, possibly sustained by epidermal growth factor receptor heterodimerization. In conclusion, SDCs and ACCs, although sharing TRK-A autocrine loop activation, have different pathologically activated RTK-deregulated profiles that may be potential targets for pharmacological RTK inhibitors.
Introduction
Receptor tyrosine kinases (RTKs) are often deregulated in human cancers, and, therefore, they represent an attractive target for selective pharmacological inhibitors. As novel pharmacological RTK inhibitors are being increasingly developed, exploiting the expression/activation patterns of RTKs in tumors poorly responsive to conventional treatments could lead to significant therapeutic advances. This group of tumors includes carcinomas of salivary gland origin, an uncommon and heterogeneous group of tumors, whose molecular and biochemical characteristics have been little investigated.
Salivary duct carcinoma is a rare salivary gland adenocarcinoma mainly affecting the parotid gland, that has striking histologic similarities to breast carcinomas and distinct aggressive clinical behavior with early nodal and visceral metastases [1]. Adenoid cystic carcinoma is the most common histotype mainly involving the parotid, the submandibular, and minor salivary glands. It has a typical protracted course with local recurrences and late hematogenous metastases. In both tumors, conventional pharmacological treatments are ineffective.
Immunophenotype analyses have found neurotrophic tyrosine kinase receptor, type 1 (TRK-A) expression in normal salivary gland tissue [2], and KITand HER-2/neu overexpression in specimens of ACCs and SDCs, along with myoepithelial carcinomas, lymphoepitheliomalike carcinomas, and mucoepidermoid carcinomas [3–6]. Furthermore, HER-2/neu gene amplification has been observed in HER-2/neuoverexpressing SDCs [7,8] and mucoepidermoid carcinomas [6].
TRK-A and nerve growth factor (NGF) have been found in normal human salivary gland by means of immunohistochemistry and Western blot analysis, respectively [2,9], and in ACC specimens by means of immunohistochemistry [10]. It has been reported that deregulated expression of TRK-A (and TRK-B, TRK-C, and their cognate ligands) correlates with malignant transformation and tumor progression in both prostate cancer cell lines and tissues [11,12] and that all the three TRKs are overexpressed in pancreatic ductal adenocarcinomas [13,14]. TRK-A is a 140-kDa RTK for NGF. It represents a pharmacological target for CEP-701 (Cephalon, Inc., West Chester, PA), an orally active compound already used in hormonerefractory prostate cancer patients [12,15–17].
HER-2/neu protein is 63% to 100% overexpressed in SDCs [18–21], and amplification of the gene has been reported in more than 50% of the SDCs overexpressing HER-2/neu [7,8]. Gene amplification is the most common pathological activation mechanism reported for HER-2/neu, mainly described in breast carcinoma [22]. The HER-2/neu product is a 185-kDa glycoprotein belonging to the family of epidermal growth factor receptor (EGFR), inhibited by herceptin (Genentech Inc., South San Francisco, CA), a monoclonal antibody directed against the extracellular domain [23], effective in breast cancer [24].
KIT expression has been reported in normal salivary gland tissue [25], among malignant epithelial carcinomas in ACC, lymphoepithelial, and myoepithelial carcinomas [3], and exceedingly rare in SDC [26]. c-kit encodes a 145-kDa RTK glycoprotein structurally related to platelet-derived growth factor receptor, whose ligand is the stem cell factor (SCF). The pathologic activation of KIT in gastrointestinal stromal tumors, sustained by activating mutations [27], is inhibited by imatinib (Novartis, Basel, Switzerland) by binding to the ATP pocket site. Little is known about KIT receptor activation in ACCs, although the lack of gain of function mutations [3,28] makes the autocrine loop the most likely activating involved mechanism.
We analyzed the expression/activation status of TRK-A, HER-2/neu, and KIT receptors in a series of SDCs and ACCs by means of immunoprecipitation (IP) and Western blot analysis experiments, and the presence of their cognate ligands by means of reverse transcription-polymerase chain reaction (RT-PCR). Eight normal glands were analyzed in the same way as reference controls. The amplification status of the HER-2/neu gene was also investigated by means of fluorescent in situ hybridization (FISH) analysis.
Materials and Methods
Patients
We studied cryopreserved material from 21 salivary gland carcinomas (9 SDCs and 12 ACCs; 19 primary tumors and 2 recurrences), collected at Istituto Nazionale Tumori of Milan between 1991 and 2004. The diagnoses were made on paraffin-embedded material. Among ACCs, six belonged to cribriform variety and one to tubular one, whereas five cases were composite tumors with two components (cribriform/tubular or cribriform/solid), four cases, or three components (cribriform/tubular/solid), one case. All the cases were reviewed by at least two pathologists and re-reviewed by one of the authors (S.P.) with a good reproducibility (100%). Seven of the SDCs originated from the parotid gland, two from the submandibular gland; the ACCs developed in the parotid gland (n = 4), submandibular gland (n = 3), sublingual gland (n = 1), and in minor salivary glands (n = 4). Their clinic-pathological features are summarized in Table 1.
Table 1.
Summary of the Clinic-Pathologic Features and Results.
| Case | Sex/Age (years) | Tumor Localization | TRK-A (IP) | NGF (RT-PCR) | NGF (WB) | HER-2 (IP) | HER-2 (FISH) | KIT (IP) | SCF (RT-PCR) |
| Control 1 | F/43 | Parotid | 2+/P | n.d. | n.d. | - | n.d. | 2+ | n.d. |
| Control 2 | M/42 | Parotid | 2+/P | + | n.d. | - | n.d. | 2+ | + |
| Control 3 | F/30 | Parotid | 2+/P | n.d. | n.d. | - | n.d. | 2+ | n.d. |
| Control 4 | F/62 | Parotid | 2+/P | n.d. | n.d. | - | n.d. | 2+ | n.d. |
| Control 5 | F/83 | Parotid | 2+/P | + | n.d. | - | n.d. | 2+ | + |
| Control 6 | M/84 | Parotid | 2+/P | + | n.d. | - | n.d. | 2+ | + |
| Control 7 | M/62 | Parotid | 2+/P | + | + | 1+/P | - | 2+ | + |
| Control 8 | F/54 | Submandibular | 2+/P | n.d. | + | 1+/P | - | 2+ | n.d. |
| SDC 1 | F/71 | Submandibular | 3+/P | + | + | - | - | 2+ | + |
| SDC 2 | F/65 | Parotid | 3+/P | + | + | 3+/PP | + (HSR) | 1+ | + |
| SDC 3 | M/63 | Parotid | 1+/P | + | + | 3+/PP | + (HSR) | - | + |
| SDC 4 | M/79 | Parotid | 3+/PP | + | + | 3+/P | + (HSR) | - | + |
| SDC 5 | M/58 | Parotid | 3+/PP | + | + | - | + (DM) | - | + |
| SDC 6 | M/69 | Parotid | 2+/PP | + | + | - | - | - | + |
| SDC 7 | F/70 | Submandibular | 1+/P | + | + | - | - | 1+ | + |
| SDC 8 | F/48 | Parotid | 3+/P | + | + | - | + (DM) | 3+/P | + |
| SDC 9 | M/62 | Parotid | 3+/PP | + | + | 1+/P | +(HSR) | 1+ | + |
| ACC 1 c | M/55 | Submandibular | 2+/P | + | + | 2+/P | - | 2+ | + |
| ACC 2 c | M/17 | Parotid | 2+/P | + | + | 2+/P | - | 2+ | + |
| ACC 3 c, t | F/41 | Submandibular | 1+/PP | n.v. | + | - | - | 2+ | n.v. |
| ACC 4 c | F/46 | Parotid | 2+/P | + | + | - | - | 3+ | + |
| ACC 5 c, t, s | F/51 | Minor | 2+/P | + | + | - | - | 3+ | + |
| ACC 6 t | F/52 | Minor | 2+/P | + | + | 2+/P | - | 3+/P | + |
| ACC 7 c, s | F/34 | Sublingual | 2+/P | + | + | 2+/P | - | 2+ | + |
| ACC 8 c | F/54 | Submandibular | 3+/PP | + | + | 1+/P | - | 3+ | + |
| ACC 9 c, t | F/55 | Minor | 2+/P | + | + | 1+/P | - | 3+/P | + |
| ACC 10 c, s | F/62 | Parotid | 2+/P | + | + | - | - | 3+/P | + |
| ACC 11 c | F/55 | Minor* | 3+/PP | + | + | 1+/P | - | 3+/P | + |
| ACC 12 c | M/39 | Parotid* | 3+/P | + | + | 1+/PP | - | 3+/P | + |
The expression and phosphorylation level detected in normal salivary glands was considered as baseline for each receptor (2+/P for TRK-A; 1+/P for HER-2/neu; 2+ for KIT).
c indicates cribriform variety; DM, double minute; F, female; HSR, homogeneously staining region; M, male; n.d., not done; n.v., not evaluable; P, phosphorylation of the receptor; PP, high level of receptor phosphorylation; s, solid variety; t, tubular variety.
Recurrence.
Normal control salivary gland specimens were obtained from eight patients who underwent surgical procedures for benign parotid conditions or submandibular gland removal for neck surgery in head and neck malignancies. Written informed consent was obtained in all cases.
Before molecular analysis, every specimen was checked for quality by hematoxylin and eosin staining of a representative section.
Biochemical Analysis
Positive controls
An NIH3T3 cell line overexpressing proto-TRK-A (E25) [29] and the SKBR3 human breast cancer cell line (American Type Culture Collection, Manassas, VA) were respectively used in the IP and Western blot analysis experiments as TRK-A and HER-2/neu-positive controls; a Δ559 cell line overexpressing a mutated KIT receptor was used as the KIT-positive control [30]; and NGF 2.5 S (01–125; Upstate, Lake Placid, NY) was used as the positive control, when evaluating the NGF expression in Western blot analysis experiments.
Immunoprecipitation and Western blot analysis
The proteins were extracted and IP experiments were performed as described elsewhere [31].
TRK-A protein was immunoprecipitated from 1 mg of protein lysate using 300 ng of monoclonal MGR12 antibody, kindly supplied by Dr. Tagliabue [32]; the positive control was 200 µg of E25 cell line lysate.
HER-2/neu protein was immunoprecipitated from 1 mg of protein lysate derived from the unbound proteins of anti-TRK-A IP using 2 µg of c-neu Ab-3 monoclonal antibody (Oncogene Research Products, San Diego, CA); the positive control was 300 µg of SKBR3 lysate.
KIT was immunoprecipitated from the unbound proteins derived from the successive IPs of TRK-A and HER-2/neu using 360 ng of Ab-3 (K45) monoclonal antibody directed against the receptor (Neomarkers, Fremont, CA); the positive control was 500 µg of µ 559 cell line lysate.
The samples were loaded on a 6.5% (TRK-A) or 8% (KIT and HER-2/neu) acrylamide gel and blotted to a polyvinylidene fluoride membrane (Millipore Corporation, Bedford, MA). To reveal the status of receptor phosphorylation, the membrane was incubated with antiphosphotyrosine mouse monoclonal antibody (Clone 4G10; Upstate). To measure receptor expression, the filters were stripped and incubated with the following antibodies: Trk (C-14) sc-11 (Santa Cruz Biotechnology, Santa Cruz, CA) for TRK-A; c-kit (c-19) sc-168 (Santa Cruz Biotechnology) for KIT; and c-neu Ab-3 for HER-2/neu.
To normalize the IP and Western blot experiments, after α-TRK-A IP, 50 µg of unbound proteins from each tissue sample was loaded onto a 10% acrylamide gel and blotted; the blots were then incubated with α-actin antibody (actin H-196: sc-7210; Santa Cruz Biotechnology). To verify NGF expression in the normal and carcinoma samples, 50 µg of protein lysate was loaded onto a 15% acrylamide gel and blotted; the blots were then incubated with α-NGF antibody (NGF H-20: sc-548; Santa Cruz Biotechnology). Nerve growth factor 2.5 S 10 µg was also loaded as a positive control.
Fluorescent In Situ Hybridization
Fluorescent in situ hybridization on cytologic specimens and paraffin-embedded tissue sections
Touch imprints were obtained from the frozen specimens of 2 of 8 normal salivary glands, 8 of 9 SDCs, and the 12 ACCs by means of apposition on precleaned slides. In one SDC case (case No. 7; Table 1) for which no cryopreserved material was available, the analysis was performed on sections obtained from tissue block. Slides and sections were treated as previously described [33,34], and the same was true for the assessment of Her-2 amplification [34].
Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted and reverse-transcribed as described elsewhere [35], and the efficiency of the reaction was assessed by amplifying the β-actin housekeeping gene. For the NGF and SCF analyses, the PCR amplifications were performed as previously described [9,35].
Results
TRK-A
Biochemical analysis
Both normal and tumor cases were analyzed for TRK-A receptor expression and phosphorylation by means of IP experiments on total protein extracts. The experiments were normalized by Western blot analysis using the same aliquots of α-TRK-A unbound proteins and incubating the membrane with α-actin antibody (Figure 1). The results are summarized in Table 1.
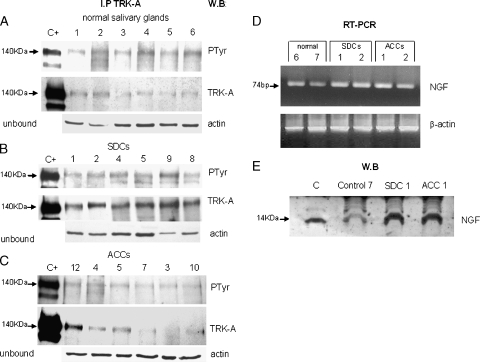
Figure 1.
TRK-A expression and activation. For each sample, total protein extracts were immunoprecipitated with α-TRK-A antibody, run on gel, and blotted with α-PTyr antibody (PTyr panel) for receptor phosphorylation status and with α-TRK-A antibody (TRK-A panel) for receptor expression. Normalization of the experiment was done using 50 µg of unbound proteins and blot analysis with α-actin antibody (actin panel). Lane C+: positive control (E25 cell line). Lane numbers correspond to the cases reported in Table 1. (A) Expression of TRK-A and its phosphorylation in six normal salivary glands. (B) Expression of TRK-A and its phosphorylation in six overexpressing SDC cases. (C) Expression of TRK-A and its phosphorylation in six ACC cases. (D) Expression detected by RT-PCR experiments of NGF transcript in normal and tumoral salivary glands. A total of 1 µl of cDNA was used as template for each NGF and β-actin gene amplification reaction; 10 µl of PCR were loaded on 2% agarose gel. (E) Expression of NGF in normal and tumoral salivary glands. A total of 50 µg of protein lysate was loaded onto a 15% acrylamide gel and blotted with α-NGF antibody. Lane C: positive control (NGF 2.5 S).
Normal tissues
TRK-A was expressed in all normal salivary gland specimens and visualized as a 140-kDa band corresponding to the fully glycosylated receptor form. Hybridization of the same membrane with α-PTyr antibody revealed that TRK-A was phosphorylated (and therefore active) in all cases.We arbitrarily indicated these cases as 2+/P. In Figure 1A, six of eight cases are represented.
Salivary duct carcinomas
TRK-A was underexpressed in two cases (marked as 1+/P), expressed as in the normal controls but highly phosphorylated in one (marked as 2+/PP), and overexpressed and phosphorylated in six (marked as 3+/P and 3+/PP), which are also shown in Figure 1B. The 140-kDa band of the wild type mature receptor was observed in all of the expressing cases.
Adenoid cystic carcinomas
Eight ACC tissue samples showed the same pattern of TRK-A expression and activation (2+/P) as the one observed in the normal controls. One showed less expressed but highly phosphorylated TRK-A (1+/PP), and three showed overexpressed and phosphorylated TRK-A (3+/P and 3+/PP). In Figure 1C, six representative cases are showed.
In brief, TRK-A expression/activation was observed in all of the normal samples (considered baseline or threshold expression); TRK-A overexpression (cases specified as 3+/P and 3+/PP) was observed in 6 of 9 SDCs and 3 of 12 ACCs.
Reverse Transcription-Polymerase Chain Reaction
Nerve growth factor expression
The presence of NGF transcript was analyzed in four normal samples and all of tumor specimens by RT-PCR. All the samples were positive for the 74-bp ligand band (Figure 1D). Unfortunately, one ACC (No. 3 of the Table 1) was not evaluable because of material unsuitability. Western blot analysis confirmed the NGF expression as a 14-kDa band in all analyzed samples (two normal and all tumor specimens; Figure 1E and Table 1).
HER-2/neu
Biochemical analysis
Due to the scarcity of normal and tumor material HER-2/neu expression and activation were analyzed by means of IP and Western blot experiments using unbound proteins derived from α-TRK-A IP. Results are summarized in Table 1.
Normal tissues
Hybridization with α-HER-2/neu antibody revealed a band of 185 kDa in two of eight normal salivary gland tissue samples, where hybridization with α-PTyr antibody showed that HER-2/neu was phosphorylated and therefore active. These two cases are reported in Figure 2A and are identified as 1+/P.
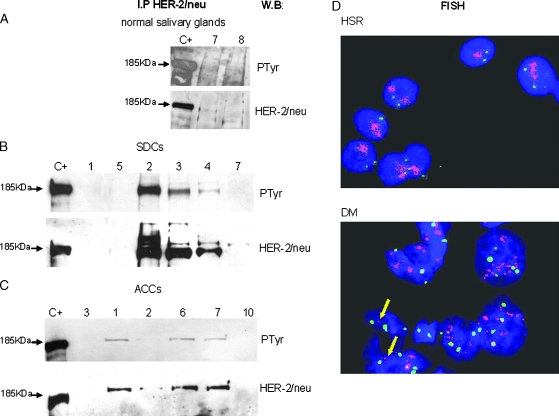
Figure 2.
HER-2/neu expression and activation. For each sample, protein lysate derived from the unbound proteins of anti-TRK-A IP was immunoprecipitated with α-HER-2/neu antibody, run on gel, and blotted with α-PTyr antibody (PTyr panel) for receptor phosphorylation status and with α-HER-2/neu antibody (HER-2/neu panel) for receptor expression. Lane C+: positive control (SKBR3 cell line). Lane numbers correspond to the cases reported in Table 1. (A) Expression of HER-2/neu and its phosphorylation in the two normal salivary glands that express the receptor. (B) Expression of HER-2/neu and its phosphorylation in six SDC cases. (C) Expression of HER-2/neu and its phosphorylation in six ACC cases. (D) HER-2/neu gene amplification analysis in SDC cases. Fluorescent in situ hybridization on imprint specimens. Chromosome 17 probe is labeled in Spectrum Green; HER-2/neu locus-specific probe is labeled in Spectrum Orange. Nuclei are counterstained with DAPI. Upper panel: amplification in HSR in SDC case No. 2 of Table 1. Lower panel: amplification in DM in SDC case No. 5 of Table 1. Single-copy gene signals are indicated by arrows.
Salivary duct carcinomas
Three of nine SDCs overexpressed phosphorylated HER-2/neu (indicated as 3+/P and 3+/PP), one had the same expression/activation status as the two normal controls (indicated as 1+/P) and five cases resulted negative. In Figure 2B, the three overexpressing cases and three negative cases are reported.
Adenoid cystic carcinomas
Four of 12 ACCs showed the same HER-2/neu expression and receptor activation as the two normal controls and were indicated as 1+/P (although No. 12 had a higher phosphorylation level), another four showed a higher level of phosphorylated HER-2/neu expression (indicated as 2+/P) and other four resulted negative. In Figure 2C, the four overexpressing cases and two negative cases are showed.
Fluorescent In Situ Hybridization
Normal tissues
A normal double signal was present in the two cases analyzed (Nos. 7 and 8 of Table 1).
Salivary duct carcinomas
HER-2/neu amplification was detected in six cases: four as homogeneously staining regions (HSR, although the signal was very low in No. 9) and two as double minutes (DM; Figure 2D).
Correlations between the SDC biochemical and FISH analyses
Three of the HSR-positive cases were associated with overexpressed and phosphorylated HER-2/neu receptors (3+/P and 3+/PP); the level of expression in No. 9 was as low as in the normal controls (1+/P). On the contrary, the two DM cases lacked HER-2/neu protein expression and phosphorylation.
Adenoid cystic carcinomas
HER-2/neu gene amplification was never detected.
In brief, HER-2/neu expression was found in only two of the normal salivary glands. HER-2/neu overexpression and phosphorylation were observed in 3 of the 9 SDCs (in which HSR amplification also occurred) and in 4 of the 12 ACCs.
KIT
Biochemical analysis
The protein extracts obtained from the 8 normal and 21 salivary carcinomas were analyzed for KITexpression and activation by means of IP and Western blot experiments using unbound proteins derived from the previous α-TRK-A and α-HER-2/neu IPs. Whereas this procedure allowed us to detect in the positive samples the related protein, in most of the cases, we failed to detect any phosphorylation of the receptor, most likely, because after two IP rounds dephosphorylation by phosphatase has occurred. The results are summarized in Table 1.
Normal tissues
Hybridization with α-KIT antibody revealed a band of 145 kDa corresponding to the mature form of the receptor (indicated as 2+); hybridization of the same blot with α-PTyr antibody showed the absence of receptor phosphorylation in all cases (data not shown). In Figure 3A, KITexpression of six of eight cases is represented.
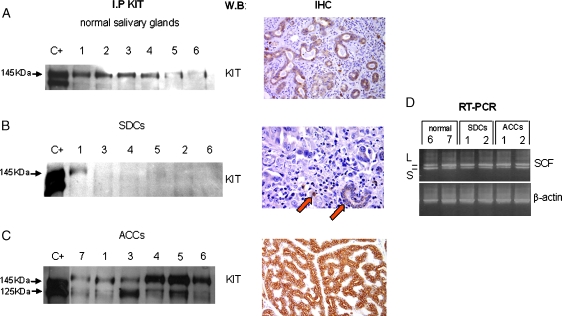
Figure 3.
KIT expression and activation. For each sample, protein lysate derived from the unbound proteins of anti-TRK-A and anti-HER-2/neu IPs was immunoprecipitated with α-KIT antibody, run on gel, and blotted with α-KIT antibody (KIT panel) for receptor expression. Lane C+: positive control (Δ559 cell line). Lane numbers correspond to the cases reported in Table 1. (A) Expression of KIT in six normal salivary glands. KIT expression detected in these samples was in keeping with the immunohistochemistry decoration we observed (right panel), using Dako CD117 antibody. (B) Expression of KIT in six SDC cases. Absence of KIT expression by immunohistochemistry experiments was also detected in tumoral specimens; normal salivary duct and mastocytes, positive in-built controls, are indicated by arrows. (C) Expression of KIT in six ACC cases. The high KIT expression is confirmed by the strong CD117 positivity detected in these tumoral samples. (D) Expression of SCF transcript detected by RT-PCR experiment in normal and tumoral salivary glands. A total of 1 µl of cDNA was used as template for each SCF and β-actin gene amplification reaction; 10 µl of PCR were loaded on 2% agarose gel. In the case of SCF amplification, two bands of the expected molecular weight were detected (L = 494 bp and S = 409 bp).
Salivary duct carcinomas
Four of the nine SDCs did not express KIT. It was expressed to the same extent as in the normal controls in one case (indicated as 2+), underexpressed in three (indicated as 1+), and overexpressed in one (indicated as 3+/P). Hybridization with α-PTyr antibody showed the absence of bands in all but one case (No. 8). In Figure 3B, some representative cases are reported.
Adenoid cystic carcinomas
All 12 specimens expressed KIT. Eight overexpressed the 145-kDa receptor form and were indicated as 3+ and 3+/P; in the remaining four, KIT expression was the same as in the normal controls (indicated as 2+), although No. 3 overexpressed the 125-kDa form. Receptor phosphorylation was observed in five cases (indicated as 3+/P). In Figure 3C, expression of KIT receptor of both expressing and overexpressing cases are showed.
In brief, KIT receptor was expressed in the normal salivary glands and, in comparison with these, was overexpressed in 1 of the 9 SDCs and 8 of the 12 ACCs.
Reverse transcription-polymerase chain reaction
Stem cell factor expression
Reverse transcription-polymerase chain reaction was used to identify SCF transcript in four normal and in all SDC and ACC samples. All of the samples (except for ACC No. 3) were positive, showing two bands with the expected molecular weights of 494 bp (SCF-L) and 409 bp (SCF-S; Figure 3D and Table 1). L-stem cell factor was more expressed than S-stem cell factor. Results in ACC No. 3 were unavailable for material unsuitability.
Discussion
The present molecular/biochemical analyses show that SDCs and ACCs, despite sharing TRK-A autocrine/paracrine loop activation, have different RTK-deregulation profiles. Salivary duct carcinoma profile is characterized by overexpression/activation of TRK-A in the presence of its ligand coupled with overexpression/activation of HER-2/neu sustained by gene amplification. Adenoid cystic carcinoma profile is characterized instead by overexpression/activation of KIT and TRK-A coupled with the presence of their cognate ligands, and no amplification-related overexpression of HER-2/neu.
The involvement of TRK-A in SDCs is not unexpected because this tumor has a number of morphologic and immunophenotype similarities with ductal prostate, pancreas and breast carcinomas, in which development and progression have been associated with TRK-A receptor, both in preclinical and clinical studies. Furthermore, TRK-A/NGF coexpression promotes malignant transformation and tumor progression in the prostate [11,12] and characterizes breast cancer cell lines [9]. Interestingly, TRK-A expression in breast cancer cell lines has been associated with HER-2/neu activation by NGF, thus suggesting that HER-2/neu is activated by means of heterodimerization [36]. Moreover, TRK-A mRNA and/or protein expression has been detected in surgical specimens of infiltrating ductal breast carcinomas investigated by means of RT-PCR/Western blot analysis [36] and in ductal adenocarcinomas of the pancreas analyzed by means of immunohistochemistry [13,14].
The present study provides the first demonstration that the molecular profile of SDCs is characterized by the overexpression of phosphorylated TRK-A and the coexpression of its cognate ligand, supporting the presence of an autocrine/paracrine loop activation of this gene, for which no activation mutations have yet been reported in carcinomas [37,38] and overexpression, due to its promoter methylation, is described in pancreatic cancer [39].
Regarding HER-2/neu, our data confirm that the results of IP/Western blot analysis experiments closely correlate with those of FISH analysis being all HER-2/neu 3+-expressing cases also HER-2/neu-amplified. These results, which recapitulated what observed in ductal breast carcinoma [40], suggest that herceptin should be considered for the treatment of SDC as already reported [41–43]. However, because TRK-A activation or coactivation is the most frequent RTK deregulation in SDCs, combined treatments based on TRK-A inhibitors and herceptin should be considered. Remarkably, in two cases of SDC, the amplification is DM-sustained and is correlated with the absence of protein expression. If confirmed, the latter finding has important implications because it is expected that herceptin will be ineffective in cases carrying DM amplifications.
Regarding ACC, our results point out that the overexpression of activated KIT and TRK-A in presence of their ligands seems to characterize this tumor profile. The lack of KIT phosphorylation in our normal and tumoral samples is probably attributable to our methodological approach, because KIT was immunoprecipitated after two rounds of IPs from unbound α-TRK-A and α-HER-2/neu proteins. This procedure could have affected KIT phosphorylation status. This assumption is supported by the fact that transcript analysis revealed the presence in all the samples of the two biologically active SCF isoforms corresponding to the soluble- (L) and membrane-associated (S) ligands in both normal and tumoral KIT-expressing tissues. Therefore, an autocrine loop also seems to be the most likely mechanism of KIT activation in ACCs, a finding that is strengthened by c-kit gene analyses showing the absence of activating mutations in overexpressing ACCs [3,28] (data not shown). We have previously demonstrated a similar KIT activation mechanism in small cell lung cancers, which expressed activated receptor in presence of SCF and wild type c-DNA [35]. Because it has been found that compounds, such as sunitinib (Sugen Inc., South San Francisco, CA), are effective on cell lines, in which KIT is activated by means of an autocrine loop [44,45], whereas imatinib (evaluated in phase II clinical trials of unresectable or metastatic KIT-expressing ACCs) has proved to be ineffective [46,47] and considering the frequent activation of TRK-A, the application of wild type KIT inhibitors in addition to TRK-A inhibitors may represent a suitable pharmacological option in ACC. Furthermore, the overexpression of HER-2/neu in the absence of gene amplification and coupled with the EGFR immunophenotypic positivity (data not shown) we observed in the same ACCs strongly suggests that HER-2/neu may also be activated by means of heterodimerization with EGFR in some cases. This finding, if confirmed by coimmunoprecipitation experiments, might further extend treatment choices to EGFR inhibitors, in particular to antibodies that block ligand binding.
In conclusion, we demonstrated that TRK-A deregulation is a characteristic shared by SDC and ACC that, however, carry different deregulated RTK profiles representing potential targets for RTK inhibitors administered alone or in combination. Future developments should include the development of drugs capable of inhibiting the secondary transducers activated by TRK-A, KIT, HER-2/neu, and EGFR shared by existing downstream pathways. However, the relevance of RTK inhibitors remains confined to advanced cases (recurrence and/or metastasis), being surgery the primary modality of treatment of primary salivary gland carcinomas.
Footnotes
Supported by grants from the Associazione Italiana per la Ricerca sul Cancro to S.P.
We have no conflict of interest to declare.
References
- 1.Guzzo M, Di Palma S, Grandi C, Molinari R. Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck. 1997;19:126–133. doi: 10.1002/(sici)1097-0347(199703)19:2<126::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.De Vicente JC, Garcia-Suarez O, Esteban I, Santamaria J, Vega JA. Immunohistochemical localization of neurotrophins and neurotrophin receptors in human and mouse salivary glands. Ann Anat. 1998;180:157–163. doi: 10.1016/S0940-9602(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 3.Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett. 2000;154:107–111. doi: 10.1016/s0304-3835(00)00387-6. [DOI] [PubMed] [Google Scholar]
- 4.Freier K, Flechtenmacher C, Walch A, Devens F, Mühling J, Lichter P, Joos S, Hofele C. Differential KIT expression in histological subtypes of adenoid cystic carcinoma (ACC) of the salivary gland. Oral Oncol. 2005;41:934–939. doi: 10.1016/j.oraloncology.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Seethala RR, Hunt JL, Baloch ZW, Livolsi VA, Leon Barnes E. Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol. 2007;31:1683–1694. doi: 10.1097/PAS.0b013e3180dc928c. [DOI] [PubMed] [Google Scholar]
- 6.Press MF, Pike MC, Hung G, Zhou JY, Ma Y, George J, Dietz-Band J, James W, Slamon DJ, Batsakis JG. Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: correlation with poor prognosis. Cancer Res. 1994;54:5675–5682. [PubMed] [Google Scholar]
- 7.Skalova A, Starek I, Vanecek T, Kucerova V, Plank L, Szepe P, Di Palma S, Leivo I. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology. 2003;42:348–356. doi: 10.1046/j.1365-2559.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 8.Dagrada GP, Negri T, Tamborini E, Pierotti MA, Pilotti S. Re: Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology. 2004;44:301–302. doi: 10.1111/j.1365-2559.2004.01781.x. [DOI] [PubMed] [Google Scholar]
- 9.Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22:5592–5601. doi: 10.1038/sj.onc.1206805. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Sun M, Jiang Y, Yang L, Lei D, Lu C, Zhao Y, Zhang P, Yang Y, Li J. Nerve growth factor and tyrosine kinase A in human salivary adenoid cystic carcinoma: expression patterns and effects on in vitro invasive behaviour. J Oral Maxillofac Surg. 2006;64:636–641. doi: 10.1016/j.joms.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Dionne CA, Camoratto AM, Jani JP, Emerson E, Neff N, Vaught JL, Murakata C, Djakiew D, Lamb J, Bova S, et al. Cell cycle-independent death of prostate adenocarcinoma is induced by the trk tyrosine kinase inhibitor CEP-751 (KT6587) Clin Cancer Res. 1998;4:1887–1898. [PubMed] [Google Scholar]
- 12.Weeraratna AT, Arnold JT, George DJ, DeMarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2000;45:140–148. doi: 10.1002/1097-0045(20001001)45:2<140::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behaviour. Int J Cancer. 1999;81:417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto Y, Kitajima Y, Edakuni G, Sasatomi E, Mori M, Kitahara K, Miyazaki K. Expression of Trk tyrosine kinase receptor is a biologic marker for cell proliferation and perineural invasion of human pancreatic ductal adenocarcinoma. Oncol Rep. 2001;8:477–484. doi: 10.3892/or.8.3.477. [DOI] [PubMed] [Google Scholar]
- 15.Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, Isaacs JT. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7:2237–2245. [PubMed] [Google Scholar]
- 16.Beckhardt S. Re: growth factor receptor tyrosine kinase inhibitors; clinical development and potential for prostate cancer therapy. J Urol. 2004;172:1545. [PubMed] [Google Scholar]
- 17.Marshall JL, Kindler H, Deeken J, Bhargava P, Vogelzang NJ, Rizvi N, Luhtala T, Boylan S, Dordal M, Robertson P, et al. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs. 2005;23:31–37. doi: 10.1023/B:DRUG.0000047103.64335.b0. [DOI] [PubMed] [Google Scholar]
- 18.Felix A, El-Naggar AK, Press MF, Ordonez NG, Fonseca I, Tucker SL, Luna MA, Batsakis JG. Prognostic significance of biomarkers (c-erbB-2, p53, proliferating cell nuclear antigen, and DNA content) in salivary duct carcinoma. Hum Pathol. 1996;27:561–566. doi: 10.1016/s0046-8177(96)90162-8. [DOI] [PubMed] [Google Scholar]
- 19.Glisson B, Colevas AD, Haddad R, Krane J, El-Naggar A, Kies M, Costello R, Summey C, Arquette M, Langer C, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10:944–946. doi: 10.1158/1078-0432.ccr-03-0253. [DOI] [PubMed] [Google Scholar]
- 20.Skalova A, Starek I, Kucerova V, Szepe P, Plank L. Salivary duct carcinoma—a highly aggressive salivary gland tumor with HER-2/neu oncoprotein overexpression. Pathol Res Pract. 2001;197:621–626. doi: 10.1078/0344-0338-00136. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Barba E, Cortes-Guardiola JA, Minguela-Puras A, Torroba-Caron A, Mendez-Trujillo S, Bermejo-Lopez J. Salivary duct carcinoma: clinicopathological and immunohistochemical studies. J Craniomaxillofac Surg. 1997;25:328–334. doi: 10.1016/s1010-5182(97)80035-2. [DOI] [PubMed] [Google Scholar]
- 22.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 23.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 24.Leyland-Jones B. Trastuzumab: hopes and realities. Lancet Oncol. 2002;3:137–144. doi: 10.1016/s1470-2045(02)00676-9. [DOI] [PubMed] [Google Scholar]
- 25.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Shimode Y, Itoi A, Ueda Y, Katsuda S. Salivary duct carcinoma of the parotid gland presenting KIT (CD117) overexpression. Histopathology. 2007;51:114–115. doi: 10.1111/j.1365-2559.2007.02709.x. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 28.Holst VA, Marshall CE, Moskaluk CA, Frierson HF., Jr KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol. 1999;12:956–960. [PubMed] [Google Scholar]
- 29.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamborini E, Bonadiman L, Greco A, Albertini V, Negri T, Gronchi A, Bertulli R, Colecchia M, Casali PG, Pierotti MA, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:294–299. doi: 10.1053/j.gastro.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Tamborini E, Bonadiman L, Greco A, Gronchi A, Riva C, Bertulli R, Casali PG, Pierotti MA, Pilotti S. Expression of ligand-activated KIT and platelet-derived growth factor receptor beta tyrosine kinase receptors in synovial sarcoma. Clin Cancer Res. 2004;10:938–943. doi: 10.1158/1078-0432.ccr-03-0059. [DOI] [PubMed] [Google Scholar]
- 32.Tagliabue E, Ghirelli C, Lombardi L, Castiglioni F, Asnaghi L, Longhi C, Borrello MG, Aiello P, Menard S. Production of a monoclonal antibody directed against the high-affinity nerve growth factor receptor. Int J Biol Markers. 1999;14:68–72. doi: 10.1177/172460089901400203. [DOI] [PubMed] [Google Scholar]
- 33.Perrone F, Tabano S, Colombo F, Dagrada G, Birindelli S, Gronchi A, Colecchia M, Pierotti MA, Pilotti S. p15INK4b, p14ARF, and p16INK4a inactivation in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. Clin Cancer Res. 2003;9:4132–4138. [PubMed] [Google Scholar]
- 34.Dagrada GP, Mezzelani A, Alasio L, Ruggeri M, Romano R, Pierotti MA, Pilotti S. HER-2/neu assessment in primary chemotherapy treated breast carcinoma: no evidence of gene profile changing. Breast Cancer Res Treat. 2003;80:207–214. doi: 10.1023/A:1024579206250. [DOI] [PubMed] [Google Scholar]
- 35.Tamborini E, Bonadiman L, Negri T, Greco A, Staurengo S, Bidoli P, Pastorino U, Pierotti MA, Pilotti S. Detection of overexpressed and phosphorylated wild-type kit receptor in surgical specimens of small cell lung cancer. Clin Cancer Res. 2004;10:8214–8219. doi: 10.1158/1078-0432.CCR-04-1013. [DOI] [PubMed] [Google Scholar]
- 36.Tagliabue E, Castiglioni F, Ghirelli C, Modugno M, Asnaghi L, Somenzi G, Melani C, Menard S. Nerve growth factor cooperates with p185 (HER2) in activating growth of human breast carcinoma cells. J Biol Chem. 2000;275:5388–5394. doi: 10.1074/jbc.275.8.5388. [DOI] [PubMed] [Google Scholar]
- 37.George DJ, Suzuki H, Bova GS, Isaacs JT. Mutational analysis of the TrkA gene in prostate cancer. Prostate. 1998;36:172–180. doi: 10.1002/(sici)1097-0045(19980801)36:3<172::aid-pros5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Pierotti MA, Greco A. Oncogenic rearrangements of the NTRK1/NGF receptor. Cancer Lett. 2006;232:90–98. doi: 10.1016/j.canlet.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto M, Kitazawa R, Maeda S, Kitazawa S. Methylation adjacent to negatively regulating AP-1 site reactivates TRK-A gene expression during cancer progression. Oncogene. 2005;24:5108–5118. doi: 10.1038/sj.onc.1208697. [DOI] [PubMed] [Google Scholar]
- 40.Couturier J, Vincent-Salomon A, Nicolas A, Beuzeboc P, Mouret E, Zafrani B, Sastre-Garau X. Strong correlation between results of fluorescent in situ hybridization and immunohistochemistry for the assessment of the ERBB2 (HER-2/neu) gene status in breast carcinoma. Mod Pathol. 2000;13:1238–1243. doi: 10.1038/modpathol.3880228. [DOI] [PubMed] [Google Scholar]
- 41.Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29:907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 42.Nashed M, Casasola RJ. Biological therapy of salivary duct carcinoma. J Laryngol Otol. 2008;11:1–3. doi: 10.1017/S0022215108002314. [DOI] [PubMed] [Google Scholar]
- 43.Prat A, Parera M, Reyes V, Peralta S, Cedrés S, Andreu J, Huguet P, del Campo JM. Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck. 2008;30:680–683. doi: 10.1002/hed.20714. [DOI] [PubMed] [Google Scholar]
- 44.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- 45.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 46.Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, Brown S, Pond GR, Murgo A, Siu LL. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23:585–590. doi: 10.1200/JCO.2005.06.125. [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43:33–36. doi: 10.1016/j.oraloncology.2005.12.026. [DOI] [PubMed] [Google Scholar]