Abstract
The current threshold for lead toxicity, defined as a blood lead level of 10 μg/dL, was adopted by the United States (US) Centers for Disease Control and Prevention (CDC) in 1991 and the World Health Organization in 1995. Since that time, adverse health outcomes at blood lead levels below this threshold have been well demonstrated. Most concern probably relates to children of pre-school age; an international pooled analysis has demonstrated lead-associated intellectual deficits at blood lead levels well below 10 μg/dL. In the case of adults, several convincing population studies have shown a positive association between blood lead and risk of death. The largest such study compared mortality information from participants with blood lead levels in the highest third of the blood lead distribution (3.6 μg/dL or greater) with those in the lowest third (less than 1.9 μg/dL). After adjustment for potential confounders, estimates of the excess risk were 25% for all cause mortality and 55% for cardiovascular mortality. The adverse consequences of lead exposure have no discernible blood lead threshold, implying there is no safety margin at existing exposure levels. Despite marked declines in population mean blood lead levels since 1980, low level environmental lead exposure remains a significant public health concern.
Introduction
Although lead ores occur naturally as minerals in the earth’s crust, human blood lead levels are quite low in the absence of industrial activities.1 It is the wide scope and long-standing pursuit of industrial activities with lead over millennia that have made lead a common industrial contaminant. The environmentally persistent nature of lead makes any attempt at remediation expensive and difficult. For example, oil companies added organic lead to petrol to improve its combustion properties from the 1920s onwards. Although the developed world no longer uses lead as a petrol additive, automotive exhaust derived lead resides as historical ceiling or attic dust within habitations and trapped in surface layers of soil surrounding them.2 Similarly although lead should not currently be added to paint, there are many Australian houses built prior to the 1970s where leaded paint awaits the unwary renovator as a deteriorated layer. Toys which have been painted with leaded paint continue to be inadvertently imported into Australia and were the subject of several worldwide product recalls in 2007.3
Although a large and constantly growing body of literature has pointed to the toxicity of lead, particularly during the period of rapid human brain development up to about five years of age, lead is sometimes treated in cavalier fashion without regard for its toxicity in trace concentrations. A recent example was the transport of granular processed lead carbonate concentrate in railway wagons and then with open conveyor belts into ships’ holds at the port of Esperance in Western Australia from 2005 to March 2007. Dispersion of airborne lead dust generated from transportation and loading by prevailing winds into the town surrounding the port gave rise to increased blood lead levels in the community, affecting both children and adults.
Fortunately, such episodes of contamination are uncommon and median blood lead concentrations in both children and adults has shown a sustained decline from about the mid 1980s, mainly due to the removal of lead from petrol. Despite this success, lead exposure continues to be a concern, as evidenced by an unabated flow of publications in the medical literature demonstrating further deleterious effects at lower and lower blood lead levels. This review summarises recent findings for a metal known and used by man for millennia and is presented in two sections, considering effects in children and adults separately. Developments in official recommendations for lead exposure are discussed, including the implications of a steady stream of new and unexpected findings.
Lead Exposure in Children
Blood Lead Levels and Intellectual Deficit
The adverse consequences of a blood lead level >10 μg/dL on brain function in children and adolescents, especially as revealed by cognitive test scores, have been documented by multiple studies in several countries and the strength of the observed effects are similar around the world. As blood lead concentrations increase from 10 μg/dL to 30 μg/dL in children aged between 15 months and 4 years, the Intelligence Quotient (IQ) measured at 7 years of age decreases by 4–5 points.4 Australian studies of cohorts of children resident in the lead smelting town of Port Pirie in South Australia4 and the lead mining town of Broken Hill in New South Wales5 were of central importance in setting a threshold for lead toxicity at blood lead levels at 10 μg/dL. The CDC and the World Health Organization adopted this threshold in 1991 and 1995 respectively. In Australia, the National Health and Medical Research Council (NHMRC) set a blood lead level of <10 μg/dL as a specific goal in its lead guidelines issued in 1993. Other harmful effects on mental health include adverse affects on behaviour, with teachers reporting that students with high lead levels in deciduous teeth were less attentive, hyperactive, disorganised, and less able to follow directions.6
Intense interest has naturally focused on whether the deficit in cognitive test scores can be reversed. A study of 375 Port Pirie children found only small and inconsistent IQ improvements in children over two years of age whose blood lead levels decreased the most, relative to those children whose blood lead declined the least.7 Treatment by chelation, traditonally with ethylenediaminetetraacetic acid (EDTA), is designed to decrease the body burden of lead, however, evidence for mental health improvements have been weak and inconclusive. In a large randomised trial of the relatively new chelating agent succimer, children were followed up at both five and seven years of age but failed to show improvements in cognitive function.8 In a 2005 policy statement, the American Academy of Pediatrics concluded there is no evidence that chelation could reverse cognitive impairment.9
No Lead Threshold for Intellectual Deficit
The well known relationship between blood lead levels in children over a baseline of 10 μg/dL and their ultimate IQ was first extended to lead levels of <10 μg/dL in a landmark 2003 paper in the New England Journal of Medicine.10 These findings were confirmed and extended in a 2005 international pooled analysis of seven population-based longitudinal cohort studies.11 The pooled analysis estimated the mean IQ point decrements associated with increases in blood lead from 2.4 to 10 μg/dL, 10 to 20 μg/dL, and 20 to 30 μg/dL to be 3.9, 1.9 and 1.1 respectively. Of great importance was the finding that for a given increase in blood lead, the lead-associated intellectual decrement for children with a maximal blood lead level <7.5 μg/dL was significantly greater than that observed for those with a maximal blood lead level ≥7.5 μg/dL.11
The inescapable conclusion is that no threshold has been identified for the adverse consequences of lead exposure. Current recommendations are that childhood lead exposure should be eliminated as far as possible by banning nonessential uses of lead and further reducing allowable lead levels in air emissions, house dust, soil, water and consumer products.9
Sustained Decline in Children’s Blood Lead Levels
Reductions in average blood lead levels in children have been observed worldwide since the 1980s, certainly in the developed world. The main reason cited is the removal of lead additives to petrol which occurred progressively in most countries from the 1980s, although some jurisdictions also took other lead abatement steps at the same time. For example, the US introduced a variety of restrictions on lead emissions and lead in canned food. Although Australia first introduced unleaded petrol in 1985, leaded petrol still accounted for 64% of sales in 1991. Each Australian state independently set dates for the final phase out, for example, Western Australia did not finally withdraw leaded petrol until January 2000.
The US National Health and Nutrition Examination Surveys (NHANES) have documented the progressive fall in median blood lead concentrations in children aged one to five years; the median was 15 μg/dL in 1976–1980, falling to 3.6 μg/dL in 1988–199112 and 1.9 μg/dL in 1999. Similar declines were recorded in other locations, for example, the mean blood lead in newborns in Taipei, Taiwan, fell from 7.5 μg/dL in 1985– 1987 to 2.3 μg/dL in 2001–2002.13
Blood Lead Levels in Australian Children
Most studies of Australian children have been conducted in environments contaminated by lead, notably lead smelting at Port Pirie in South Australia and lead mining at Broken Hill in New South Wales. However, data is available for urban children aged from one to five years living in metropolitan Sydney over a 29 year period from 1974 to 2003. The mean blood lead concentrations declined from 19–21 μg/dL in 1974–75 to 14 μg/dL in 1980–82 and 11 μg/dL in 199114 when leaded petrol still accounted for 64% of sales, and by 2003 the mean was 3.1 μg/dL.15
Two cohorts of preschool children (0 to 5 years old) living in the port city of Fremantle, Western Australia, were studied in 199316 and 2005.17 Interestingly, the sale of leaded petrol in Western Australia ceased at the approximate midpoint of this period in early 2000. The geometric mean blood lead concentration fell from 6.8 μg/dL in 1993 to 1.8 μg/dL in 2005 and the proportion of children with lead concentrations ≥ 10 μg/dL fell from 25% in 1993 to 0% in 2005.
A steady fall in mean blood lead concentrations has also been documented for Australian children living in leadcontaminated environments, albeit from higher initial levels. In preschool children from Broken Hill, where lead ore is mined, there was a drop of the geometric mean blood lead concentration from 16.7 μg/dL in 1991 to 7.0 μg/dL in 2002.18 Children living in the lead smelting town of Port Pirie have had a comparable drop in the mean blood lead concentrations of 22.4 μg/dL in 1984 to 10 μg/dL in 2004.19
Sources of Lead
Automobile exhaust has not contributed to airborne lead since the final withdrawal of leaded petrol (e.g. 1996 in the US and 2000 in Western Australia). However, lead levels in ceiling or attic dusts represent a historical record of automobile exhaust and an Australian study demonstrated that ceiling dust lead levels measured in Sydney homes displayed an average 10- fold enrichment over background soil lead levels. If the ceiling dust was disturbed and allowed to plume within the living areas it could expose children to sufficient lead to exceed the currently recommended maximum blood level of 10 μg/dL.2
Dust within the living areas of habitations and in the surrounding soil partly arises from degraded paint and automobile exhaust and is a potential source of lead which has been demonstrated to contribute to elevating blood lead levels in children who play in bare contaminated soil.20 Although the sale of leaded paint intended for interior use was banned in Australia in the early 1970s, it continued to be available in marine and other specialised paint. Children who live in houses with deteriorating leaded paint can achieve lead concentrations of 20 μg/dL or higher without showing pica, the appetite disorder characterised by a craving for nonfood substances.21 Renovators of Australian dwellings built pre-1970s also face the problem of dust and chips during the attempted removal of leaded paint.
Herbal Medicines as a Lead Source
Heavy metals, such as lead and mercury, are commonly incorporated into herbal remedies, particularly Indian Ayurvedic preparations, although lead has also been found in preparations of Chinese origin. Patients ingesting these preparations can develop fully symptomatic lead poisoning with blood lead levels over 120 μg/dL.22,23 A recommendation has been made to question patients with abdominal symptoms or anaemia regarding possible intake of herbal remedies, especially if they have recently returned from Asian destinations.23
Transplacental Lead Exposure
Blood lead concentrations in newborn infants are similar to those of the mothers, therefore lead crosses the placenta.24 Lead carries a distinct isotopic signature according to its source from different ore deposits around the world and this isotopic signature can be determined by inductively coupled plasma mass spectrometry, provided the laboratory has the necessary expertise. Lead isotope studies of European women who migrated to Australia have shown that the sources of lead in newborns included lead mobilised from the maternal skeleton with an isotope signature pre-dating their migration.25
Other Sources
Lead in drinking water can contribute to blood lead and most international concern relates to particular cities where lead plumbing remains in use. A particular problem in Australia relates to collection of rain water from roof runoff into tanks for use as potable water, if lead contaminates the catchment roof area or storage tank, the rain water may be contaminated. The Australian Drinking Water Guidelines set a maximum lead level of 0.01 mg/L (1 μg/dL), based on estimates of water intake for a young child and data which attribute up to 20% of total lead intake to drinking water.26
Less common sources which should be considered include hobbies such as making lead light glass, contaminated work clothes, ceramics (particularly low cost imported products), cosmetics and imported canned food. Children should not be present during renovation projects in older housing and adults should take precautions especially against generating airborne lead dust.
Lead Exposure in Adults
Demographics of Adult Blood Lead Levels
A consistent feature of blood lead levels in community surveys of adults is thefinding of higher lead levels in men compared to women across all age groups. In both genders, lead levels rise continuously with age from a minimum in young adults reaching a maximum in the elderly. Table 1 shows the prevalence of lead levels ≥ 5 μg/dL in the 1999–2002 NHANES community survey of US adults by age groups and gender.27
Table 1.
Age specific prevalence of lead levels ≥5 μg/dL for US Adults NHANES Survey 1999–2002. Compiled using data extracted from Muntner et al., Arch Intern Med 2005;165:2155–61.27
| Blood lead ≥5 μg/dL | |
|---|---|
| 18–39 y | 2.8% |
| 40–59 y | 5.4% |
| 60 –74 y | 7.9% |
| ≥75 y | 9.2% |
| Overall | 5.0% |
| Men | 8.1% |
| Women | 2.2% |
Decline in Adult Blood Lead Levels
Substantial declines in blood lead levels in adults have occurred worldwide from about the late 1980s, coinciding with the widespread phasing out of leaded petrol together with various nation specific interventions. For example, in the US, lead solder was banned in canned food and industrial lead emissions reduced. Data from the US NHANES surveys have documented the progressive fall in blood lead concentrations in US adults. A dramatic fall was recorded from the NHANES III survey of 1988–1994 to the NHANES 1999–2002 survey27 as shown in Table 2, which compares the prevalences of lead levels ≥ 5 μg/dL and ≥10 μg/dL both overall and for men and women.
Table 2.
Prevalence of lead levels ≥5 μg/dL and ≥10 μg/dL for US Adults NHANES Surveys of 1988–1994 and 1999–2002. Compiled using data extracted from Muntner et al., Arch Intern Med 2005;165:2155–61.27
| 1988–1994 | 1999–2002 | |
|---|---|---|
| Lead Level ≥5 μg/dL | ||
| Overall | 20.5% | 5.0% |
| Men | 30.9% | 8.1% |
| Women | 11.0% | 2.2% |
| Lead Level ≥10 μg/dL | ||
| Overall | 3.3% | 0.7% |
| Men | 5.7% | 1.2% |
| Women | 1.3% | 0.3% |
Adult Blood Lead Levels in a Lead Exposed Australian Community
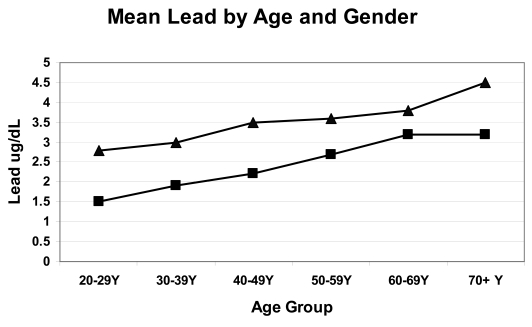
For a period of two years from 2005 to 2007, granular lead carbonate concentrate was shipped from the port of Esperance in Western Australia, a town of approximately 13,000 people. The ore was loaded into ships via a conveyor belt which was open to the surrounding air and air currents. To ascertain the extent of the community’s lead exposure the population was offered participation in a blood lead survey and approximately 20% of the population responded. A preliminary statistical analysis of blood lead levels in adult Esperance residents conformed to the patterns described in the US NHANES surveys; mean blood lead levels were higher in men and increased with age, with the highest levels seen in the over 70 years age group. Figure 1 shows the mean blood lead levels by age group and gender in 1839 adult Esperance residents.
Figure 1.
Mean blood lead levels by age group and gender from 1839 Esperance adults, 676 males [▴] and 1163 females[▪]. Preliminary data from participants in the Esperance Blood Lead Survey 2007.
The extent of the Esperance community lead exposure can be estimated by comparing Esperance data to the NHANES Survey of US adults 1999–2002, 27 as shown in Table 3. It is assumed that in the absence of lead contamination, the Esperance community lead levels would have approximated those observed in the 1999–2002 US survey. At the current lead level of concern of ≥10 μg/dL, there were 3.7 times more Esperance men and two times more Esperance women reaching the level of concern compared to participants in the 1999–2002 survey of US adults.
Table 3.
Prevalence of Lead Levels ≥5 μg/dL and ≥10 μg/dL of NHANES Survey of US Adults 1999–2002 and Esperance Western Australia Survey 2007. US data extracted from Muntner et al., Arch Intern Med 2005;165:2155–61.27 Esperance data are from a preliminary analysis of the Esperance Blood Lead Survey of 2007.
| US Adults 999–2002 | Esperance Adults 2007 | |
|---|---|---|
| Lead Level ≥5 μg/dL | ||
| Overall | 5.0% | 14.4% |
| Men | 8.1% | 22.8% |
| Women | 2.2% | 9.5% |
| Lead Level ≥10 μg/dL | ||
| Overall | 0.7% | 2.0% |
| Men | 1.2% | 4.4% |
| Women | 0.3% | 0.6% |
Blood Lead Levels and Mortality
Recent epidemiologic studies have demonstrated a positive association of blood lead and mortality, whether due to all causes, to circulatory disease and, in some studies, to cancer.28–30 One of the first studies reported on a 12–16 year follow up of mortality among US adults aged 30–74 years who had blood lead levels determined in the second NHANES survey of 1976–1980.28 After adjustment for potential confounders, individuals with baseline blood lead levels of 20–29 μg/dL had a 46% increase in all cause mortality, a 39% increase in circulatory mortality and a 68% increase in cancer mortality when compared with those with blood lead levels <10 μg/dL.28
Two subsequent studies published in 2006 were based on the third NHANES US community survey from 1988 to 1994, and confirmed the association of blood lead levels with all cause and circulatory mortality. Of particular relevance, these studies extended the association to blood lead levels considerably lower than the current 10 μg/dL level of concern.29,30 The first study analysed mortality information from 9,757 participants who were ≥40 years of age at baseline using blood lead levels categorised as <5 (the reference category), 5 to <10 and ≥10 μg/dL.29 The relative risk of mortality from all causes was 1.24 for those with levels of 5 to <10 μg/dL and 1.59 for those with levels of ≥10 μg/dL. The magnitude of risk was similar for deaths due to circulatory disease and cancer and the relative risks persisted after adjustment for sex, race, education and smoking status.
The second study also used data from the third NHANES survey and analysed mortality information from 13,946 participants who were followed for up to 12 years using blood lead levels categorised in tertiles as <1.9 (the reference category), 1.9 to 3.5 and ≥3.6 μg/dL.30 After multivariate adjustment, the hazard ratios for participants with blood lead levels ≥3.6 μg/dL were 1.25 for all cause mortality and 1.55 for cardiovascular mortality, however the association with cancer was not significant. Blood lead levels were significantly associated with mortality from both myocardial infarction and stroke and the association was evident at lead levels ≥2 μg/dL.30
Limitations of Mortality Data
Two potential confounding factors are especially relevant when considering the association of blood lead and mortality. Firstly, blood lead levels declined relatively rapidly in the US after 1980. Participants in the second NHANES survey (1976–1980) had a median blood lead level of 13 μg/dL and 15% of the population had blood lead levels of 20–29 μg/dL whereas the median blood lead level was 3 μg/dL at the third NHANES survey (1988–1994). Thus the increased mortality risks observed in participants who were aged 30–74 years in 1976–1980 may reflect exposure to the relatively elevated blood lead levels of that era.
Secondly the possibility exists that the blood lead and mortality relationship observed in these studies may be explained by lead being a surrogate for some other factor associated with an increase in mortality. The influence of these possible confounding factors deserves careful consideration. The comprehensive nature of information gathered in the NHANES surveys allowed stratified analysis according to several potential confounding variables; age, sex, race, smoking, education, rural/suburban/urban location, income, exercise and body mass index.28 The association of blood lead level and mortality proved to be consistent overall and across sub-groups, arguing against confounding by these variables as an explanation of the data. If the observed association is the product of confounding, the confounder would seem to be something other than a measured socioeconomic factor e.g. education, income or race, or a measured lifestyle factor such as residential location, smoking or physical activity level.
Lead Action Levels in Australia
Evidence from recent publications suggests the current blood lead threshold of 10 μg/dL is set too high. In the case of children, an international pooled analysis of population-based studies has clearly demonstrated a lead associated intellectual decrement at maximal blood lead levels below the 10 μg/dL threshold.11 In adults, epidemiologic studies of mortality have concluded that blood lead levels were significantly associated with increases in both all cause and cardiovascular mortality and was evident at lead levels ≥2 μg/dL.30 As no specific threshold has been identified, these findings have created a problem for regulatory agencies such as the US CDC and the NHMRC in Australia who are faced with the task of incorporating these findings into policy.
The NHMRC issued Australian guidelines in 1993, aiming for blood lead levels of <10 μg/dL. However these guidelines were rescinded on 31 December 2005 and are now available on the NHMRC Internet Archives site for historic and research reasons only.31 Although extensive disclaimers at the website advise that the publication no longer represents the NHMRC position, no alternative action levels have been substituted. An additional problem is the accessibility of the rescinded guidelines through other official portals, such as the New South Wales government Environmental Protection Authority website.32
Limitations of a Single Blood Lead Level
All the epidemiological studies of mortality cited have relied on a single blood lead level to assess lead exposure and categorise participants.28–30 The half-life of blood lead is approximately 30 days and therefore reflects recent exposure, typically the blood lead level reverts to normal once the exposure ceases. Lead is ultimately stored in bone which holds approximately 90% of the body burden and has a range of biological halflives ranging from seven years to several decades.33 Lead can be released into blood by bone remodelling, a process which is especially active in pregnancy, lactation and postmenopause. In addition, the gradual rise in adult blood lead levels with increasing age observed in both genders may reflect an increase in bone remodelling with age. Thus older participants in mortality studies are more likely to have higher cumulative lead exposure and their blood levels may be more influenced by the release of lead from bone compared to younger persons.
Therefore, it is unknown whether the adverse affects on mortality observed in the epidemiological studies are associated with either current or cumulative lead exposure, as blood lead data reflects both exposures. As lead levels have been declining for some decades, it is unclear whether the observed increase in mortality was due to lead exposure at baseline or lead mobilised from the skeleton from prior exposure. A more accurate picture of the association between long term exposure to lead and mortality would be given by a cumulative measure of lead exposure such as bone lead or an indirect measure of bone lead such as urine lead measured post EDTA chelation. Advances in measuring bone lead by X-ray fluorescence have allowed the evaluation of health effects with both blood and bone lead measures. For example, one study found bone lead predicted the onset of hypertension whereas blood lead levels did not.33 Although more studies are required, we can say that bone lead is the more valuable measurement of internal dose as it represents cumulative exposure, however, it seems impractical for large scale population studies.
Postulated Mechanisms for the Effect of Lead
The association of lead and increased mortality is biologically plausible. Indeed, so many possible mechanisms have been documented that multiple mechanisms are probably at work. The following brief summary is not exhaustive, for a more complete explanation see.28–30 Often the data supporting possible mechanisms is incomplete and controversial, for example there is no consensus on the possible relationship between of low level lead and hypertension despite several recent publications. However, a meta-analysis of 31 studies concluded a doubling of blood lead was associated with blood pressure increases averaging 1 mmHg for systolic and 0.6 mmHg for diastolic pressure.34
Changes in renal function might represent a more plausible explanation for the effect of low level lead on mortality than hypertension, and evidence exists for deleterious effects of lead on both glomerular and tubular renal function. A study of adolescents with a mean blood lead level of 2 μg/dL found a positive association of serum cystatin C (a measure of glomerular filtration) and urine beta-2-microglobulin (a measure of tubular function) with blood lead.35 An alternative but less likely hypothesis ascribes the positive association between renal dysfunction and blood lead levels to renal dysfunction reducing the renal excretion of lead and thus increasing blood lead levels.36
Conclusion
The public health consequences of blood lead levels below 10 μg/dL are progressively being recognised. In children the list includes intellectual deficits, reading problems, school failure and delinquent behaviour; all these effects have substantial public health impact and are apparently long lasting and irreversible. In adults, lead levels below 10 μg/dL are now convincingly associated with increases in all cause and vascular mortality. Research is now being directed at further defining the biological mechanisms for the effects of lead on mortality. Among the candidate mechanisms, the strong associations between blood lead levels and chronic diseases such as hypertension, kidney disease and peripheral arterial disease all need to be investigated.
The adverse impact of lead occurs at levels still considered by many as acceptable, especially for adult exposures. The US CDC has maintained 10 μg/dL as the level of concern, however, it is increasingly acknowledged there is no threshold for the adverse consequences of lead exposure and hence no safety margin. Although huge improvements in population blood lead levels have been made since about 1980, these should be viewed in an historical context. Evidence exists that preindustrialised human societies had substantially lower blood levels than our current mean population lead levels of approximately 2 μg/dL in both children and adults.1
In practice, primary prevention of lead exposure is the only viable strategy. Lead exposure should be eliminated as far as possible by banning all nonessential uses of lead and further reducing the allowable levels of lead in air emissions, house dust, soil, water and consumer products.
Footnotes
Competing Interests: None declared.
References
- 1.Flegal AR, Smith DR. Lead levels in preindustrial humans. N Engl J Med. 1992;326:1293–4. [PubMed] [Google Scholar]
- 2.Davis JJ, Gulson BL. Ceiling (attic) dust: a “museum” of contamination and potential hazard. Environ Res. 2005;99:177–94. doi: 10.1016/j.envres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3. [(Accessed 21 February 2008)];Mattel issues third recall. http://www.choice.com.au/viewArticle.aspx?id=105951&catId=100570&tid=100011&p=1&title=Mattel+issues+third+recall.
- 4.Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, et al. Environmental exposure to lead and children’s intelligence at the age of seven years. The Port Pirie Cohort Study. N Engl J Med. 1992;327:1279–84. doi: 10.1056/NEJM199210293271805. [DOI] [PubMed] [Google Scholar]
- 5.Gulson BL, Davis JJ, Mizon KJ, Korsch MJ, Law AJ, Howarth D. Lead bioavailability in the environment of children: blood lead levels in children can be elevated in a mining community. Arch Environ Health. 1994;49:326–31. doi: 10.1080/00039896.1994.9954982. [DOI] [PubMed] [Google Scholar]
- 6.Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990;322:83–8. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- 7.Tong S, Baghurst PA, Sawyer MG, Burns J, McMichael AJ. Declining blood lead levels and changes in cognitive function during childhood: the Port Pirie Cohort Study. JAMA. 1998;280:1915–9. doi: 10.1001/jama.280.22.1915. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, Rhoads GG, et al. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics. 2004;114:19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics Committee on Environmental Health. Lead exposure in children: prevention, detection, and management. Pediatrics. 2005;116:1036–46. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- 10.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994;272:284–91. [PubMed] [Google Scholar]
- 13.Hwang YH, Ko Y, Chiang CD, Hsu SP, Lee YH, Yu CH, et al. Transition of cord blood lead level, 1985–2002, in the Taipei area and its determinants after the cease of leaded gasoline use. Environ Res. 2004;96:274–82. doi: 10.1016/j.envres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Fett MJ, Mira M, Smith J, Alperstein G, Causer J, Brokenshire T, et al. Community prevalence survey of children’s blood lead levels and environmental lead contamination in inner Sydney. Med J Aust. 1992;157:441–5. doi: 10.5694/j.1326-5377.1992.tb137301.x. [DOI] [PubMed] [Google Scholar]
- 15.Gulson B, Mizon K, Taylor A, Korsch M, Stauber J, Davis JM, et al. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline-preliminary results. Environ Res. 2006;100:100–14. doi: 10.1016/j.envres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Willis FR, Rossi E, Bulsara M, Slattery MJ. The Fremantle Lead Study. J Paediatr Child Health. 1995;31:326–31. doi: 10.1111/j.1440-1754.1995.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 17.Guttinger R, Rossi E, Pascoe E, Kotecha R, Willis F. The Fremantle Lead Study Part 2. J Paediatr Child Health. doi: 10.1111/j.1440-1754.2008.01413.x. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Burke H, Balding B. Reducing lead exposure in children in Broken Hill. NSW Public Health Bull. 2003;14:52–4. doi: 10.1071/nb03016. [DOI] [PubMed] [Google Scholar]
- 19.Maynard EG, Franks LJ, Malcolm MS. The Port Pirie Lead implementation program: Future Focus and Directions. Adelaide: Government of South Australia, Department of Health; 2005. [Google Scholar]
- 20.Centers for Disease Control and Prevention. Managing elevated blood lead levels among young children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Atlanta: CDC; 2002. [(Accessed 13 February 2008)]. www.cdc.gov/nceh/lead/CaseManagement/caseManage_main.htm. [Google Scholar]
- 21.Charney E, Sayre J, Coulter M. Increased lead absorption in inner city children: where does the lead come from? Pediatrics. 1980;65:226–31. [PubMed] [Google Scholar]
- 22.Roche A, Florkowski C, Walmsley T. Lead poisoning due to ingestion of Indian herbal remedies. N Z Med J. 2005;118:U1587. [PubMed] [Google Scholar]
- 23.van Schalkwyk J, Davidson J, Palmer B, Hope V. Ayurvedic medicine: patients in peril from plumbism. N Z Med J. 2006;119:U1958. [PubMed] [Google Scholar]
- 24.Graziano JH, Popovac D, Factor-Litvak P, Shrout P, Kline J, Murphy MJ, et al. Determinants of elevated blood lead during pregnancy in a population surrounding a lead smelter in Kosovo, Yugoslavia. Environ Health Perspect. 1990;89:95–100. doi: 10.1289/ehp.908995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. Mobilization of lead from human bone tissue during pregnancy and lactation-a summary of long-term research. Sci Total Environ. 2003;303:79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 26.Australian Drinking Water Guidelines. [(Accessed 24 January 2008)]; http://www.nhmrc.gov.au/publications/synopses/eh19syn.htm.
- 27.Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–61. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- 28.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–9. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 29.Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006;114:1538–41. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–94. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 31.Revision of the Australian guidelines for lead in blood and lead in ambient air. [(Accessed 13 February 2008)];Extract from the 115th and 116th Sessions of Council, October and November 1993. http://www.nhmrc.gov.au/publications/synopses/withdrawn/eh8.pdf.
- 32.New South Wales Government Environmental Protection Authority Recommendations on lead poisoning. [(Accessed 13 February 2008)]; http://www.environment.nsw.gov.au/leadsafe/nhmrc.htm.
- 33.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am J Epidemiol. 2001;153:164–71. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 34.Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. J Hum Hypertens. 2002;16:123–31. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- 35.Staessen JA, Nawrot T, Hond ED, Thijs L, Fagard R, Hoppenbrouwers K, et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:1660–9. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]