Abstract
Water confined into the interior channels of narrow carbon nanotubes or transmembrane proteins forms collectively oriented molecular wires held together by tight hydrogen bonds. Here, we explore the thermodynamic stability and dipolar orientation of such 1D water chains from nanoscopic to macroscopic dimensions. We show that a dipole lattice model accurately recovers key properties of 1D confined water when compared to atomically detailed simulations. In a major reduction in computational complexity, we represent the dipole model in terms of effective Coulombic charges, which allows us to study pores of macroscopic lengths in equilibrium with a water bath (or vapor). We find that at ambient conditions, the water chains filling the tube are essentially continuous up to macroscopic dimensions. At reduced water vapor pressure, we observe a 1D Ising-like filling/emptying transition without a true phase transition in the thermodynamic limit. In the filled state, the chains of water molecules in the tube remain dipole-ordered up to macroscopic lengths of ≈0.1 mm, and the dipolar order is estimated to persist for times up to ≈0.1 s. The observed dipolar order in continuous water chains is a precondition for the use of nanoconfined 1D water as mediator of fast long-range proton transport, e.g., in fuel cells. For water-filled nanotube bundles and membranes, we expect anti-ferroelectric behavior, resulting in a rich phase diagram similar to that of a 2D Coulomb gas.
Keywords: 1D confinement, antiferro-electric, carbon nanotubes, proton transfer, phase transition
The 1D wires formed by water in molecularly narrow pores are central to the function of many biomolecules, offer new possibilities for technological applications, and provide model systems to study the unique properties of dimensionally confined fluids (1). Proteins filled by water wires, such as aquaporins and gramicidin A, mediate the transport of water, protons, or ions across biological membranes (2, 3). Inspired in part by biology, narrow water-filled pores have been suggested as promising building blocks for high-selectivity/high-flux membranes in molecular separation devices and fuel cells (4–8). As an example of the rich properties of nanoscopically confined fluids, 1D water wires in carbon nanotubes have been found to exhibit first-order like drying transitions (9).
Carbon nanotubes provide nearly ideal systems to study water in 1D confinement. Their smooth interior cavity surface interacts in a relatively nonspecific way with water molecules, confining them to a narrow, almost cylindrical volume. In pores with subnanometer diameters, the water molecules arrange themselves in a single-file structure, linked by hydrogen bonds. Such ordered chains of water molecules were found to permit rapid water flow (4, 9), and mediate proton transfer with mobilities exceeding those in bulk water (10–13).
A key factor for the unique properties of 1D confined water is the nearly perfect molecular order, both translationally and orientationally, with uninterrupted chains of water molecules whose dipoles collectively point either up or down along the pores (9, 14). However, such strong ordering was observed in simulations of pores of up to a few nm in length only. Based on the statistical mechanics of 1D fluids, one would expect this microscopic order to disappear on macroscopic length scales. In a single-file arrangement, dipole–dipole interactions dominate the energetics, decaying as 1/r3 with distance r. For a true order/disorder phase transition in the thermodynamic limit, that decay would have to be slower than 1/r2 (15, 16). Thus, strictly speaking, long-range orientational order cannot exist in 1D water chains, and defects destroying the order are bound to occur at any finite temperature for a sufficiently large system. But despite this exact result, valid in the thermodynamic limit, water chains in narrow pores may support order to large distances.
Here, we show that dipolar order persists for water chains in pores up to macroscopic lengths. We develop a dipole model that accurately reproduces the results of detailed molecular simulations, and use it to study the order/disorder transitions of single-file water chains in long and narrow pores. We quantify the statistics of translational and orientational defects in filled pores, and the particle-number fluctuations associated with the first-order like filling/emptying transition. A charge representation of the energy derived from the dipole model simplifies the analysis of such chains and their defects. We also discuss possible collective effects in 3D carbon nanotube bundles, where true phase transitions may occur in the thermodynamic limit.
The results of this study not only shed light on the phase-like properties of dimensionally confined fluids, but are also relevant for practical applications. In particular, for fast water–mediated proton transport it is essential that water molecules form tightly connected chains in which all hydrogen bonds point into the same direction, up or down (11). At ambient conditions of 298 K and ≈1 bar pressure, both requirements are found to be fulfilled for short (6,6) carbon nanotubes immersed in water (9) and the filling/emptying of the hydrophobic tube interior has been studied in detail recently (3, 17–20). We find here that subnanometer-wide tubes up to ≈0.1 mm length are densely filled by ordered water chains, thus providing excellent mediators of proton transfer over macroscopic dimensions.
This article is organized as follows. We first briefly introduce the dipole model, with a detailed description provided in Models and Methods. [A comparison of the dipole lattice model with the corresponding molecular system, provided together with an explanation of the parameterization in the supporting information (SI) Text, Figs. S1–S3, and Table S1, demonstrates that the model recovers the results of atomistic molecular simulations with great accuracy.] We then characterize the filling/emptying transition in terms of particle-number fluctuations and the adsorption isotherm. After showing that at ambient conditions the tube is completely filled with a continuous chain of water molecules, we examine the order/disorder transition by determining the tube lengths up to which the orientational order of water is maintained. In conclusion, we discuss possible issues in experimental realizations and implications on the formation of orientationally ordered “anti-ferroelectric” water wires in 3D nanotube bundles.
Results and Discussion
Dipole Model.
To explore the properties of 1D confined water on macroscopic length scales, we develop a dipole lattice model. The dipole model is designed and parameterized to accurately capture the key structural and thermodynamic properties of 1D water, including the formation of hydrogen-bonding defects, and validated against atomistic Monte Carlo simulations. Central to the success of such a model is that 1D-confined water chains exhibit tight nearest-neighbor hydrogen bonds. The resulting local order limits the number of relevant conformational states of individual water molecules (Fig. 1A). Indeed, a 1D dipole model has been shown to quantitatively reproduce the interactions between dipolar and charge defects (i.e., excess protons) in a fully occupied short pore (11).
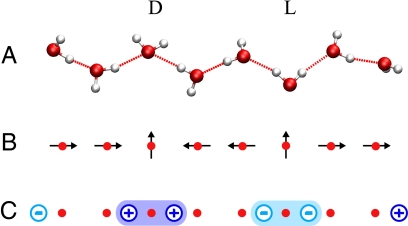
Fig. 1.
1D water wires in nanopores. (A) Chain configuration with a D-defect and an L-defect. Shown is the corresponding lattice model in the dipole representation (B) and the charge representation (C). In the charge representation, the dipoles are replaced by effective charges, indicated by circles, at the end points of the ordered-chain segments. D-defects and L-defects are located between two positive and two negative charges, respectively.
Here, we extend the dipole lattice model to take into account the free energies of hydrogen-bond defect generation/recombination, water-chain rupture, and tube-filling/emptying. As depicted schematically in Fig. 1B, the sites of a 1D lattice, spaced at a distance a, are either empty or occupied by an electric dipole of strength μ. The dipole points either up or down parallel to the tube axis, or is orthogonal to it in the case of defect molecules. In a D-defect, a water molecule accepts two hydrogen bonds without donating any; in an L-defect, a water molecule donates two hydrogen bonds but accepts none [with typical molecular configurations shown in Fig. 1A; but other configurations can occur as well (J.K. and C.D., unpublished results)]. Correspondingly, D-defects and L-defects always occur in the interior of hydrogen-bonded water chains, separating two ordered segments of opposing dipole directions that both point toward a D-defect and away from an L-defect, respectively (Fig. 1B). We note that for both L- and D-defects, the overall number of hydrogen bonds remains unchanged with respect to the perfectly ordered chain.
Charge Picture.
A major methodological result of this study is that the total energy of the dipole lattice can also be represented as a sum over long-range Coulomb-like interactions between effective charges placed at the ends of ordered segments (see Models and Methods, Eqs. 1 and 2, respectively, and Fig. 1C). With its greatly reduced computational cost, this charge representation permits us to evaluate thermodynamic and structural properties even for systems of macroscopic lengths. Furthermore, and perhaps more importantly, this charge picture offers a powerful framework to study 1D confined water as it occurs, for instance, in biological transmembrane pores. In the charge picture, hydrogen-bonding defects carry effective charges, as illustrated in Fig. 1C. This particular chain of water molecules consists of three ordered segments. Each of these segments is two lattice constants long and carries a positive and a negative charge at its ends with signs that depend on the chain orientation. Thus, each defect effectively carries two charges of the same sign located between the defect and the two neighboring water molecules. Whereas for the L-defect the two charges are negative, they are positive for the D-defect. From far, these hydrogen-bonding defects appear as Coulomb charges, with absolute values twice those of the effective charges at the chain ends. A special case of this particular Coulombic interaction was found earlier for two defects in a single chain (11), and is here derived rigorously from the dipole model (see Models and Methods). Because of their structure, L- and D-defects have to alternate within a chain, thereby forming an alternating series of positive and negative charges. The energetics and order properties of water chains can be easily understood in terms of the interactions of such charges.
By using the dipole model in the charge representation, we first study the filling of the pore and then investigate the dipolar order of the water molecules in the completely filled pore at ambient conditions.
Emptying/Filling Transitions.
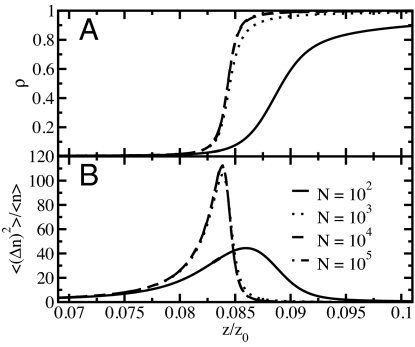
To probe the emptying/filling transition, we have determined the adsorption isotherms (i.e., the average particle density) as a function of the fugacity z relative to the fugacity at ambient conditions, z0, for different system sizes (see Fig. 2A). The transition from an empty to a full tube occurs in a narrow fugacity range around z/z0 ≈ 0.084, corresponding to ≈8.4% relative humidity at ambient conditions. The adsorption isotherm becomes steeper with increasing system size but remains continuous even in the thermodynamic limit, as is required by the impossibility of a true first-order phase transition.
Fig. 2.
Filling/emptying transition. (A) Adsorption isotherms: particle density ρ = 〈n〉/N, as a function of the scaled fugacity, z/z0, for different system sizes. The filling transition occurs at ≈8% of the fugacity at ambient conditions. The adsorption isotherms become steeper with increasing system size. At N = 104 the adsorption isotherms have reached their large system limit. (B) Relative variance of the particle number fluctuations, 〈(Δn)2〉/〈n〉 = (〈n2〉 − 〈n〉2) /〈n〉, as a function of the scaled fugacity, z/z0, for different system sizes.
Corresponding behavior is observed in the relative variance of the particle number, n, as shown in Fig. 2B. For macroscopic volumes, the relative particle number fluctuations are related to the isothermal compressibility κT via (〈n2〉 − 〈n〉2)/〈n〉 = ρkBT κT, where ρ is the particle density and angle brackets indicate the ensemble average. The relative variance is peaked at the filling transition. Even though the peak height initially grows with increasing system size, it eventually converges to a finite value. We find that both the adsorption isotherms and the relative variance are nearly converged to their thermodynamic limits for a system size of 104 sites.
We conclude that at ambient temperature and relative humidity >10% long pores are almost completely filled. In the following we study the long-range orientational order in such full tubes and show that the order persists to macroscopic tube lengths.
Macroscopic Dipolar Ordering.
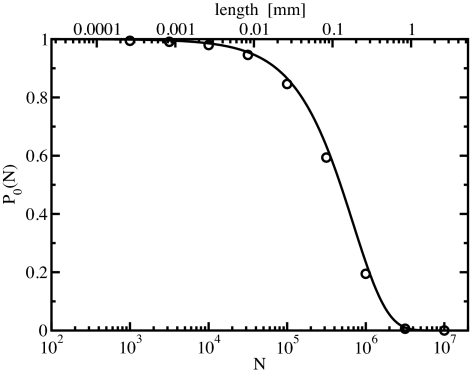
In completely filled tubes at ambient conditions, narrow gaps one lattice-spacing wide occur but do not influence the ordering behavior of the chain, which is purely determined by the occurrence of orientational defects. Thus, we performed canonical Monte Carlo simulations of a full tube at ambient conditions for system sizes up to 107 water molecules, which become feasible in the charge representation. As a measure of the dipolar ordering of the system, we define the probability P0(N) that all water dipoles point in the same direction, for a system with N sites. As shown in Fig. 3, the order probability decays only slowly with increasing system size and reaches a 50%-ordered state at a macroscopic tube length of ≈0.1 mm.
Fig. 3.
Order probability P0(N) obtained from canonical Monte Carlo simulations (circles) of a single chain as a function of the number of sites, N (lower x axis). Also shown is the order probability calculated according to Eq. 1 (solid line). The labels on the upper x axis indicate the corresponding tube length.
This macroscopic ordering can be explained with a simplified model of uncorrelated defects. Let p = exp(−βE𝒟)/[1 + exp(−βE𝒟)] be the probability of an isolated defect within a long water chain, with E𝒟 the free energy of creating the defect, and β−1 = kBT, where kB is Boltzmann's constant and T is the absolute temperature. The probability P0(N) of seeing no defect in a fully occupied system of N sites can then be approximated as
with the dipole model parameterized for water in (6,6) carbon nanotubes (see Models and Methods, SI Text, and Fig. 4A), we find that E𝒟 ≈ 13.4 kBT. As shown in Fig. 3, the order probability resulting from Eq. 1 agrees very well with the results of the canonical Monte Carlo simulations up to lengths of 1 mm.
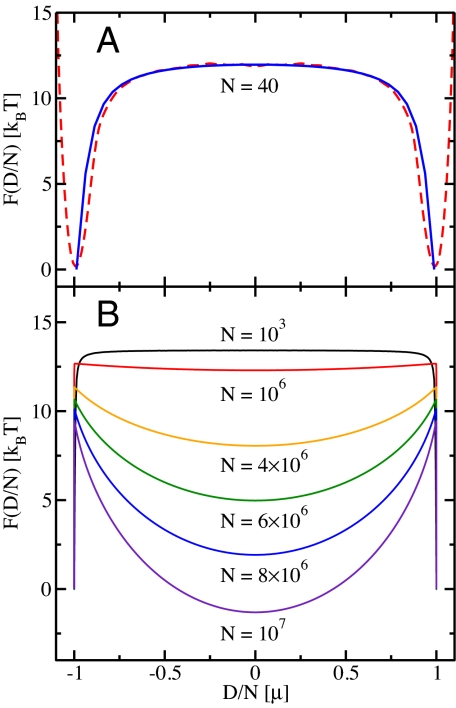
Fig. 4.
Free energy, F(D/N), as a function of the total dipole moment, D, per site, N, of a fully water-filled tube at ambient conditions. (A) Comparison of F(D/N) from atomistic Monte Carlo simulations (dashed line) and the dipole lattice model (solid line) for N = 40 water molecules. Details of the atomistic calculations and results for other system sizes are given in SI Text and Fig. S2. (B) F(D/N) from the dipole lattice model for different system sizes. The curves are shifted so that the free energy vanishes for the ordered states (D/N = ±μ). For N ≲ 105 the free-energy barrier between the ordered states is flat, whereas for larger system sizes the curves exhibit a minimum for vanishing total dipole moment.
The transition from dipole-ordered to disordered systems at tube lengths of >0.1 mm is reflected in the free energy (or potential of mean force), F(D/N), as a function of the dipole moment per site, D/N. Fig. 4A and Fig. S2 in SI Text show that the dipole lattice model accurately reproduces the free-energy profiles obtained from atomistic simulations, and thus the energetics of dipole defect formation. Free energies obtained for the dipole model for large system sizes are depicted in Fig. 4B. For N < 106 water molecules, the system is either in an up or down state, with sharp minima at the two fully ordered states (D/N = ±μ). For intermediate total dipole moments the free-energy profile is flat because different values of the total dipole moment correspond to different positions of a single defect moving along the chain at no free energetic cost. With increasing system size, the ordered states become less stable because defect numbers increase. Eventually, for large systems, the dipole moment distributions become essentially Gaussians centered at zero, with additional local maxima at D/N = ±μ corresponding to residual ordered states. The width of the Gaussian distributions (for the dipole moment per site) decreases with increasing system size. Free-energy curves obtained under the assumption of noninteracting hydrogen-bonding defects (not shown) differ by <2kBT from the exact results.
In summary, we find that at ambient conditions single-file water chains remain ordered up to macroscopic lengths of almost millimeter size. Next, we estimate the life times of such ordered states.
Temporal Persistence of Collective Dipole Orientation.
From the free energy as a function of the total dipole moment (Fig. 4), the kinetics of dipole flips of the entire chain can be estimated. Here we assume that dipole inversion occurs via transport of a single defect through the chain, as seen in molecular dynamics simulations (11, 22). The diffusion of a defect is treated as a nonlinear one-step hopping process (23). With a defect diffusion constant 𝒟 = 5 Å2 ps−1, as found in earlier work (11), we obtain first passage times for the inversion of the total dipole moment of τ ≈ 1 ms, τ ≈ 10 ms, and τ ≈ 100 ms for system sizes of N = 103, 104, and 105, respectively. For N < 105, the free energies as a function of the dipole moment (Fig. 4) have approximately rectangular shapes. To a good approximation the mean first passage time is then given by τ(N) = eβΔF Na2/𝒟, where ΔF is the free-energy difference between the disordered states with D ≈ 0 and the ordered states with D/N = ±μ. We conclude from this estimate that a particular dipole orientation can persist for almost seconds in water chains of macroscopic length.
Water-Chain Fragmentation.
So far, we have focused primarily on tubes that are (nearly) completely filled. As the water fugacity (relative humidity) is reduced and the tube begins to empty, an interesting coupling between hydrogen-bonding defect formation and chain fragmentation is revealed. Both aspects are of considerable relevance in practical applications of 1D-confined water chains. In particular, for water-mediated proton transfer, (for example, in nanotube-based fuel-cell membranes), dipolar order and continuity of the water chains are both essential.
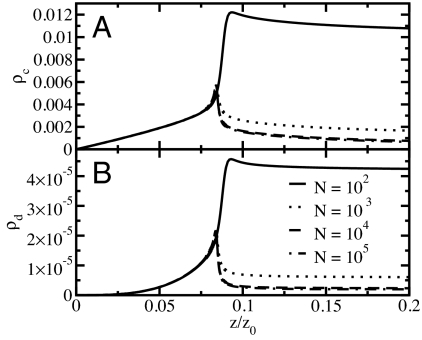
The formation and dynamics of hydrogen-bonding defects in 1D water is a major factor for the break-up of long ordered chains into shorter segments. With the energetic cost of forming a defect at the end of a chain being lower than in the interior, defects facilitate chain fragmentation, and vice versa. Indeed, Fig. 5 A and B shows that the fragment and hydrogen-bonding defect densities closely mirror each other when plotted as a function of the water fugacity. Here, fragments are defined as separate chains of hydrogen-bonded molecules, or single molecules. We find that both the fragment density and the defect density are peaked at the filling transition. The peak in the fragment density reflects the entropic gain through fragmentation for low particle densities. For fugacities z below the filling transition, the average fragment number decays linearly toward zero. At low fugacity, the system behaves ideally with a particle density ρ ≈ 2z exp(−βSc), where Sc is the entropic correction (see Models and Methods). The fragment number is then approximately equal to the number of particles, explaining the observed linear decay for small z. Moreover, with fragments too short to carry a defect, the average defect density ρd in Fig. 5B decays faster than linear.
Fig. 5.
Water-chain fragmentation. (A) Fragment density, ρc = 〈nc〉/N, as a function of the scaled fugacity, z/z0, for different system sizes. The average number of fragments 〈nc〉 has a maximum at the filling transition. (B) Hydrogen-bonding defect density, ρd = 〈nd〉/N, as a function of the scaled fugacity, z/z0, for different system sizes.
In essence, it is the entropic fragmentation that prevents a true phase transition in the thermodynamic limit (15) and destroys the dipolar order at the filling transition.
Concluding Remarks.
By using a dipole lattice model calibrated against detailed molecular simulation results, we found that 1D water confined into long carbon nanotubes is expected to form continuous dipole-ordered chains over macroscopic dimensions of up to ∼0.1 mm. The reasons for this remarkable behavior are: (i) the strong thermodynamic preference for fully filled tubes up to macroscopic lengths at ambient conditions, (ii) the large energetic penalty for inserting hydrogen-bonding defects, and (iii) the relatively small number of water molecules needed in 1D to reach macroscopic lengths (compared with Avogadro's number), which limits the entropic gain of defect insertion.
From the defect mobility and the free-energy surface for the total dipole moment, we estimated that the collective dipole orientations persist for long times of up to ≈0.1 s. With external electric fields being strongly coupled to the collective dipole moment (14), the slow, two-state–like dipole reorientation could possibly be detected experimentally by measuring the frequency-dependent dielectric loss.
An important concern in future experimental studies of the predicted effects is the presence of defects in the nanotube walls. If such defects interact strongly with the water molecules they could, in effect, trap a hydrogen-bonding defect in the water chain, similar to what is observed in aquaporin water channels (24, 25). As in aquaporins, such trapped hydrogen-bonding defects may not interfere with fast water flow (4, 7, 9), but would affect the long-range dipolar order.
Our results for the behavior of water in a single narrow tube also have implications for water in bundles or membrane arrays of parallel tubes, in particular with respect to dielectric properties (26, 27). The dipolar interactions between water molecules in adjacent pores are expected to lead to interesting collective behavior. For instance, in a hexagonally packed membrane of parallel carbon nanotubes, neighboring water wires should (if they stay perfectly ordered within the tube) be oriented in opposite directions. Perfect anti-ferroelectric order, however, is frustrated by the lattice geometry. Hints about what may happen are provided by earlier studies of 2D lattice Coulomb gases (28). The link of this model to the membrane system is established by the observation that two perfectly ordered dipole chains interact like charges attached to their endpoints (note, however, that the charges interact like 1/r and not logarithmically as in true 2D electrostatics). Thus, the membrane system is nearly equivalent to a system of charges arranged in two planes (the two surfaces of the membrane) and coupled by the requirement of global charge neutrality. It will be interesting to study whether the membrane system displays a similarly rich phase diagram as the 2D Coulomb gas, with first-order and Kosterlitz–Thouless transition lines. Simulations for different arrangements of water-filled carbon nanotubes (parallel carbon nanotubes on a surface, honeycomb lattice, square lattice, etc.) with different degrees of frustration will indicate whether water-filled membranes could be used to experimentally realize 2D lattice Coulomb gases, which are models for vortex interactions in thin superconducting films (28) and the roughening transition (29), and in which lattice geometry the carbon nanotube bundles must be arranged to obtain particularly interesting and experimentally observable phase behavior.
Models and Methods
The Hamiltonian for a dipole lattice system with N sites numbered from left to right, n particles (i.e., occupied sites), nd defects, and nc chains (i.e., fragments) is given by
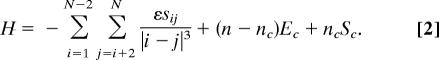 |
Here, ε = 2μ2/(4πε0a3) sets the energy scale for the dipolar interactions (ε0 is the vacuum permittivity). The double sum in Eq. 2, running over pairs of lattice sites, arises from the dipole–dipole interactions. For a given pair of dipoles, sij = 1 if the dipoles are parallel and sij = −1 if the dipoles are antiparallel. sij = 0 if at least one of the sites is unoccupied or occupied by a defect. In addition to the dipole–dipole interactions, the model includes the contact energy, Ec, that is, the interaction energy of next-neighbor molecules within a chain (including defects). The contact energy is the total average pair interaction energy of two hydrogen-bonded molecules within a chain. Therefore, in Eq. 2, the dipole–dipole interaction is calculated only for dipoles more than a site apart. There are a total of (n − nc) hydrogen-bonded pairs, each contributing the contact energy Ec. The last term in the effective Hamiltonian in Eq. 2, ncSc, is an entropic correction that accounts for the different size of configuration space available to water molecules at chain ends and defect sites, compared to single molecules. A detailed discussion of the entropic correction is provided in the SI Text. In the following, we rewrite the Hamiltonian in a completely equivalent formulation in which the sums extend over ordered chain segments rather than particle numbers. As these ordered chain segments typically consist of many particles, this reformulation is computationally advantageous and permits us to carry out Monte Carlo simulations of systems of macroscopic size. Perhaps even more importantly, the reformulated Hamiltonian provides a physically transparent and appealing picture of the energetics of the system in which contributions of long-range interactions to the total energy are given by a sum of Coulombic interactions of effective charges located at the positions next to defects and at chain ends.
In this charge picture, the Hamiltonian can be written as
 |
where the double sum is the interaction energy of all pairs of effective charges qi = ±1 located at the end points xi of the ns ordered segments in the chain (see Fig. 1C). The prime next to the sum symbol indicates that the interaction energy of the charge pair forming a defect, Φ(1), is excluded from the double sum and absorbed in the defect excitation energy E𝒟. Resummation results in a Coulomb-like interaction potential Φ(xij) of two charges qi and qj, separated by a distance xij = |xi − xj|, given by
where ψ′(x) and ψ″(x) are polygamma functions (30). For large distances, this interaction potential converges to the Coulomb interaction, i.e., Φ(xij) = ε/(2xij) for xij ≫ 1. We can rewrite this interaction as qiqjΦ(xij) = QiQj/(4πε0xija), with the magnitude of the charges then given by |Qi| = μ/a. The remaining part of the Hamiltonian is linear in the number of defects nd, the number of fragments nc, and the number of occupied sites n. The corresponding coefficients E𝒟, cc, and c, which depend on the contact energy Ec, the entropic correction Sc, and ε, are given by E𝒟 = 2ε[ζ(2) − 1], cc = ε[ζ(2) − 1] − Ec + Sc, and c = ε[1 − ζ(3)] + Ec, where ζ(m) is Riemann's zeta function (30). By comparison with the results of fully atomistic Monte Carlo simulations of TIP3P water (31) inside a (6,6) carbon nanotube (14) with free boundary conditions we have determined the lattice spacing a = 2.65 Å, the dipole moment μ = 1.9975 D, the energy constant ε = 25.8236 kJ/mol, the contact energy Ec = −20.8 kJ/mol, the fugacity z0 = 0.000327 at ambient conditions and 100% relative humidity, and the entropic correction of βSc = −3.96. For details see the SI Text. Note that in both the lattice model and the atomistic simulations, the nanotube is assumed to be rigid. In previous simulation studies, the structure and dynamics of water inside nanotubes showed little sensitivity to the amplitude of wall fluctuations in artificially softened (6,6)-type tubes (32). Therefore, we do not expect a strong effect from the assumption of rigid nanotubes.
To study the thermodynamics of filling/emptying transitions in the dipole model, we use biased grand-canonical Monte Carlo simulations (33), the Wang-Landau algorithm (21), and reweighting techniques (33).
Supplementary Material
Acknowledgments.
We thank Andreas Tröster for useful discussions. This work was supported by the Austrian Science Fund (FWF) Grant P17178-N02 and Science College “Computational Materials Science” Grant W004 (to J.K. and C.D.), by a short-visit grant within the activity SimBioMa: ‘Molecular Simulations in Biosystems and Material Science’ from European Science Foundation (J.K.), and by the National Institutes of Health. G.H. was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801448105/DCSupplemental.
References
- 1.Rasaiah JC, Garde S, Hummer G. Water in nonpolar confinement: From nanotubes to proteins and beyond. Annu Rev Phys Chem. 2008;59:713–740. doi: 10.1146/annurev.physchem.59.032607.093815. [DOI] [PubMed] [Google Scholar]
- 2.Hille B. Ion Channels in Excitable Membranes. 3rd Ed. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 3.Zhu F, Schulten K. Water and proton conduction through carbon nanotubes as models for biological channels. Biophys J. 2003;85:236–244. doi: 10.1016/S0006-3495(03)74469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalra A, Garde S, Hummer G. Osmotic water transport through carbon nanotube membranes. Proc Natl Acad Sci USA. 2003;100:10175–10180. doi: 10.1073/pnas.1633354100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt JK, Noy A, Huser T, Eaglesham D, Bakajin O. Fabrication of a carbon nanotube-embedded silicon nitride membrane for studies of nanometer-scale mass transport. Nano Lett. 2004;4:2245–2250. [Google Scholar]
- 6.Kreuer KD, Paddison SJ, Spohr E, Schuster M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem Rev. 2004;104:4637–4678. doi: 10.1021/cr020715f. [DOI] [PubMed] [Google Scholar]
- 7.Holt JK, et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science. 2006;312:1034–1037. doi: 10.1126/science.1126298. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Bigioni TP, Xu Y, Cassel AM, Cruden BA. Vertically aligned dense carbon nanotube growth with diameter control by block copolymer micell catalyst templates. J Phys Chem B. 2006;110:20102–20106. doi: 10.1021/jp0647378. [DOI] [PubMed] [Google Scholar]
- 9.Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–190. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- 10.Hummer G. Water, proton, and ion transport: from nanotubes to proteins. Mol Phys. 2007;105:201–207. [Google Scholar]
- 11.Dellago C, Naor MM, Hummer G. Proton transport through water-filled carbon nanotubes. Phys Rev Lett. 2003;90:105902. doi: 10.1103/PhysRevLett.90.105902. [DOI] [PubMed] [Google Scholar]
- 12.Dellago C, Hummer G. Kinetics and mechanism of proton transport across membrane nanopores. Phys Rev Lett. 2006;97:245901. doi: 10.1103/PhysRevLett.97.245901. [DOI] [PubMed] [Google Scholar]
- 13.Dellago C, Naor MM. Dipole moment of water molecules in narrow pores. Comput Phys Commun. 2005;169:36–39. [Google Scholar]
- 14.Vaitheeswaran S, Rasaiah JC, Hummer G. Electric field and temperature effects on water in the narrow nonpolar pores of carbon nanotubes. J Chem Phys. 2004;121:7955–7965. doi: 10.1063/1.1796271. [DOI] [PubMed] [Google Scholar]
- 15.Ruelle D. Statistical Mechanics of a one-dimensional lattice gas. Commun Math Phys. 1968;9:67–278. [Google Scholar]
- 16.Luijten E, Blöte HWJ. Classical critical behavior of spin models with long-range interactions. Phys Rev B. 1997;56:8945–8958. [Google Scholar]
- 17.Striolo A, Chialvo AA, Gubbins KE, Cummings PT. Water in carbon nanotubes: Adsorption isotherms and thermodynamic properties from molecular simulation. J Chem Phys. 2005;122:234712. doi: 10.1063/1.1924697. [DOI] [PubMed] [Google Scholar]
- 18.Maibaum L, Chandler D. A coarse-grained model of water confined in a hydrophobic tube. J Phys Chem B. 2003;107:1189–1193. [Google Scholar]
- 19.Waghe A, Rasaiah JC, Hummer G. Filling and emptying kinetics of carbon nanotubes in water. J Chem Phys. 2002;117:10789–10795. [Google Scholar]
- 20.Berezhkovskii A, Hummer G. Single-file transport of water molecules through a carbon nanotube. Phys Rev Lett. 2002;89 doi: 10.1103/PhysRevLett.89.064503. 064503. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Landau DP. Determining the density of states for classical statistical models: A random walk algorithm to produce a flat histogram. Phys Rev E. 2001;64 doi: 10.1103/PhysRevE.64.056101. 056101. [DOI] [PubMed] [Google Scholar]
- 22.Best RB, Hummer G. Reaction coordinates and rates from transition paths. Proc Natl Acad Sci USA. 2005;102:6732–6737. doi: 10.1073/pnas.0408098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Kampen NG. Stochastic Processes in Physics and Chemistry. 1st Ed. Amsterdam: Elsevier; 1992. [Google Scholar]
- 24.De Groot BL, Grubmüller H. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- 25.Tajkhorshid E, et al. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–530. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- 26.Matyushov DV. Dielectric response of one-dimensional polar chains. J Chem Phys. 2007;127 doi: 10.1063/1.2756841. 054702. [DOI] [PubMed] [Google Scholar]
- 27.Saha SK, Chakravorty D. One-dimensional organic giant dielectrics. Appl Phys Lett. 2006;89 043117. [Google Scholar]
- 28.Lee J, Teitel S. Phase transitions in classical two-dimensional lattice Coulomb gases. Phys Rev B. 1992;46:3247–3262. doi: 10.1103/physrevb.46.3247. [DOI] [PubMed] [Google Scholar]
- 29.Chui ST, Weeks JD. Phase transitions in the two dimensional Coulomb gas, and the interfacial roughening transition. Phys Rev B. 1976;14:4978–4982. [Google Scholar]
- 30.Abramowitz M, Stegun IA. Handbook of Mathematical Functions. 1st Ed. New York: Dover Publications; 1965. [Google Scholar]
- 31.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 32.Andreev S, Reichman D, Hummer G. Effect of flexibility on hydrophobic behavior of nanotube water channels. J Chem Phys. 2005;123:194502. doi: 10.1063/1.2104529. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel D, Smit B. Understanding Molecular Simulation: From Algorithms to Applications. 1st Ed. San Diego: Academic; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.