Abstract
During cardiomyocyte development, early embryonic ventricular cells show spontaneous activity that disappears at a later stage. Dramatic changes in action potential are mediated by developmental changes in individual ionic currents. Hence, reconstruction of the individual ionic currents into an integrated mathematical model would lead to a better understanding of cardiomyocyte development. To simulate the action potential of the rodent ventricular cell at three representative developmental stages, quantitative changes in the ionic currents, pumps, exchangers, and sarcoplasmic reticulum (SR) Ca2+ kinetics were represented as relative activities, which were multiplied by conductance or conversion factors for individual ionic systems. The simulated action potential of the early embryonic ventricular cell model exhibited spontaneous activity, which ceased in the simulated action potential of the late embryonic and neonatal ventricular cell models. The simulations with our models were able to reproduce action potentials that were consistent with the reported characteristics of the cells in vitro. The action potential of rodent ventricular cells at different developmental stages can be reproduced with common sets of mathematical equations by multiplying conductance or conversion factors for ionic currents, pumps, exchangers, and SR Ca2+ kinetics by relative activities.
Keywords: Cardiac ventricular cell, Computer modeling, Development, Electrophysiology, Ion channels, Spontaneous electrical activity
Background
During vertebrate embryonic development, the heart is the first functional organ to provide nourishment to the developing vertebrate, as an indispensable part of the circulatory system. At a cellular level, early embryonic ventricular cells fire spontaneous action potentials, which eventually disappear at later stages of development (Yokoshiki and Tohse 2001). Dramatic changes in action potential are mediated by the ion channels of the cells. Although developmental changes of such electrophysiological properties have been reported, most have been investigated in individual ion channels; a detailed scheme of cardiomyocyte development has yet to be drawn up.
Developmental changes in ionic currents are reported both at a transcript level and as electrophysiological data. At a transcript level, the ionic channels undergo complex regulation, including subtype switching (Ferron et al. 2002; Nagashima et al. 2001; Franco et al. 2001; Niwa et al. 2004) and alternative splicing (Klugbauer et al. 2002). Despite this complex regulation, the current–voltage (I–V) relationships of the ionic channels do not change significantly among different developmental stages (Ferron et al. 2002; Davies et al. 1996; Kato et al. 1996; Liu et al. 2002; Masuda and Sperelakis 1993). In addition, the I–V relationships of the ionic channels are well conserved among different rodents (Linz and Meyer 2000; Zhang et al. 1994). On the basis of these reports, we make the reasonable assumption that developmental changes in the ion channels can be represented quantitatively as the activities of the channels in the developing rodent relative to those in the adult.
Simulation of cardiac action potential with electrophysiological models has provided a wealth of novel knowledge over the past few decades (Puglisi et al. 2004). Hence, reconstruction of the electrophysiological properties of the individual ionic currents into an integrated mathematical model facilitates our understanding of the developmental changes in cardiac action potential. Here, we show that action potential at different developmental stages can be reproduced with common sets of mathematical models, wherein quantitative changes in the ionic currents, pumps, exchangers, and sarcoplasmic reticulum (SR) Ca2+ kinetics are expressed as relative activities. The models constructed are available online at http://www.ecdn.e-cell.org.
Methods
General approach to modeling of different developmental stages
Simulating of action potentials at different developmental stages were constructed on the basis of the Kyoto model, an electrophysiological model of the guinea pig cardiomyocyte (Matsuoka et al. 2003). In it, all ionic currents, pumps, exchangers, and SR Ca2+ kinetics are expressed in mathematical equations, which include either a conversion factor (pA/mM) or conductance (pA/mV) as one of the parameters.
Various in vitro experimental data, including I–V curves and Western blot analyses, were utilized to estimate the relative activities of ionic currents, pumps, exchangers, and SR Ca2+ kinetics. Those in vitro experimental studies using guinea pigs were preferentially adopted because the Kyoto model was constructed using the adult guinea pig (Matsuoka et al. 2003). Although this was the preferred experimental animal, data from the rat and mouse were also utilized on the basis of the reported observation that the I–V relationships of the ionic channels are well conserved among different rodents (Linz and Meyer 2000; Zhang et al. 1994). In addition, the target stages for simulation of action potentials were set to early embryonic, late embryonic, and neonatal, because plenty of literature was available for these stages. The early embryonic stage represents approximately the mouse at 9.5 days postcoitum (dpc) and the rat at 11.5 dpc; the late embryonic and neonatal stages correspond to 1–5 days before and after birth, respectively.
Ionic currents
Developmental changes of ionic currents are usually reported at a transcript level and as electrophysiological data. Although ionic channels undergo complex regulation at a transcript level, the I–V relationship of most currents does not change among different developmental stages (Ferron et al. 2002; Davies et al. 1996; Kato et al. 1996; Liu et al. 2002; Masuda and Sperelakis 1993). Hence, we assumed that developmental changes in ionic currents are determined mainly by their quantitative changes (Fig. 1, Tables 1–3), which can be represented as the activities of the current in developing stages relative to that in the adult stage.
Fig. 1.
Schematic diagram for modeling rodent ventricular cells at different stages of development. Early embryonic stage corresponds to approximately 9.5 dpc mouse and 11.5-dpc rat. Late embryonic stage corresponds to 1–5 days before birth. Neonatal stage corresponds to 1–5 days after birth. The developmental changes are represented as relative activities, which are obtained or estimated from various in vitro experimental data. [All the relative activities are listed in Tables 1–3]
Table 1.
Relative activities for ionic currents, as obtained from the literature
| Current | EE | Ref. | LE | Ref. | N | Ref. |
|---|---|---|---|---|---|---|
| INa | 0.08 | Davies et al. (1996) | 1.00 | Davies et al. (1996) | 1.00 | Davies et al. (1996) |
| ICaL | 0.46 | Liu et al. (2002) | 0.78 | Kato et al. (1996, Liu et al. 2002) | 0.78 | Kato et al. (1996) |
| ICaT | 4.50 | Ferron et al. (2002) | 4.50 | Ferron et al. (2002) | 2.90 | Ferron et al. (2002) |
| IK1 | 0.11 | Masuda and Sperelakis (1993) | 1.00 | Kato et al. (1996) | 1.00 | Kato et al. (1996) |
| IKATP | 0.32 | Xie et al. (1997) | 0.88 | Xie et al. (1997) | 1.60 | Xie et al. (1997) |
Relative activities of INa, ICaL, ICaT, IK1, and IKATP for the early embryo (EE), late embryo (LE), and neonatal (N) stage were estimated from the current-voltage (I–V) curves of the cells in vitro. I–V curve of INa was obtained from 11- to 13-dpc (early embryonic), and 17- to 20-dpc (late embryonic) mice; expression of INa reached the adult level in the late embryonic stage (Davies et al. 1996). For ICaL, the early embryonic I–V curve was obtained from 9.5-dpc mice (Liu et al. 2002); the late embryonic I–V curve was obtained from both 18-dpc mice (Liu et al. 2002) and fetal guinea pigs 1–7 days before birth (Kato et al. 1996); the neonatal I–V curve was obtained from neonatal guinea pigs t 1–5 days after birth (Kato et al. 1996). Relative activities of ICaT were obtained on the basis of data for the 14-dpc rat, 18-dpc rat, and 1-day-old rat (Ferron et al. 2002), which corresponded to EE, LE, and N, respectively. For IK1, I–V curves of the 12-dpc rat (Masuda and Sperelakis 1993), the fetal guinea pig 1–7 days before birth (Kato et al. 1996), and the neonatal guinea pig 1–5 days after birth (Kato et al. 1996) were obtained. Relative activities of IKATP were obtained on the basis of data for the 12-dpc rat, 18-dpc rat, and 1-day-old rat (Xie et al. 1997)
Table 3.
Relative ratios of ion fluxes of exchangers, pumps, and sarcoplasmic reticulum (SR) Ca2+ kinetics
| Current | EE | Ref. | LE | Ref. | N | Ref. |
|---|---|---|---|---|---|---|
| Na+/Ca2+ exchange | 4.95 | Liu et al. (2002) | 1.74 | Liu et al. (2002), Artman (1992) | 1.00 | Liu et al. (2002), Artman et al. (1995), Artman (1992) |
| SR Ca2+ pump | 0.03 | Liu et al. (2002) | 0.21 | Liu et al. (2002), Chen et al. (2000) | 0.21 | Liu et al. (2002), Chen et al. (2000) |
| RyR channel | 0.05 | Liu et al. (2002) | 0.40 | Liu et al. (2002) | 0.40 | Liu et al. (2002) |
| SR transfer | 0.04 | Liu et al. (2002) | 0.30 | Liu et al. (2002) | 0.30 | Liu et al. (2002) |
| SR leak | 0.04 | Liu et al. (2002) | 0.30 | Liu et al. (2002) | 0.30 | Liu et al. (2002) |
Developmental change in Na+/Ca2+ exchange (INaCa) is reported as I–V curves in both the rabbit and guinea pig (Artman et al. 1995) and by Western blots of NCX1 protein in the mouse (Liu et al. 2002) and rabbit (Artman 1992); on the basis of the literature implying that postnatal quantitative changes in the density of INaCa are in good agreement with the changes in protein production level, we assumed that the relative production level of the proteins directly reflected the relative ratios of ion fluxes of INaCa, the SR Ca2+ pump (ISR,uptake), and the RyR channel (IRyR). Hence, we computed the relative ratios of the current fluxes from Western blots of SR-related proteins (Liu et al. 2002; Chen et al. 2000). The average relative production levels of SR-related proteins in the EE stage (0.04), LE stage (0.30), and N stage (0.30) were adopted as the relative activities of ISR,transfer and ISR,leak at these stages
The relative activities of ionic currents were either computed from I–V curves (Table 1) or estimated on the basis of qualitative observations (Table 2). We confirmed that the I–V relationship did not change among different developmental stages for ICaL (Kato et al. 1996), ICaT (Ferron et al. 2002), IK1 (Kato et al. 1996; Masuda and Sperelakis 1993), IKr (Davies et al. 1996), IKs (Davies et al. 1996), and INa (Davies et al. 1996). The relative activities were multiplied by the conversion factor or the conductance of the corresponding ionic current. In addition, all currents listed in Table 1 were normalized by the ratio of the cell capacitance (Cm) of individual myocytes at the corresponding developmental stages (Table 4) to that of adult ventricular cells (132 pF), because I–V relationships are usually reported as current density (pA/pF), and the Kyoto model presents current in pA units. The ratios were 28/132 for the early embryonic ventricular cell model, 35/132 for the late embryonic ventricular cell model, and 40/132 for the neonatal ventricular cell model.
Table 2.
Estimated relative activities of ionic currents
| Current | EE | Ref. | LE | Ref. | N | Ref. |
|---|---|---|---|---|---|---|
| Iha | 100.00 | Yasui et al. (2001) | 18.00 | Yasui et al. (2001) | 0.00 | n/a |
| IKr | 10.00 | Spence et al. (1994), Chun et al. (2004) | 2.00 | Kato et al. (1996), Wang et al. (1996) | 1.50 | Kato et al. (1996), Wang et al. (1996) |
| IKs | 0.01 | Davies et al. (1996) | 0.01 | Davies et al. (1996), Kato et al. (1996) | 2.00 | Kato et al. (1996) |
| Ito | 0.01 | Davies et al. (1996) | 0.27 | Kilborn and Fedida (1990) | 0.27 | Kilborn and Fedida (1990) |
| IbNSC | 0.35 | n/a | 0.43 | n/a | 0.49 | n/a |
Relative activities of Iha, IKr, IKs, and IbNSC were estimated from various qualitative observations. The conversion factor of Iha was set to 0 in the adult guinea pig ventricular cell model (Matsuoka et al. 2003); thus the expression levels of Iha in the early embryonic stage and late embryonic stage were estimated from the I–V curves of Iha in 9.5- and 18-dpc mice (Yasui et al. 2001). Relative activities of IKr and IKs were estimated from various in vivo and in vitro experimental data: I–V curve of IK, sum of IKr and IKs in fetal and neonatal guinea pigs (Kato et al. 1996); I–V curves of IKs in 11–13-dpc and 17–20-dpc mice (Davies et al. 1996); qualitative observation using the selective IKr blocker dofetilide in 11- and 14.5-dpc rats (Spence et al. 1994; Chun et al. 2004), and in 18-dpc and 1-day-old mice (Wang et al. 1996). For Ito, I–V curves of the 11-dpc mice (Davies et al. 1996) and 1-day-old rat after birth (Kilborn and Fedida 1990) were obtained for the estimation. Because we found that IbNSC plays an important role in the spontaneous action potential of early embryonic ventricular myocytes, relative activities were estimated from the activity of the current in SA node cells, which was normalized according to the cell capacitances
Table 4.
Cell capacitances and volumes of cell compartments
| Parameter(unit) | EE | Ref. | LE | Ref. | N | Ref. |
|---|---|---|---|---|---|---|
| Cm (pF) | 28 | Yasui et al. (2001) | 35 | Kato et al. (1996), Yasui et al. (2001) | 40 | Kato et al. (1996) |
| Vi (μl) | 1.697 × 10−3 | Huynh et al. (1992) | 2.121 × 10−3 | Huynh et al. (1992) | 2.424 × 10−3 | Huynh et al. (1992) |
| Vrel (μl) | 1.357 × 10−6 | Liu et al. (2002) | 1.273 × 10−5 | Liu et al. (2002) | 1.454 × 10−5 | Liu et al. (2002) |
| Vup (μl) | 3.394 × 10−6 | Liu et al. (2002) | 3.182 × 10−5 | Liu et al. (2002) | 3.636 × 10−5 | Liu et al. (2002) |
Cm values of mouse early embryonic ventricular cells (28 pF), guinea pig late embryonic cells (35 pF), and guinea pig neonatal ventricular cells (40 pF) were adopted (Kato et al. 1996; Yasui et al. 2001). Cell volume (Vi) was estimated by multiplying adult Vi (8.0 × 10−3 μl) by the corresponding Cm (28, 35, or 40 pF) over adult Cm (132 pF), on the basis of the report that there is a positive linear correlation between membrane capacitance and cell volume (Huynh et al. 1992). The volume fraction of Vrel and that of Vup to Vi were set on the basis of the average relative expression levels of these proteins in the EE stage (0.04), LE stage (0.30), and N stage (0.30), as estimated from Western blots of SR-related proteins (Liu et al. 2002)
Background ionic currents
IbNSC is known to have a higher current density in sinoatrial (SA) node cells than in ventricular cells (Kiyosue et al. 1993). Because we found that IbNSC plays an important role in the spontaneous action potential of both SA node cells and early embryonic ventricular myocytes, the current amplitudes at different stages were scaled down according to the cell capacitances of the fetal and neonatal cells (Table 2).
IKACh is known to have negligible effects on the action potential of ventricular cells during the course of development (Davies et al. 1996; Xie et al. 1997). In addition, IKACh is not included in adult ventricular cell models (Matsuoka et al. 2003). Hence, we excluded IKACh from the models. Other background currents, including IKpl, II(Ca), and ICab were assumed to have steady current densities; as such, these currents were normalized to the corresponding cell capacitance by the method described above.
Exchangers, pumps, and SR Ca2+ kinetics
Developmental changes in exchangers, pumps, and SR Ca2+ kinetics are mostly reported as Western blots of the corresponding proteins. Here, we assumed that the relative production levels of the proteins directly reflected the relative ratios of ion fluxes of Na+/Ca2+ exchange, the SR Ca2+ pump, the ryanodine receptor (RyR) channel, and other SR Ca2+ kinetics; this assumption can be partly supported by the results of previous reports, which imply that the postnatal change in the current density of Na+/Ca2+ exchange (Artman et al. 1995) is in good agreement with that in the production level of the corresponding protein (Liu et al. 2002; Mahony 1996; Artman 1992). The average relative production values of SR-related proteins in the early embryonic stage (0.04), late embryonic stage (0.30), and neonatal stage (0.30) were adopted for estimating those values for ISR,transfer and ISR,leak.
Cell capacitance and volume of cell compartments
Cm and volumes (Vi, Vrel, Vup) were computed on the basis of quantitative data from the literature (Table 4). No significant differences were observed between the Cm of mouse ventricular cells (31 ± 3.3 pF) and that of guinea pig ventricular cells (34.5 ± 2.72 pF) in the late embryonic stage (Kato et al. 1996; Yasui et al. 2001). As such, the Cm values for mouse early embryonic ventricular cells (28 pF), guinea pig late embryonic ventricular cells (35 pF), and guinea pig neonatal ventricular cells (40 pF) were adopted.
The developmental change in Vi in rabbit ventricular cells is roughly proportional to that of cell capacitance (Huynh et al. 1992). In addition, a positive linear correlation was found between membrane capacitance and cell volume in several species (Satoh et al. 1996). Hence, cell volume was estimated by multiplying the adult Vi (8.0 × 10−3 μl) by the corresponding Cm (28, 35, or 40 pF) over the adult Cm (132 pF).
In the Kyoto model, the volume fractions of Vrel and Vup were set to 2% and 5% of Vi, respectively (Matsuoka et al. 2003). The SR-mediated Ca2+ transient is modeled by multiplying an estimated value called the “SA factor” by Vrel, Vup, and SR-related currents in the Kyoto model (Matsuoka et al. 2003). The same approach has been adopted for estimating Vrel and Vup in different developmental stages of ventricular cells; on the basis of quantitative changes in SR-related proteins (Liu et al. 2002), the average relative expression values of those proteins in the early embryonic stage (0.04), late embryonic stage (0.30), and neonatal stage (0.30) were utilized for the estimation.
Simulation of action potential at three different developmental stages
The E-Cell simulation environment version 3.1 (Takahashi et al. 2004) was utilized to simulate the action potential. All of the models were simulated for 200 s to confirm that the spontaneous action potentials were stable or that the membrane potential had reached a quasi-steady state. Hence, the simulation results presented in this report were recorded after simulating the corresponding models for 200 s. In addition, an external current (Iext) was applied in the late embryonic and neonatal ventricular cell models in order to fire the action potential of the cells.
To determine the factors that contribute to the dramatic changes in action potential between early and late embryonic stages, the relative activity of each current was shifted from early embryonic to late embryonic by 10% increments. We assumed that all of the changes in the current components proceeded steadily between the representative stages. The action potentials were obtained 200 s after changing the parameters of the original condition.
Modeling and simulation of action potentials in the Luo–Rudy model
Another electrophysiological model, the Luo–Rudy model (Faber and Rudy 2000), was utilized to further validate the simulated result with the Kyoto model. The Luo–Rudy model included all the ionic currents listed in Fig. 1 except Ito, IKATP, and Iha; all relative activities except those of these currents were implemented by the same procedure as used in the Kyoto model. Several background currents were left unchanged, because ionic currents in Luo–Rudy model are presented as current density (μA/μF). Cell volumes (Vi, Vrel, Vup) were computed accordingly by the procedure described above. Because the Luo–Rudy model requires “pacing” of the action potential, the model was simulated for 600 s as instructed in the report (Faber and Rudy 2000).
Results
Simulated action potential at different developmental stages
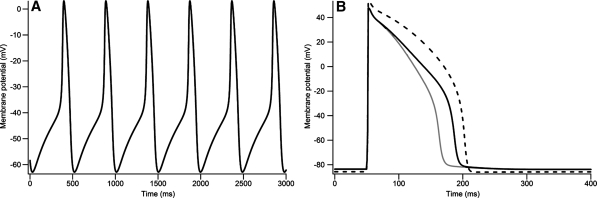
The early embryonic ventricular cell model exhibited a spontaneous action potential (Fig. 2A). After the maximum diastolic potential (MDP) at −62.86 mV, the membrane potential slowly depolarized until it reached approximately −40 mV, when spontaneous action potential was triggered. The membrane potential then started to repolarize at 3.13 mV, and completed repolarization in less than 100 ms. The whole action potential was completed in a basic cycle length (BCL) of 492 ms.
Fig. 2.
Simulated action potentials at different developmental stages with the constructed models. (A) Simulated action potential at early embryonic stage. (B) Simulated action potential at late embryonic stage (dark line) and neonatal stage (light line). Action potential at adult stage is shown as dashed line
The spontaneous action potential ceased in the later stages of development (Fig. 2B). Both late embryonic and neonatal ventricular cells showed resting membrane potentials that were more hyperpolarized (−83.60 mV) than the MDP of the early embryonic ventricular cell. Repolarization of the action potential occurred more slowly in the late embryonic ventricular cell than in the neonatal ventricular cell; the action potential duration (APD) at 90% was 140 ms in the late embryonic ventricular cell and 117 ms in the neonatal ventricular cell.
Simulated action potential at the early embryonic stage in the Kyoto and Luo–Rudy models
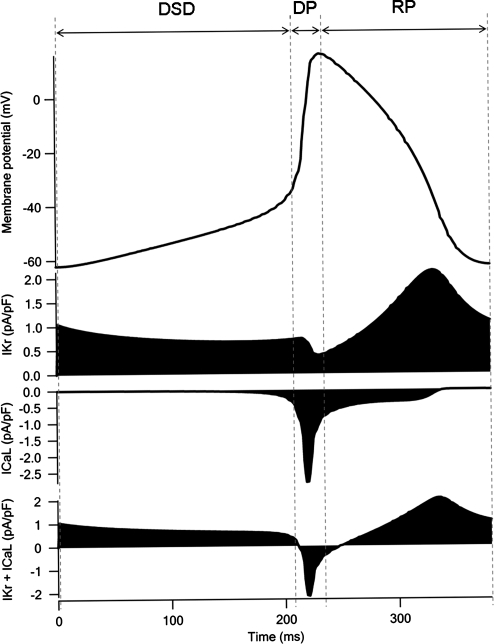
A spontaneous action potential was observed in both the Kyoto (Fig. 3A) and Luo–Rudy models (Fig. 3B). The MDP was more negative in the Luo–Rudy model (−71.16 mV) than in the Kyoto model (−62.86 mV). Repolarization of the spontaneous action potential started from the overshoot at 13.74 mV in the Luo–Rudy model. Both the depolarization phase (DP) and the repolarization phase (RP) were faster in the Luo–Rudy model, resulting in a shorter BCL (414 ms); differences in simulated action potential were determined by differences in ionic currents.
Fig. 3.
Simulated action potential and ionic currents at early embryonic stage with two different electrophysiological models. Simulated action potential can be divided into three phases: diastolic slow depolarization (DSD) phase, depolarization phase (DP), and repolarization phase (RP). (A) Simulated action potential, IKr current, and ICaL current in the Kyoto model. (B) Simulated action potential, IKr current, and ICaL current in the Luo–Rudy model. Sum of IKr and ICaL shows that the increase in outward (positive) current is slower in the Kyoto model than in the Luo–Rudy model
The dynamic behavior of the ionic currents (ICaL and IKr) observed during action potential simulation is shown in Fig. 3. Ionic currents were expressed by different mathematical equations in the Kyoto and Luo–Rudy models, so the simulated behavior of the ionic currents differed between the models. The sums of ICaL and IKr are shown in Fig. 3. Apparently, activation of IKr in RP was faster in the Luo–Rudy (Fig. 3B) than in the Kyoto model (Fig. 3A). Although activation of ICaL in DP occurred at approximately the same rate in both models, the peak of ICaL in the Kyoto model was smaller than that of ICaL in the Luo–Rudy model. In addition to the fact that the ICaL was larger than the Kyoto model, reactivation of the ICaL was observed in RP in the Luo–Rudy model.
Effects of shifting relative activities of current components
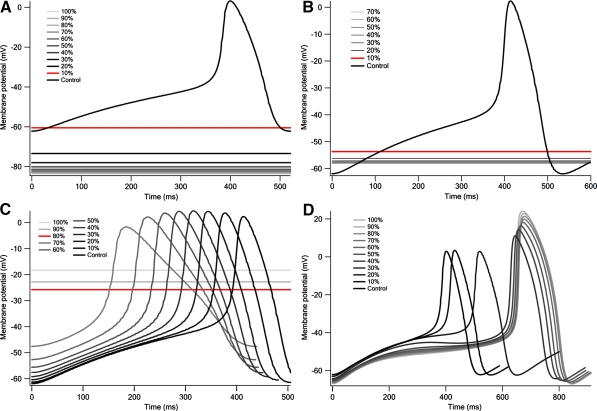
The spontaneous action potential of early embryonic ventricular cells ceased when the relative activities of all current components were shifted by 10% or more toward the late embryonic stage value (Fig. 4A).
Fig. 4.
Effects of shifting relative activities of all current components (A), IK1 (B), IKr (C), and of all current components except IK1 and IKr (D). (A) Changes in action potential when shifting relative activities of all currents by 10% increments. (B) Changes in action potential when shifting from relative activity of early embryonic IK1 (darkest line) to that of late embryonic IK1 (lightest line) by 10% increments. Spontaneous action potential ceased when relative activity of IK1 was shifted by 10% (red line) or more toward late embryonic stage value. Dataset could not be obtained when the current was shifted by 90% and 100% toward the late embryonic stage value, owing to collapse in the balance of the ionic concentration. (C) Changes in action potential when shifting from relative activity of early embryonic IKr (darkest line) to that of late embryonic IKr (lightest line) by 10% increments. Spontaneous action potential ceased when relative activity of IKr was shifted by 80% (red line) or more toward late embryonic stage value. (D). Changes in action potential when relative activities of all currents except IK1 and IKr were shifted from early embryonic to late embryonic values by 10% increments
Shifting the relative activities of IK1 and IKr from early embryonic to late embryonic values contributed to the disappearance of the spontaneous action potential (Figs. 4B, C). The spontaneous action potential ceased when the relative activity of IK1 was shifted by 10% or more toward the late embryonic stage values (Fig. 4B). When the relative activity of IK1 was shifted from 10% to 80% toward the late embryonic stage value, the resting membrane potential became more negative (−53.69 to −58.15 mV). In contrast, the spontaneous action potential ceased when the relative activity of IKr was shifted by 80% or more toward the late embryonic stage value (Fig. 4C), at which time the resting membrane potential was −25.85 mV.
The spontaneous action potential did not disappear when the relative activities of all current components except IK1 and IKr were shifted toward the late embryonic stage value (Fig. 4D). The MDP shifted positively from −61.28 to −47.55 mV when the relative activity was shifted by 10 to 70% toward the late embryonic stage value. These results further indicate that developmental changes in the relative activities of IK1 and IKr are the sole factors in the disappearance of the spontaneous action potential.
Discussion
This study provides for the first time computational models simulating the action potential at 3 different developmental stages. We have shown that developmental changes in action potential can be exhibited with the same set of mathematical equations by multiplying equations for ionic currents, pumps, exchangers, and SR Ca2+ kinetics by their corresponding relative activities.
Simulated action potential at the early embryonic stage
The early embryonic ventricular cell model reproduced well the spontaneous action potential that is generated by ventricular cells in 12-dpc rats (Nagashima et al. 2001). Species-specific differences in spontaneous action potential waveforms have been observed between ventricular cells in 9.5-dpc mice (Yasui et al. 2001) and 12-dpc rats (Nagashima et al. 2001). The ventricular cells in 9.5-dpc mice generate a more hyperpolarized MDP (−71.2 ± 0.4 mV) than in 12-dpc rats (−66.7 ± 3.6 mV). A spontaneous action potential is triggered when the membrane potential reaches approximately −60 mV in 9.5-dpc mice and approximately −40 mV in 12-dpc rats (Nagashima et al. 2001; Yasui et al. 2001). The simulated action potential in our early embryonic ventricular cell model (Fig. 2A) was very similar to the action potential waveforms generated by the automatically beating cells in 12-dpc rats (Nagashima et al. 2001). In addition, the MDP of the simulated action potential (−62.86 mV) was approximately equal to that of the ventricular cells in 12-dpc rats. Hence, our early embryonic ventricular cell model could reproduce an action potential that was in reasonable agreement with those of previous studies (Nagashima et al. 2001; Yasui et al. 2001).
The speed of the spontaneous action potential in the early embryonic stage is a controversial issue. Unfortunately, the action potential of early embryonic guinea pig ventricular cells has not been reported. Early embryonic hearts have shown a large range of heart rates, from 61 to 219 min−1 in 11.5-dpc rats (Couch et al. 1969). The BCL of the simulated action potential of our early embryonic ventricular cell model (492 ms) was roughly consistent with that of ventricular cells in 9.5-dpc mice, which is 510.8 ± 32.8 ms (Yasui et al. 2001). Although the BCL of the action potential of our model was approximately consistent with that of previous study (Yasui et al. 2001), it should be noted that the BCL of the simulated action potential might not be quantitatively accurate, because early embryonic hearts have a large range of rates in vivo.
Simulated action potential at the late embryonic stage and neonatal stage
Our models reproduced the qualitative characteristics of late embryonic and neonatal ventricular cells that have been reported (Kato et al. 1996; Kojima et al. 1990). Such characteristics include disappearance of spontaneous action potential and hyperpolarized resting membrane potential. Disappearance of the spontaneous action potential in the late embryonic stage is consistent with the results of previous studies. Spontaneous activity has not been recorded from the ventricular muscles of 16-dpc or older rats (Kojima et al. 1990). The membrane potential of ventricular cells became more hyperpolarized in 18-dpc rats than in 12-dpc rats (Nagashima et al. 2001). In addition, recent studies have reported that the resting membrane potential has no statistically significant differences between late embryonic and neonatal ventricular cells (Kato et al. 1996), which is consistent with our simulated cell action potential.
Developmental changes in APD are totally different among rodents. The action potentials of the neonatal rabbit and guinea pig have a long plateau phase (Kato et al. 1996; Sanchez-Chapula et al. 1994), whereas those of the neonatal mouse and rat are immediately repolarized (Nagashima et al. 2001; Wang et al. 1996). One of the well-recognized factors that contributes to this difference is the transient outward current (Ito), which is considered absent in the ventricular cells of the adult guinea pig under physiological conditions. A recent report classified the Ito as either a 4-aminopyridine (AP)-sensitive K+ current or a 4-AP-resistant Ca2+-activated current, and showed the presence of the latter current under conditions of Ca2+ overload (Nakajima et al. 2002). In the Kyoto model, Ito was implemented as having a negligibly small amplitude, and we confirmed that decreased activity of Ito had hardly any effect on the simulated action potential in the late embryonic, neonatal, and adult ventricular cell models. In simulation with early embryonic ventricular cells, however, we noticed that Ito subtly affected diastolic depolarization, although the current did not block the spontaneous action potential (data not shown). Unfortunately, the presence of the Ito in the early embryonic guinea pig has not been reported in the literature. Although we admit that the characteristics of the Ito in embryonic mice may not be applicable to guinea pigs, we adopted the relative activity of the current in the mouse and rat for application to early embryonic, late embryonic, and neonatal ventricular cell models. The issue of Ito therefore requires further assessment and discussion.
Despite the prominent differences in the shape of the action potential, shortening of the APD between the late embryonic stage and the neonatal stage has been observed in both guinea pigs (Nagashima et al. 2001) and rats (Kojima et al. 1990). The rapid component (IKr) and slow component (IKs) of the delayed rectifier K+ current play important roles in repolarization and thus control the length of the APD; both IKr and IKs undergo very complex changes in their activities between the late embryonic and neonatal stages (Fig. 1). We estimated the relative activities of both IKr and IKs on the basis of the qualitative characteristics of these currents, including the changes in APD in response to a selective IKr blocker. Although qualitatively consistent, APD may not be quantitatively accurate because several relative activities were estimated on the basis of qualitative characteristics.
Roles of individual ionic currents in spontaneous action potential
Disappearance of spontaneous firing of action potential is one of the important changes in electrical activity of the ventricular cells during embryonic development. Our simulation has shown that the spontaneous action potential of early embryonic ventricular cells ceased when the relative activities of all current components were shifted by 10% or more toward the late embryonic stage value (Fig. 4A), which was roughly consistent with the previous report that spontaneous activity has not been recorded from the ventricular muscles of 16-dpc or older rats (Kojima et al. 1990). To determine the factors that contribute to the disappearance of spontaneous action potential during embryonic development, the relative activity of each current was shifted from early embryonic to late embryonic by 10% increments.
Yasui et al. (2001) implied that Iha is important for the autonomic modulation of heart rate; the relative activity of Iha is decreased by 82% during embryonic development and absent in adult ventricular cells. Although Iha is not included in the adult ventricular cell model, the Kyoto model implemented the current as part of its SA node cell model. The developmental changes in Iha were thus simulated by shifting the relative activity from 100 to 18; the decrease in Iha subtly affected the DSD phase and increased the BCL by 20 ms, but did not induce disappearance of the spontaneous action potential (data not shown).
Hyperpolarization of the membrane potential via an increase in IK1 is considered to be a major factor contributing to the disappearance of spontaneous beating (Nagashima et al. 2001). This previous finding is consistent with our result that a 10% increase in IK1 shifted the membrane potential more negatively and stopped the spontaneity (Fig. 4B). Although hyperpolarization was not as strong when the relative activities of all current components were shifted 10% toward the late embryonic stage value (Fig. 4A), this result suggests that hyperpolarization of the membrane potential by an increase in IK1 is the most important factor in the disappearance of the spontaneous action potential.
Application of defetilide, an IKr specific blocker, is reported to strongly impair the rhythm of embryonic hearts with lethal effect (Spence et al. 1994). Although the simulation may not be quantitatively accurate, the spontaneous action potential ceased when the relative activity of IKr was shifted by 80% or more toward the late embryonic stage value (Fig. 4C). Additional simulation showed that spontaneity did not disappear when shifted relative activities of all current components except IK1 and IKr to the late embryonic stage value (Fig. 4D), which further indicated that developmental changes in the relative activities of IK1 and IKr are the key factors in the disappearance of the spontaneous action potential.
Comparison of the spontaneous action potentials of early embryonic ventricular cells and differentiated SA node cells
The spontaneous action potential is one of the important features of electrical activity in early embryonic ventricular cells. It is well known that fully differentiated SA node cells show spontaneous action potential. Figure 5 shows the action potential of the SA node cells, as simulated by using common sets of mathematical equations; the SA node cells had a longer RP phase than early embryonic ventricular cells. The amplitude parameters of the current components in early embryonic ventricular cells were similar to those in SA node cells, but one prominent difference is that the early embryonic ventricular cells had more ICaT and INaCa than did the SA node cells.
Fig. 5.
Simulated action potential and ionic currents of SA node cells. Simulated action potential and changes in IKr current, ICaL current, and sum of IKr and ICaL accompanying the spontaneous action potential are indicated. BCL of the action potential was 382 ms, which was shorter than that of early embryonic ventricular cells (492 ms). The MDP was approximately the same in the SA node cells (−62.17 mV) and early embryonic ventricular cells (−62.86 mV). The overshoot was 16.14 mV in the SA node cells: this value was more positive than that in the early embryonic ventricular cells (3.13 mV)
Recently, the roles of both the ICaT and INaCa, as well as that of Ca2+ release through the RyR channel in the SR, have been noted as important factors in diastolic depolarization of the SA node cells (Lakatta et al. 2003). In contrast, the role of these components in the early embryonic ventricular cells is a controversial issue. Most previous studies agree with the observation that the SR is scarce and poorly organized in early embryonic ventricular cells (Olivetti et al. 1980); in rats the t-tubules of the ventricular cells begin to form during postnatal development (Seki et al. 2003). A recent report showed that application of ryanodine to 9.5-dpc mice did not affect the spontaneous Ca2+ transient (Takeshima et al. 1998), whereas another report stated that the magnitude of the Ca2+ transient was decreased after the application of ryanodine to 12-dpc rats (Seki et al. 2003).
In addition, the role of abundant Na+/Ca2+ exchange in early embryonic ventricular cells remains unclarified. One previous study generated NCX1-deficient mice and showed that the absence of NCX1 was lethal to the embryo at approximately 9.0 dpc as a result of abnormal cardiac development (Cho et al. 2000). Moreover, application of an inhibitor of the reverse mode of Na+/Ca2+ exchange to 9.5-dpc mice had no substantial effect on the Ca2+ transient of the ventricular cells, indicating that Ca2+ entry by the reverse mode of Na+/Ca2+ exchange is not a main source of the Ca2+ transient in early embryonic ventricular cells (Liu et al. 2002). As a future prospect, by the expansion of our models it will be possible to contrive models that reflect each of these contended concepts, and we believe that comparative simulation with such models will provide novel insights into these controversial issues.
Simulation of action potential with the Kyoto and Luo–Rudy models
Spontaneous action potentials have been observed in simulations with the Kyoto and Luo–Rudy models (Fig. 3). This result strongly supports our claim that spontaneous action potential in the early embryonic stage can be reproduced with the same sets of mathematical equations as used in the adult model by multiplying by the corresponding relative activities. This result also supports our simulated result that lack of Iha and Ito does not considerably affect spontaneous firing of the action potential, because those currents are absent in the Luo–Rudy model. Although both models have simulated spontaneous action potentials, the Kyoto model reproduced both the quantitative characteristics and qualitative behavior of previously reported experimental data (Nagashima et al. 2001) more consistently than the Luo–Rudy model.
Faster RP was observed in the Luo–Rudy model (Fig. 3B) than in the Kyoto model (Fig. 3A). Repolarization of the spontaneous action potential is mediated mostly by IKr; therefore, fast repolarization in the Luo–Rudy model is determined by fast activation of IKr. Whereas IKr in the Luo–Rudy model is described by one activation gate (Faber and Rudy 2000), IKr in the Kyoto model involves two activation gates and one inactivation gate (Matsuoka et al. 2003); the kinetics and parameters of IKr in the Kyoto model were obtained on the basis of IKr in SA node cells (Ono and Ito 1995).
The late half of the RP was slower in the Luo–Rudy model than in the Kyoto model; this was caused by reactivation of ICaL. ICaL in the Kyoto model is immediately inactivated when the membrane potential has started to repolarize from the peak of the action potential, whereas ICaL is reactivated in the middle of the RP in the Luo–Rudy model. ICaL in the Luo–Rudy model contains a voltage-dependent activation gate, a voltage-dependent inactivation gate, and a Ca2+-dependent gate. In contrast, the Kyoto model uses a 4-state model (Shirokov et al. 1993) for both the voltage-dependent gate and Ca2+-dependent gate, which can be adjusted on the basis of the kinetics of ICaL and the action potential of rabbit SA node cells (Hagiwara et al. 1988).
The DSD phase is faster in the Luo–Rudy model than the Kyoto model. The Luo–Rudy model lacks several currents that are included in the Kyoto model; such currents include Iha and IbNSC. The balance of IKr and IbNSC determines the MDP of the spontaneous action potential in the Kyoto model (Sarai et al. 2003). Although it has been reported that Iha plays an important role in the spontaneous activity of early embryonic ventricular cells (Yasui et al. 2001), Iha plays a minor role in diastolic depolarization in the Kyoto model (Sarai et al. 2003). Hence, the difference in IbNSC between the two models is the most important difference contributing to the increased speed of the DSD phase.
All of the our observations we have described thus far indicate that the Kyoto model reproduced the action potential in the early embryonic stage more consistently than did the Luo–Rudy model. Although the Luo–Rudy model was developed for a single cell type, the model equations in the Kyoto model were developed for two cell types: SA node cells and ventricular cells (Matsuoka et al. 2003); in this report, Matsuoka et al. intentionally used common mathematical equations, because no obvious difference has been observed in the electrophysiological properties of the currents, in terms of their kinetics. We may have taken advantage of their intention, because most currents in ventricular cells change quantitatively with similar kinetics throughout the stages of development.
Limitations
Although our primary target species for the modeling was guinea pigs and we attempted to preferentially adopt those in vitro experimental data on the guinea pigs, the model included experimental data on the rat and mouse, especially in the case of the early embryonic ventricular cell model. We intended to utilize the term “rodent” to represent all species from which we obtained experimental data, but this does not imply that the models constructed represent rodent ventricular cells in general. Hence, we admit that the results of our simulations only roughly represent developmental changes in the electrophysiological activity of rodent ventricular cells at the representative stages.
In addition, in light of our initial assumption that developmental changes in ionic currents are determined mainly by the quantitative changes in these currents, further application of the models we have constructed may need to be cautious, especially in terms of quantitative interpretation of the simulation results. Our assumption may be too abstract in regard to the ways in which several ionic channels undergo complex regulation, such as by subtype switching and alternative splicing. Despite the fact that such complex regulations affect ionic channel kinetics, the results of our simulations using models constructed only on the basis of quantitative changes were roughly consistent with action potentials previously reported in vitro (Nagashima et al. 2001; Kato et al. 1996). We thus believe that one of the important points of this study is that the developmental changes in action potential were well represented by the same sets of mathematical equations as used in the adult model, simply by multiplying by the corresponding relative activities.
Conclusions
We assumed that developmental changes of the ion channels could be represented quantitatively as the activities of the channels in the developing rodent relative to those in the adult. Multiplication of the relative activities by the corresponding mathematical equations reproduced the developmental changes in the action potential of the rodent ventricular cell. Although both the Kyoto and Luo–Rudy models represented spontaneous action potentials, the Kyoto model reproduced action potentials in the early embryonic stage more consistently than did the Luo–Rudy model.
Authors' contributions
HI conceived the study, drafted the design, analyzed the collected data, and wrote the manuscript. YN participated both in the design of the study and implementation of the model by the E-Cell system. MT was involved in coordination and supervision of the study. Both YN and MT helped to draft the manuscript. All the authors read and approved the final manuscript.
Acknowledgements
This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (the Leading Project for Biosimulation and the 21st Century Center of Excellence [COE] Program: Understanding and Control of Life’s Function via Systems Biology).
Abbreviations
- ICaL
L-type Ca2+ current
- ICaT
T-type Ca2+ current
- INaCa
Na+/Ca2+ exchange current
- INaK
Na+/K+ pump current
- INa
Na+ current
- IbNSC
background non-selective cation current
- II(Ca)
Ca2+-activated background cation current
- ICab
background Ca2+ current
- IKATP
ATP-sensitive K+ current
- IK1
inward rectifier K+ current
- IKr
delayed rectifier K+ current, rapid component
- IKs
delayed rectifier K+ current, slow component
- IKpl
non-specific, voltage-dependent outward current
- Ito
transient outward current
- Iha
hyperpolarization-activated cation current
- IKACh
ACh-activated K+ current
- SR
sarcoplasmic reticulum
- ISR,uptake
Ca2+ uptake in the SR
- ISR,leak
Ca2+ leak from the SR
- ISR,transfer
Ca2+ transfer from the SR uptake site to the release site
- IRyR
Ca2+ release through the RyR channel in SR
- Iext
current applied externally through the electrode
- Cm
cell capacitance
- Vi
cell volume accessible for ion diffusion
- Vrel
volume of SR release site
- Vup
volume of SR uptake site
- MDP
maximum diastolic potential
- BCL
basic cycle length
- DP
depolarization phase
- RP
repolarization phase
- DSD
diastolic slow depolarization
- APD
action potential duration
Contributor Information
Hitomi Itoh, Email: ducky@sfc.keio.ac.jp.
Yasuhiro Naito, Email: ynaito@sfc.keio.ac.jp.
Masaru Tomita, Email: mt@sfc.keio.ac.jp.
References
- Artman M (1992) Sarcolemmal Na(+)–Ca2+ exchange activity and exchanger immunoreactivity in developing rabbit hearts. Am J Physiol 263(5 Pt 2):H1506–H1513 [DOI] [PubMed]
- Artman M, Ichikawa H, Avkiran M, Coetzee WA (1995) Na+/Ca2+ exchange current density in cardiac myocytes from rabbits and guinea pigs during postnatal development. Am J Physiol 268(4 Pt 2):H1714–H1722 [DOI] [PubMed]
- Chen F, Ding S, Lee BS, Wetzel GT (2000) Sarcoplasmic reticulum Ca(2+)ATPase and cell contraction in developing rabbit heart. J Mol Cell Cardiol 32(5):745–755 [DOI] [PubMed]
- Cho CH, Kim SS, Jeong MJ, Lee CO, Shin HS (2000) The Na+–Ca2+ exchanger is essential for embryonic heart development in mice. Mol Cells 10(6):712–722 [DOI] [PubMed]
- Chun KR, Koenen M, Katus HA, Zehelein J (2004) Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp Mol Med 36(4):367–371 [DOI] [PubMed]
- Couch JR, West TC, Hoff HE (1969) Development of the action potential of the prenatal rat heart. Circ Res 24(1):19–31 [DOI] [PubMed]
- Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS (1996) Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res 78(1):15–25 [DOI] [PubMed]
- Faber GM, Rudy Y (2000) Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J 78(5):2392–2404 [DOI] [PMC free article] [PubMed]
- Ferron L, Capuano V, Deroubaix E, Coulombe A, Renaud JF (2002) Functional and molecular characterization of a T-type Ca(2+) channel during fetal and postnatal rat heart development. J Mol Cell Cardiol 34(5):533–546 [DOI] [PubMed]
- Franco D, Demolombe S, Kupershmidt S, Dumaine R, Dominguez JN, Roden D, Antzelevitch C, Escande D, Moorman AF (2001) Divergent expression of delayed rectifier K(+) channel subunits during mouse heart development. Cardiovasc Res 52(1):65–75 [DOI] [PubMed]
- Hagiwara N, Irisawa H, Kameyama M (1988) Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol 395:233–253 [DOI] [PMC free article] [PubMed]
- Huynh TV, Chen F, Wetzel GT, Friedman WF, Klitzner TS (1992) Developmental changes in membrane Ca2+ and K+ currents in fetal, neonatal, and adult rabbit ventricular myocytes. Circ Res 70(3):508–515 [DOI] [PubMed]
- Kato Y, Masumiya H, Agata N, Tanaka H, Shigenobu K (1996) Developmental changes in action potential and membrane currents in fetal, neonatal and adult guinea-pig ventricular myocytes. J Mol Cell Cardiol 28(7):1515–1522 [DOI] [PubMed]
- Kilborn MJ, Fedida D (1990) A study of the developmental changes in outward currents of rat ventricular myocytes. J Physiol 430:37–60 [DOI] [PMC free article] [PubMed]
- Kiyosue T, Spindler AJ, Noble SJ, Noble D (1993) Background inward current in ventricular and atrial cells of the guinea-pig. Proc Biol Sci 252(1333):65–74 [DOI] [PubMed]
- Klugbauer N, Welling A, Specht V, Seisenberger C, Hofmann F (2002) L-type Ca2+ channels of the embryonic mouse heart. Eur J Pharmacol 447(2–3):279–284 [DOI] [PubMed]
- Kojima M, Sada H, Sperelakis N (1990) Developmental changes in beta-adrenergic and cholinergic interactions on calcium-dependent slow action potentials in rat ventricular muscles. Br J Pharmacol 99(2):327–333 [DOI] [PMC free article] [PubMed]
- Lakatta EG, Maltsev VA, Bogdanov KY, Stern MD, Vinogradova TM (2003) Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res 92(3):e45–e50 [DOI] [PubMed]
- Linz KW, Meyer R (2000) Profile and kinetics of L-type calcium current during the cardiac ventricular action potential compared in guinea-pigs, rats and rabbits. Pflugers Arch 439(5):588–599 [DOI] [PubMed]
- Liu W, Yasui K, Opthof T, Ishiki R, Lee JK, Kamiya K, Yokota M, Kodama I (2002) Developmental changes of Ca(2+) handling in mouse ventricular cells from early embryo to adulthood. Life Sci 71(11):1279–1292 [DOI] [PubMed]
- Mahony L (1996) Regulation of intracellular calcium concentration in the developing heart. Cardiovasc Res 31 Spec No:E61–E67 [DOI] [PubMed]
- Masuda H, Sperelakis N (1993) Inwardly rectifying potassium current in rat fetal and neonatal ventricular cardiomyocytes. Am J Physiol 265(4 Pt 2):H1107–H1111 [DOI] [PubMed]
- Matsuoka S, Sarai N, Kuratomi S, Ono K, Noma A (2003) Role of individual ionic current systems in ventricular cells hypothesized by a model study. Jpn J Physiol 53(2):105–123 [DOI] [PubMed]
- Nagashima M, Tohse N, Kimura K, Yamada Y, Fujii N, Yabu H (2001) Alternation of inwardly rectifying background K+ channel during development of rat fetal cardiomyocytes. J Mol Cell Cardiol 33(3):533–543 [DOI] [PubMed]
- Nakajima I, Watanabe H, Iino K, Saito T, Miura M (2002) Ca2+ overload evokes a transient outward current in guinea-pig ventricular myocytes. Circ J 66(1):87–92 [DOI] [PubMed]
- Niwa N, Yasui K, Opthof T, Takemura H, Shimizu A, Horiba M, Lee JK, Honjo H, Kamiya K, Kodama I (2004) Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol 286(6):H2257–2263 [DOI] [PubMed]
- Olivetti G, Anversa P, Loud AV (1980) Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. II. Tissue composition, capillary growth, and sarcoplasmic alterations. Circ Res 46(4):503–512 [DOI] [PubMed]
- Ono K, Ito H (1995) Role of rapidly activating delayed rectifier K+ current in sinoatrial node pacemaker activity. Am J Physiol 269(2 Pt 2):H453–H462 [DOI] [PubMed]
- Puglisi JL, Wang F, Bers DM (2004) Modeling the isolated cardiac myocyte. Prog Biophys Mol Biol 85(2–3):163–178 [DOI] [PubMed]
- Sanchez-Chapula J, Elizalde A, Navarro-Polanco R, Barajas H (1994) Differences in outward currents between neonatal and adult rabbit ventricular cells. Am J Physiol 266(3 Pt 2):H1184–H1194 [DOI] [PubMed]
- Sarai N, Matsuoka S, Kuratomi S, Ono K, Noma A (2003) Role of individual ionic current systems in the SA node hypothesized by a model study. Jpn J Physiol 53(2):125–134 [DOI] [PubMed]
- Satoh H, Delbridge LM, Blatter LA, Bers DM (1996) Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J 70(3):1494–1504 [DOI] [PMC free article] [PubMed]
- Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N (2003) Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res 58(3):535–548 [DOI] [PubMed]
- Shirokov R, Levis R, Shirokova N, Rios E (1993) Ca(2+)-dependent inactivation of cardiac L-type Ca2+ channels does not affect their voltage sensor. J Gen Physiol 102(6):1005–1030 [DOI] [PMC free article] [PubMed]
- Spence SG, Vetter C, Hoe CM (1994) Effects of the class III antiarrhythmic, dofetilide (UK-68,798) on the heart rate of midgestation rat embryos, in vitro. Teratology 49(4):282–292 [DOI] [PubMed]
- Takahashi K, Kaizu K, Hu B, Tomita M (2004) A multi-algorithm, multi-timescale method for cell simulation. Bioinformatics 20(4):538–546 [DOI] [PubMed]
- Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M (1998) Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. Embo J 17(12):3309–3316 [DOI] [PMC free article] [PubMed]
- Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ (1996) Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res 79(1):79–85 [DOI] [PubMed]
- Xie LH, Takano M, Noma A (1997) Development of inwardly rectifying K+ channel family in rat ventricular myocytes. Am J Physiol 272(4 Pt 2):H1741–H1750 [DOI] [PubMed]
- Yasui K, Liu W, Opthof T, Kada K, Lee JK, Kamiya K, Kodama I (2001) I(f) current and spontaneous activity in mouse embryonic ventricular myocytes. Circ Res 88(5):536–542 [DOI] [PubMed]
- Yokoshiki H, Tohse N (2001) Developmental changes of ion channels. In: Sperelakis N, Kurachi Y, Terzic A, Cohen M (eds) Heart physiology and pathophysiology, vol 4. Academic Press, New York, pp 719–735
- Zhang ZJ, Jurkiewicz NK, Folander K, Lazarides E, Salata JJ, Swanson R (1994) K+ currents expressed from the guinea pig cardiac IsK protein are enhanced by activators of protein kinase C. Proc Natl Acad Sci U S A 91(5):1766–1770 [DOI] [PMC free article] [PubMed]