Abstract
Telomerase, a ribonucleoprotein important to neoplastic immortality, is up-regulated in approximately 85% of cancers, including leukemias. In this study, 9cUAB30, a novel retinoic acid, resulted in differentiation of HL60 leukemia cells as indicated by morphologic changes characteristic of granulocytes. It also caused a down-regulation of hTERT gene expression and a decrease in telomerase activity. Telomerase inhibition was followed by loss of proliferative capacity, induction of apoptosis, and partial differentiation. These findings demonstrate the effectiveness of 9cUAB30 at inhibiting telomerase activity by down-regulating hTERT gene expression in human leukemic cells.
Introduction
The ability to replicate indefinitely is a characteristic of both stem and cancer cells, but not normal somatic cells. This proliferative immortality is achieved through the activity of the ribonucleoprotein telomerase. Approximately 85% of cancers display an aberrant increase in telomerase activity, and as a result, anticancer therapeutics aimed at exploiting this cancer-specific activation of telomerase expression promise a high degree of translational potential [1]. Telomerase activity is minimal, almost undetectable, in most normal tissues [2]; however, leukemic cells display much higher levels of telomerase activity [3,4]. Telomerase functions by adding hexameric repeats of DNA nucleotides to the 5′ ends of linear chromosomes, compensating for the slow erosion of DNA that results from the inability of DNA polymerase II to effectively replicate the end of the DNA strand. The strong correlation between hTERT mRNA expression and telomerase activity has made inhibition of hTERT expression important to preventing cellular immortalization.
Retinoids such as all-trans retinoic acid (ATRA) and 9-cis retinoic acid are derivatives of vitamin A that play a pivotal role in a diverse group of biologic processes including, but not limited to, cellular proliferation, differentiation, apoptosis, and development [5]. Retinoic acids (RAs) have been studied intensively for their anticancer effects, which are exerted through a wide range of mechanisms. Inhibition of telomerase in leukemic cells using RAs has been shown to slow proliferation and induce a differentiation to a more benign phenotype [6]. Leukemias are not the only cancer in which retinoids have been used to inhibit telomerase activity, as RAs have been shown to effectively treat or prevent breast, cervical, neural, and many of the subtypes of hematological cancers [7–10].
Acute promyelocytic leukemias (APLs) respond readily to ATRA, which induces differentiation and apoptosis [11]. In HL60 cells, it has been shown that ATRA induces a repression of hTERT expression resulting in telomere shortening and cell death [3]. Despite these findings, the negative effect of ATRA treatment on normal healthy cells has hindered the development of ATRA-based anticancer therapies. As a result, much focus has been placed on the development of synthetic retinoids that display the same anticancer properties but lack the off-target effects of ATRA and other RAs. Retinoids freely pass through the cell and nuclear membrane, where once inside the nucleus, they bind to two classes of nuclear receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in addition to cytoplasmic binding proteins. Once bound, the RA-receptor complexes directly and indirectly regulate gene transcription. Retinoic acid receptors and RXRs are related to the steroid/thyroid hormone superfamily of receptors that primarily function as ligand-inducible transcription factors [12]. A vast number of gene promoters contain RAR elements (RAREs) and RXR elements (RXREs) that provide the means for nuclear receptor complexes to transcriptionally regulate the downstream gene.
hTERT is one example of a gene that has been shown to be regulated by RAs but lacks both an RARE and a RXRE. The hTERT promoter contains an estrogen response element and a variety of binding sites for both activating and inhibiting transcription factors, and it is the direct effect of RAs on these regulating proteins that allows RAs to indirectly affect hTERT gene expression [13]. Retinoid X receptors such as Targretin have been shown to have a lower toxicity than retinoid agonists for RARs and thus are the primary form of synthetic retinoid currently being investigated for treatment or chemoprevention in many cancer cell lines. 9cUAB30 is a synthetic analog of 9-cis RA, but unlike 9-cis RA, is an RXR-selective retinoid [14]. This novel retinoid (Figure 1) has shown great potential as a chemopreventive/treatment agent in breast cancer differentiation therapy and is far less toxic than ATRA [15]. In mice, topical treatment with high doses of 9cUAB30 has been demonstrated to inhibit formation of papillomas induced by dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol 13-acetate by 54% [16]. It has also been shown to be orally active in the prevention of N-methyl-N-nitrosourea-induced mammary cancers in rats without resulting in signs of toxicity [16]. Additional studies on 9cUAB30 have demonstrated that this agent reduces cellular proliferation in mammary cancer [17]. This retinoid is currently in phase I clinical trials for pharmacology and toxicology. In the current study, the potential of 9cUAB30 to induce differentiation and inhibit telomerase in human leukemia HL60 cells was assessed because its mechanisms and efficacy have not yet been evaluated in leukemia cells.
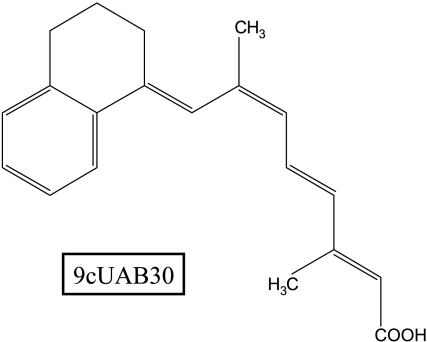
Figure 1.
Structure of 9cUAB30. 9cUAB30 is a synthetic retinoid related to 9-cis retinoic acid.
Materials and Methods
Cell Culture and Proliferation Assessment
HL60 cells (American Type Culture Collection, Manassas, VA) were cultured in 5% CO2 at 37°C in Iscove's medium supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 1% antibiotic solution: amphotericin B, penicillin, and streptomycin (Gibco, Carlsbad, CA), and 1% l-glutamine. HL60 cells were seeded at 3 x 106 cells per 25-cm2 culture flask and were exposed to 5 µM 9cUAB30. This dose was determined through dose-response analyses (data not shown).
Assessment of Differentiation
Cellular differentiation was determined using fluorescence-activated cell-sorting (FACS) analyses of CDllb expression, a cell surface protein of granulocytes. Cells (1 x 106) were washed with cold phosphate-buffered solution (PBS) and incubated with 2 µg of fluorochrome-conjugated monoclonal antibody fluorescein isiothiocyanate against human CD11b antigen (Sigma, St. Louis, MO). After antibody incubation, the cells were washed with PBS containing 1% bovine serum albumin and 0.1% NaN3, then fixed with 2% paraformaldehyde. The cells were analyzed on a Becton Dickinson (San Jose, CA) FACScalibur flow cytometer with CellQuest software.
Assessment of hTERT Expression
Genomic RNA was purified from thawed cells using the RNeasy Minikit (Qiagen Sciences, Gaithersburg, MD). Approximately 2 µg of RNA was reverse-transcribed using the SuperScript Preamplification system (Invitrogen, Carlsbad, CA). From this reaction, 1 µl was used as the template for polymerase chain reaction (PCR) using a primer set specific for a 216-bp region of the hTERT gene [18] as well as a 330-bp glyceraldehyde-3-phosphate dehydrogenase amplicon as the internal control. The PCR products were resolved on a 2% agarose gel, stained with ethidium bromide (0.5 µg/ml), and visualized under UV light.
Assessment of Telomerase Activity
The telomeric repeat amplification protocol (TRAP) assay was performed with the TRAPeze XL kit (Millipore, Temecula, CA) as previously [19]. Samples underwent PCR amplification (94°C for 30 seconds, 59°C for 30 seconds, and 72°C for 1 minute) for 36 cycles. The PCR product was visualized on a 10% polyacrylamide nondenaturing gel stained with SYBR Green (Molecular Probes, Eugene, OR) and analyzed with Kodak Digital Science software (Kodak, New Haven, CT).
Analysis of Apoptosis
The degree of apoptosis was measured using the ApopTag Plus Peroxidase In Situ Apoptosis Detection kit (Chemicon International, Temecula, CA). Briefly, the harvested HL60 cells were fixed in 1% paraformaldehyde, quenched with endogenous peroxidase, treated with anti-digoxigenin peroxidase conjugate, and stained with methyl green dye. Cells undergoing apoptosis stained brown, whereas nonapoptotic cells stained green.
Apoptosis was also determined using FACS analyses. According to the protocol of Vibrant Apoptosis Detection Kit (Invitrogen), 1 million cells were washed with cold PBS, and incubated with Alexa488 and with propidium iodide. The cells were analyzed on a Becton Dickinson FACScalibur flow cytometer with CellQuest software.
Results
Decrease in Proliferation and Induction of Differentiation
HL60 cells treated with 5 µM 9cUAB30 (optimal dose determined by dose-response analysis; data not shown) took on morphologic characteristics of granulocytes, becoming larger and more internally complex. Clumping was apparent by day 6 and was the dominant cell distribution by day 12 (Figure 2A). An increase in cells exhibiting characteristic apoptotic features was observed including chromatin condensation, nuclear fragmentation, cell shrinkage, and the presence of apoptotic bodies (data not shown). After peaking at day 6, the proliferation of the treated cells gradually declined during the remainder of the treatment period, whereas the control cells grew exponentially throughout the treatment period (Figure 2B). The viability of 5 µM 9cUAB30-treated cells decreased to 65.6% on day 12 (Figure 2C). In addition to morphologic changes associated with differentiation, the expression of CD11b increased from 2.3% for controls to 59.5% for treated cells on day 12 (Figure 2D).
Figure 2.
Effects of 5 µM 9cUAB30 on HL60 cellular morphology. (A) Original magnification, x200. Cell photographs were obtained using a Nikon CoolPix digital camera. (B) Effects of 5 µM 9cUAB30 on proliferation of HL60 cells. Cells were cultured for 0, 3, 6, 9, and 12 days and counted on a hemacytometer. The mean values of cell counts in triplicate were plotted. Bars, SEM. (C) Changes in cell viability determined by ratio of live to total cells; (D) Assessment of differentiation after 5 µM 9cUAB30 treatment of HL60 cells. Fluorescence-activated cell-sorting analyses of HL60 after incubation with fluorochrome conjugates of monoclonal antibody against human CD11b antigen. CD11b served as a marker of granulocyte differentiation. The values indicate the percentage of CD11b-positive cells.
Inhibition of hTERT Gene Expression and Telomerase Activity
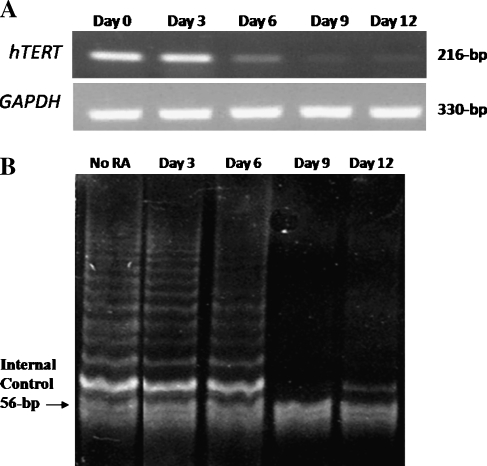
hTERT expression was down-regulated after day 6 of 5 µM 9cUAB30 treatment of HL60 cells, whereas glyceraldehyde-3-phosphate dehydrogenase expression remained constant (Figure 3A). Although the first 3 days of 9cUAB30 treatment of HL60 cells resulted in no reduction of hTERT expression, by 6 days of treatment, hTERT gene expression was attenuated, and by 9 days of treatment with 9cUAB30, it was not detectable. The effect of 9cUAB30 on telomerase activity was examined using the TRAP assay method [19]. The amount of telomerase activity of cells treated with 5 µM 9cUAB30 was similar to the untreated controls on day 3 and only slightly decreased by day 6 due to the 24-hour half-life of the telomerase enzyme after mRNA attenuation. There was a sharp decline in telomerase activity in response to 9cUAB30 by days 9 and 12. This loss of band intensity is indicative of a loss of telomerase activity (Figure 3B).
Figure 3.
(A) Effects of 5 µM 9cUAB30 on hTERT mRNA expression determined by reverse transcription-polymerase chain reaction. (B) Effects of 5 µM 9cUAB30 on telomerase activity by TRAP assay. The band of 56-bp served as the internal control for the PCR.
Induction of Apoptosis
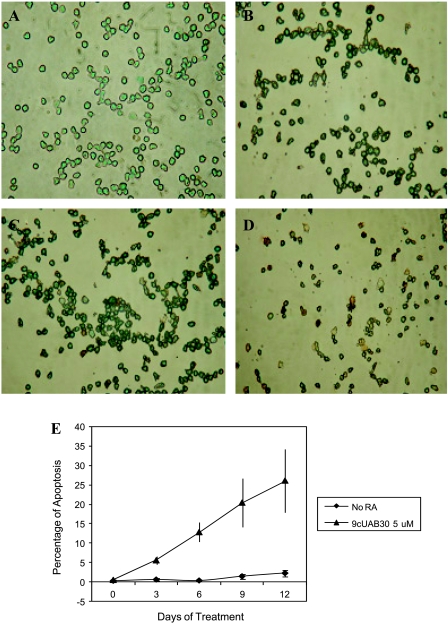
Figure 4A depicts the morphology of HL60 cells treated with 5 µM 9cUAB30 after incubation with anti-digoxigenin peroxidase conjugate and stained with methyl green dye according to the ApopTag Apoptosis Assay protocol. The cells undergoing apoptosis stain brown, whereas the nonapoptotic cells stain green (Figure 4, A–D). The untreated control cells displayed little or no apoptosis (∼1%; Figure 4, A and E). In the first 6 days of treatment, a gradual increase in the number of apoptotic cells occurred. By day 9, however, there is a considerable amount of apoptosis, and this increased through day 12. The greatest number of apoptotic HL60 cells was seen on day 12 (26.2%; Figure 4E).
Figure 4.
Analysis of apoptosis determined by the ApopTag Apoptosis assay for 5 µM 9cUAB30. Brown cells are apoptotic and green cells are not apoptotic. (A) No RA; (B) day 6 of 5 µM 9cUAB30; (C) day 9 of 5 µM 9cUAB30; (D) day 12 of 5 µM 9cUAB30. (E) Summary graph of apoptosis determined by ApopTag Apoptosis assay. Percentage of apoptosis for HL60 cells treated with 5 µM 9cUAB30.
Discussion
In this study, we have shown that 9cUAB30, the novel retinoid that is much less toxic than ATRA [16], down-regulated telomerase activity in HL60 leukemia cells and this loss of telomerase activity resulted in an induction of apoptosis in more than 25% of these cells. A safe and effective means of telomerase inhibition is a much sought-after therapy for cancer [20]. Because telomerase is down-regulated during embryonic development as stem cells differentiate into normal somatic cells and is up-regulated in up to 95% of leukemias, it serves as an excellent target for antileukemia therapies. 9cUAB30 RA is an RXR-selective synthetic analogue of 9-cis RA [14,15] and, as such, should be less toxic than RAR-selective retinoids such as ATRA or pan-agonists of RARs and RXRs such as 9-cis RA.
Many genes have RAREs and RXREs in their promoter. Other genes may be downstream targets of the retinoids. Although hTERT has not been found to have an RARE or an RXRE, hTERT suppression may be a downstream effect of retinoid receptor activation. Downstream targets of RA that could facilitate hTERT suppression involve the c-Myc/Max heterodimer, p53, DNA methyltransferases, and histone deacetylases.
Like ATRA, 9cUAB30 has an antiproliferative effect on HL60 cells (Figure 2). The changes in cellular morphology shown in Figure 2 reveal a progression toward a generally differentiated morphology. 9cUAB30 displayed an ability to induce apoptosis and inhibit telomerase activity comparable to that of ATRA (data not shown), whereas its ability to induce differentiation as measured by CD11b expression was one third less than ATRA (Figure 4 and data not shown). This finding is in agreement with those of previous studies [21,22] demonstrating that the ability of a retinoid to induce differentiation in NB4 leukemia cells correlates with activation of RARα. 9cUAB30 binds and activates only RXR retinoid receptors, unlike 9-cis RA, which is a pan-agonist [16,17]. 9cUAB30 treatment inhibited hTERT expression before either a decrease in proliferative ability or terminal differentiation, suggesting that hTERT inhibition may be mediated by a different pathway other than induction of differentiation. The effects demonstrated by 9cUAB30 on apoptosis and telomerase activity may be induced through RXR homodimers or RAR-RXR heterodimers activated in an RXR-selective manner [23].
In summary, hTERT up-regulation has long been known as a key element in tumorigenesis, vital to the immortality of cancer cells. Here we have shown that 9cUAB30 treatment leads to down-regulation of hTERT expression, decrease in telomerase activity, and induction of apoptosis of HL60 human leukemia cells. These findings strongly support 9cUAB30 as a treatment/chemopreventive agent. Future studies will be needed to elucidate the exact mechanisms responsible for the role 9cUAB30 plays in hTERT gene expression. Introduction of 9cUAB30 into breast cancer cells has been shown to cause a down-regulation of DNA methyltransferase expression, and it is this loss of DNA methylation activity that may cause a decrease in hTERT expression [24]. It is well known that hTERT expression is regulated by both genetic and epigenetic mechanisms, and this fact may suggest that 9cUAB30 asserts it anticancer effects through epigenetic mechanisms.
Footnotes
This work was supported in part by grants from the National Cancer Institute, Susan G. Komen for the Cure, and the Glenn Foundation for Medical Research.
References
- 1.Shay J, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 2.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 3.Pendino F, Flexor M, Delhommeau F, Buet D, Lanotte M, Segal-Bendirdjian E. Retinoids down-regulate telomerase and telomere length in a pathway distinct from leukemia cell differentiation. Proc Natl Acad Sci USA. 2001;98:6662–6667. doi: 10.1073/pnas.111464998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu D, Gruber A, Björkholm M, Peterson C, Pisa P. Suppression of telomerase reverse transcriptase (hTERT) expression in differentiated HL-60 cells: regulatory mechanisms. Br J Cancer. 1999;80:1156–1161. doi: 10.1038/sj.bjc.6690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn M, Roberts A. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983;43:3034–3040. [PubMed] [Google Scholar]
- 6.Reichman T, Albanell J, Wang X, Moore M, Studzinski G. Downregulation of telomerase activity in HL60 cells by differentiating agents is accompanied by increased expression of telomerase-associated protein. J Cell Biochem. 1997;67:13–23. [PubMed] [Google Scholar]
- 7.Choi S, Kang H, Im E, Kim Y, Bae Y, Choi Y, Lee K, Chung H, Chang H, Kim N. Inhibition of cell growth and telomerase activity of breast cancer cells in vitro by retinoic acids. Int J Oncol. 2000;17:971–976. doi: 10.3892/ijo.17.5.971. [DOI] [PubMed] [Google Scholar]
- 8.Sanborn C, O'Connor A, Sawin R, Moore K, Dehart M, Azarow K. Comparison of telomerase levels before and after differentiation of two cell lines of human neuroblastoma. J Surg Res. 2000;93:206–210. doi: 10.1006/jsre.2000.5982. [DOI] [PubMed] [Google Scholar]
- 9.Ding Z, Green A, Yang X, Chernenko G, Tang S, Pater A. Retinoic acid inhibits telomerase activity and downregulates expression but does not affect splicing of hTERT: correlation with cell growth rate inhibition in an in vitro cervical carcinogenesis/multidrug-resistance model. Exp Cell Res. 2002;272:185–191. doi: 10.1006/excr.2001.5412. [DOI] [PubMed] [Google Scholar]
- 10.Casillas M, Brotherton S, Andrews L, Ruppert J, Tollefsbol T. Induction of endogenous telomerase (hTERT) by c-Myc in WI-38 fibroblasts transformed with specific genetic elements. Gene. 2003;316:57–65. doi: 10.1016/s0378-1119(03)00739-x. [DOI] [PubMed] [Google Scholar]
- 11.Lo Coco F, Nervi C, Avvisati G, Mandelli F. Acute promyelocytic leukemia: a curable disease. Leukemia. 1998;12:1866–1880. doi: 10.1038/sj.leu.2401230. [DOI] [PubMed] [Google Scholar]
- 12.Mangelsdorf D. Vitamin A receptors. Nutr Rev. 1994;52:S32–S44. doi: 10.1111/j.1753-4887.1994.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 13.Poole J, Andrews L, Tollefsbol T. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT) Gene. 2001;269:1–12. doi: 10.1016/s0378-1119(01)00440-1. [DOI] [PubMed] [Google Scholar]
- 14.Muccio D, Brouillette W, Alam M, Vaezi M, Sani B, Venepally P, Reddy L, Li E, Norris A, Simpson-Herren L, et al. Conformationally defined 6-s-trans-retinoic acid analogs. 3. Structure-activity relationships for nuclear receptor binding, transcriptional activity, and cancer chemopreventive activity. J Med Chem. 1996;39:3625–3635. doi: 10.1021/jm9603126. [DOI] [PubMed] [Google Scholar]
- 15.Muccio D, Brouillette W, Breitman T, Taimi M, Emanuel P, Zhang X, Chen G, Sani B, Venepally P, Reddy L, et al. Conformationally defined retinoic acid analogues. 4. Potential new agents for acute promyelocytic and juvenile myelomonocytic leukemias. J Med Chem. 1998;41:1679–1687. doi: 10.1021/jm970635h. [DOI] [PubMed] [Google Scholar]
- 16.Grubbs C, Hill D, Bland K, Beenken S, Lin T, Eto I, Atigadda V, Vines K, Brouillette W, Muccio D. 9cUAB30, an RXR specific retinoid, and/or tamoxifen in the prevention of methylnitrosourea-induced mammary cancers. Cancer Lett. 2003;201:17–24. doi: 10.1016/s0304-3835(03)00461-0. [DOI] [PubMed] [Google Scholar]
- 17.Grubbs C, Lubet R, Atigadda V, Christov K, Deshpande A, Tirmal V, Xia G, Bland K, Eto I, Brouillette W, et al. Efficacy of new retinoids in the prevention of mammary cancers and correlations with short-term biomarkers. Carcinogenesis. 2006;27:1232–1239. doi: 10.1093/carcin/bgi308. [DOI] [PubMed] [Google Scholar]
- 18.Elmore L, Forsythe H, Ferreira-Gonzalez A, Garrett C, Clark G, Holt S. Real-time quantitative analysis of telomerase activity in breast tumor specimens using a highly specific and sensitive fluorescent-based assay. Diagn Mol Pathol. 2002;11:177–185. doi: 10.1097/00019606-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Saldanha S, Andrews L, Tollefsbol T. Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Anal Biochem. 2003;315:1–21. doi: 10.1016/s0003-2697(02)00663-2. [DOI] [PubMed] [Google Scholar]
- 20.Tollefsbol T, Andrews L. Mechanisms for telomerase gene control in aging cells and tumorigenesis. Med Hypotheses. 2001;56:630–637. doi: 10.1054/mehy.2000.1241. [DOI] [PubMed] [Google Scholar]
- 21.Kizaki M, Dawson M, Heyman R, Elster E, Morosetti R, Pakkala S, Chen D, Ueno H, Chao W, Morikawa M, et al. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood. 1996;87:1977–1984. [PubMed] [Google Scholar]
- 22.Chen Z. Differentiation therapy of acute promyelocytic leukemia. Chin Med J (Engl) 1996;109:179–182. [PubMed] [Google Scholar]
- 23.Zhang X, Hoffmann B, Tran P, Graupner G, Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992;355:441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- 24.Hansen NJ, Wylie RC, Phipps SM, Love WK, Andrews LG, Tollefsbol TO. The low-toxicity 9-cis UAB30 novel retinoid down-regulates the DNA methyltransferases and has anti-telomerase activity in human breast cancer cells. Int J Oncol. 2007;30:641–650. [PMC free article] [PubMed] [Google Scholar]