Abstract
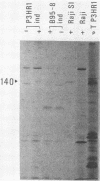
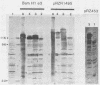
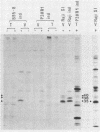
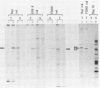
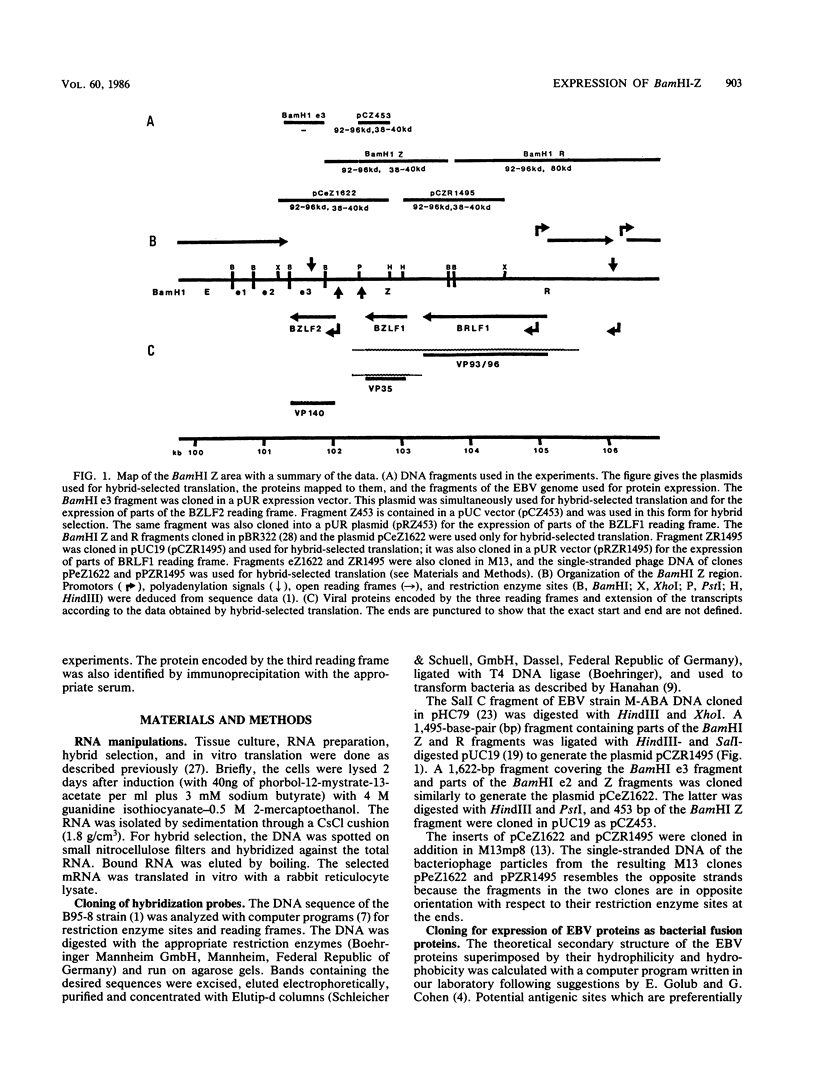
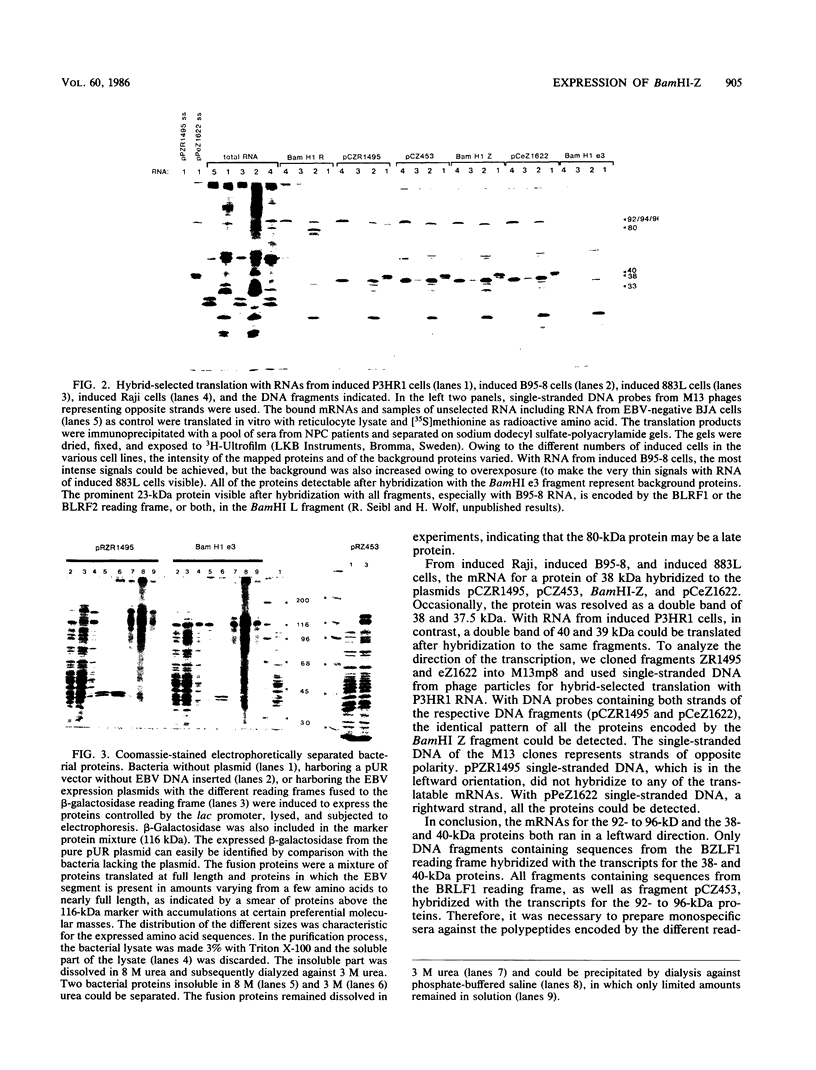
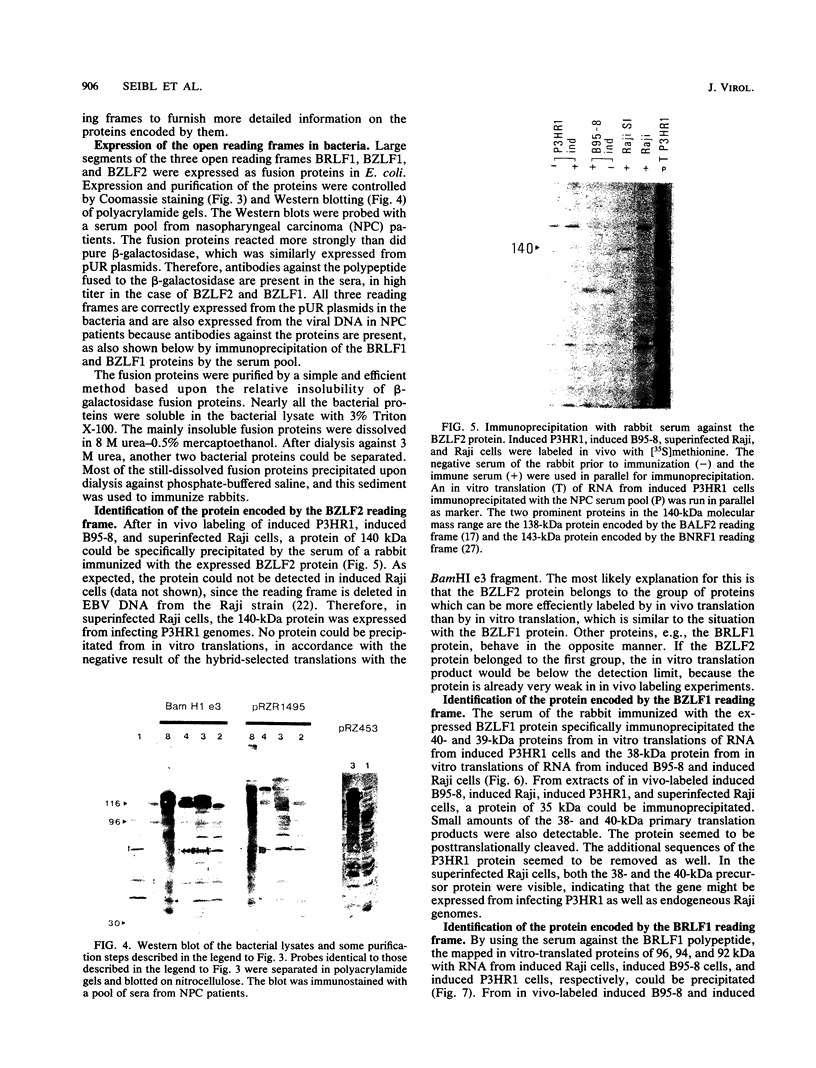
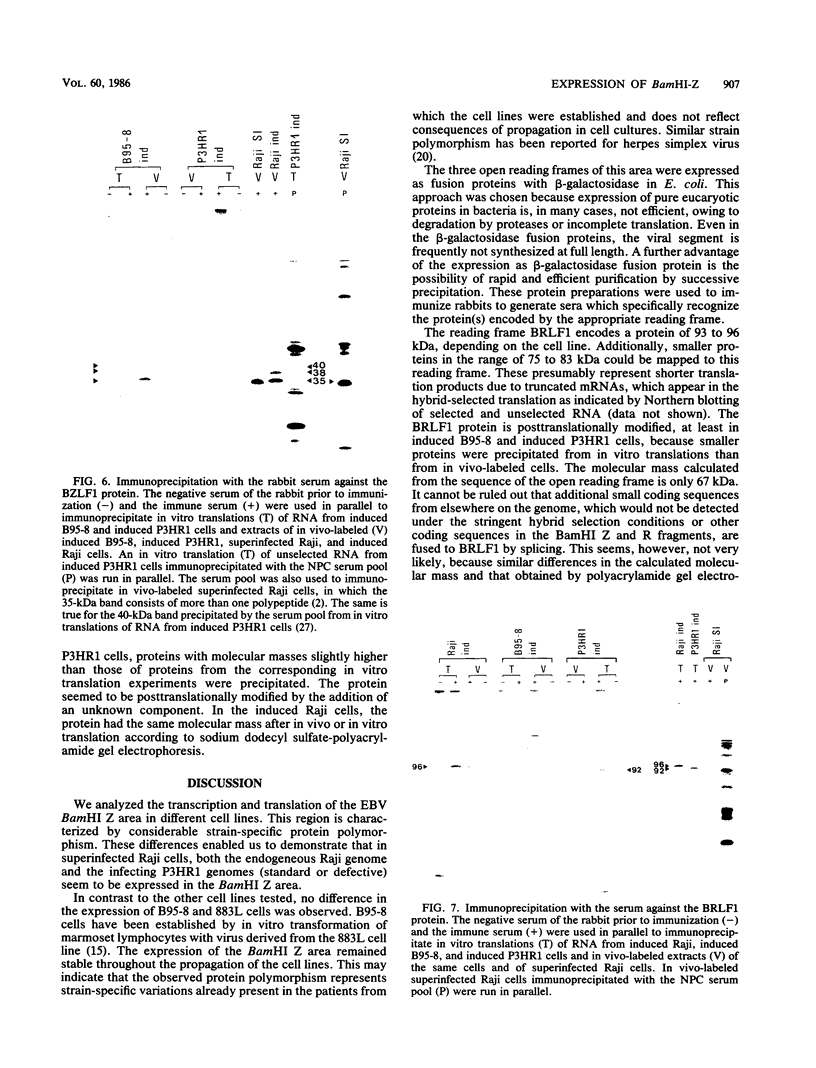
The expression of the 1,800-base-pair BamHI Z region of Epstein-Barr virus DNA was analyzed by hybrid-selected translation with several DNA subclones and RNA from different cell lines. Furthermore, large segments of the three reading frames extending in this area were expressed as fusion proteins into Escherichia coli. The fusion proteins were partially purified and used to immunize rabbits. These sera were used to confirm our mapping assignments and to identify the respective posttranslationally modified proteins in in vivo labeling experiments. The reading frame BRLF1 (the first reading frame starting in the BamHI R fragment in leftward orientation) encoded a 93- to 96-kilodalton (kDa) protein depending on the cell line. The molecular weight of in vivo-labeled proteins was increased relative to that of in vitro-translated proteins, indicating that a posttranslational modification had occurred. The BZLF1 reading frame encoded a 35-kDa protein. It was posttranslationally cleaved from a 38-kDa precursor in induced B95-8 and induced Raji cells and from a 40-kDa precursor in induced P3HR1 cells. In Raji cells superinfected with virus derived from P3HR1 cells, the protein seemed to be expressed both from endogenous Raji genomes and from infecting genomes. The transcripts for the 93- to 96-kDa and the 35-kDa protein overlapped partially. The serum against the expressed third reading frame BZLF2 specifically precipitated a 140-kDa protein. This reading frame contains only 650 nucleotides, and therefore further coding sequences were presumably spliced to BZLF2. The latter is deleted in the Raji cell line; therefore, the observed 140kDa protein in superinfected Raji cells was expressed from infecting P3HR1 genomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Wolf H. The regulated expression of Epstein-Barr virus. III. Proteins specified by EBV during the lytic cycle. J Gen Virol. 1981 Sep;56(Pt 1):105–118. doi: 10.1099/0022-1317-56-1-105. [DOI] [PubMed] [Google Scholar]
- Cho M. S., Bornkamm G. W., zur Hausen H. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol. 1984 Jul;51(1):199–207. doi: 10.1128/jvi.51.1.199-207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. K., Speck S. H., Roberts B. E., Strominger J. L. Identification and mapping of polypeptides encoded by the P3HR-1 strain of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4183–4187. doi: 10.1073/pnas.81.13.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. J Virol. 1981 Jul;39(1):172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meuller-Lantzsch N., Georg B., Yamamoto N., zur Hausen H. Epstein-Barr virus-induced proteins. II. Analysis of surface polypeptides from EBV-producing and -superinfected cells by immunoprecipitation. Virology. 1980 Apr 30;102(2):401–411. doi: 10.1016/0042-6822(80)90107-5. [DOI] [PubMed] [Google Scholar]
- Miller G., Rabson M., Heston L. Epstein-Barr virus with heterogeneous DNA disrupts latency. J Virol. 1984 Apr;50(1):174–182. doi: 10.1128/jvi.50.1.174-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. G., Niederman J. C., Miller G., Smith H. W., Dowaliby J. M. Site of Epstein-Barr virus replication in the oropharynx. Lancet. 1979 Dec 1;2(8153):1154–1157. doi: 10.1016/s0140-6736(79)92384-5. [DOI] [PubMed] [Google Scholar]
- Motz M., Fan J., Seibl R., Jilg W., Wolf H. Expression of the Epstein-Barr virus 138-kDa early protein in Escherichia coli for the use as antigen in diagnostic tests. Gene. 1986;42(3):303–312. doi: 10.1016/0378-1119(86)90234-9. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pereira L., Cassai E., Honess R. W., Roizman B., Terni M., Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976 Jan;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Polack A., Delius H., Zimber U., Bornkamm G. W. Two deletions in the Epstein-Barr virus genome of the Burkitt lymphoma nonproducer line Raji. Virology. 1984 Feb;133(1):146–157. doi: 10.1016/0042-6822(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Polack A., Hartl G., Zimber U., Freese U. K., Laux G., Takaki K., Hohn B., Gissmann L., Bornkamm G. W. A complete set of overlapping cosmid clones of M-ABA virus derived from nasopharyngeal carcinoma and its similarity to other Epstein-Barr virus isolates. Gene. 1984 Mar;27(3):279–288. doi: 10.1016/0378-1119(84)90072-6. [DOI] [PubMed] [Google Scholar]
- Rabson M., Heston L., Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Lancz G., Nonoyama M. Mapping of genes in BamHI fragment M of Epstein-Barr virus DNA that may determine the fate of viral infection. J Virol. 1986 Jan;57(1):145–154. doi: 10.1128/jvi.57.1.145-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibl R., Wolf H. Mapping of Epstein-Barr virus proteins on the genome by translation of hybrid-selected RNA from induced P3HR1 cells and induced Raji cells. Virology. 1985 Feb;141(1):1–13. doi: 10.1016/0042-6822(85)90177-1. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Nedrud J. G., Raab-Traub N., Hanes R. A., Pagano J. S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med. 1984 May 10;310(19):1225–1230. doi: 10.1056/NEJM198405103101905. [DOI] [PubMed] [Google Scholar]
- Skare J., Strominger J. L. Cloning and mapping of BamHi endonuclease fragments of DNA from the transforming B95-8 strain of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3860–3864. doi: 10.1073/pnas.77.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A., Koide N., Klein G. Two large virion envelope glycoproteins mediate Epstein-Barr virus binding to receptor-positive cells. J Virol. 1982 Jan;41(1):286–297. doi: 10.1128/jvi.41.1.286-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Haus M., Wilmes E. Persistence of Epstein-Barr virus in the parotid gland. J Virol. 1984 Sep;51(3):795–798. doi: 10.1128/jvi.51.3.795-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]