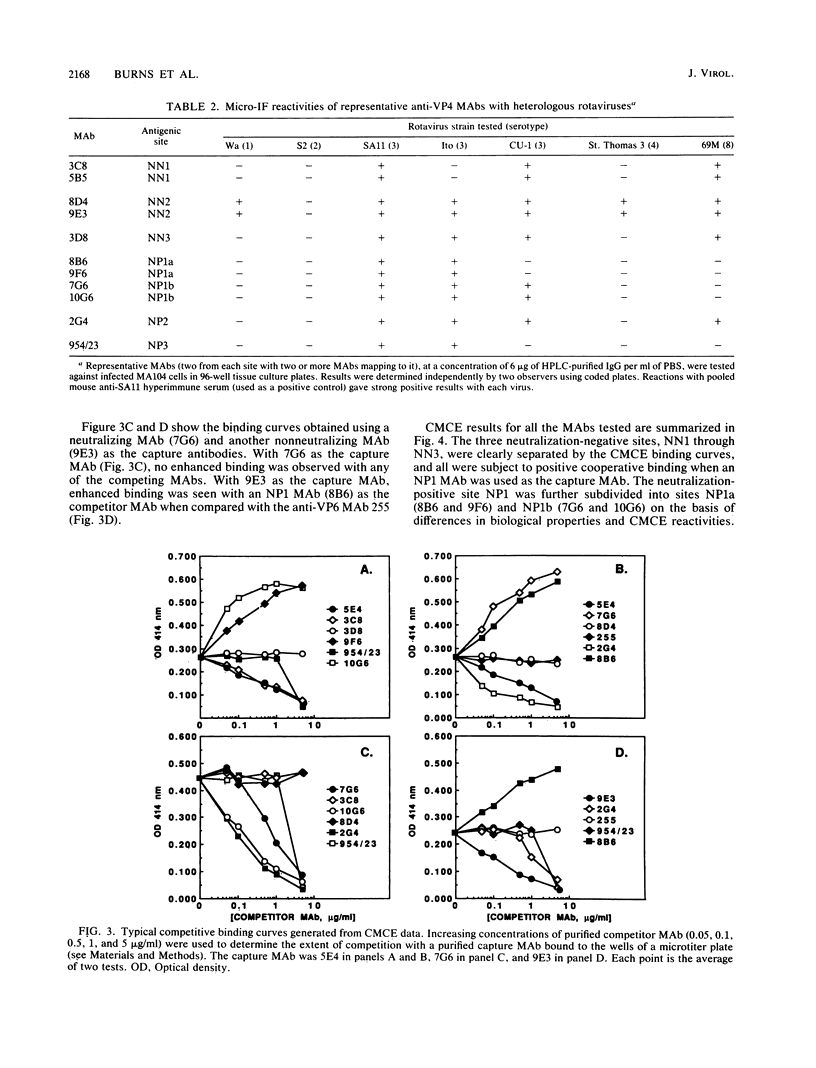
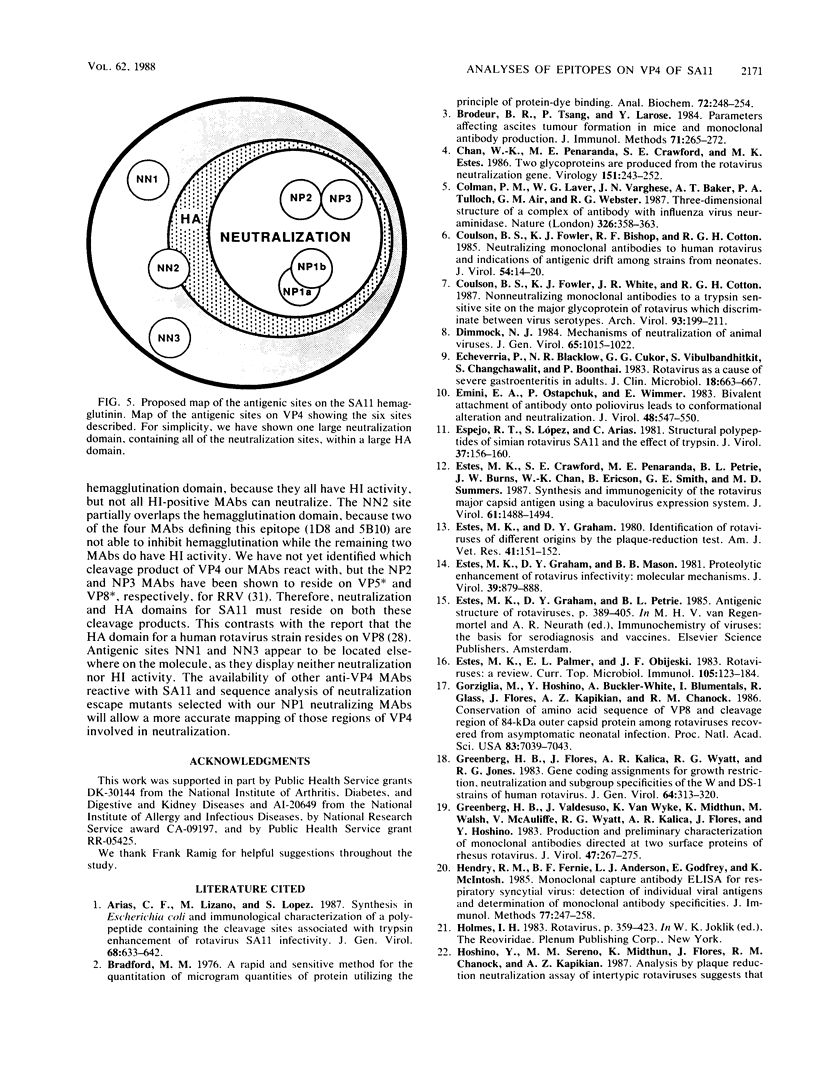
Abstract
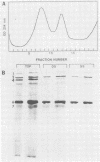
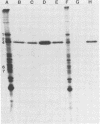
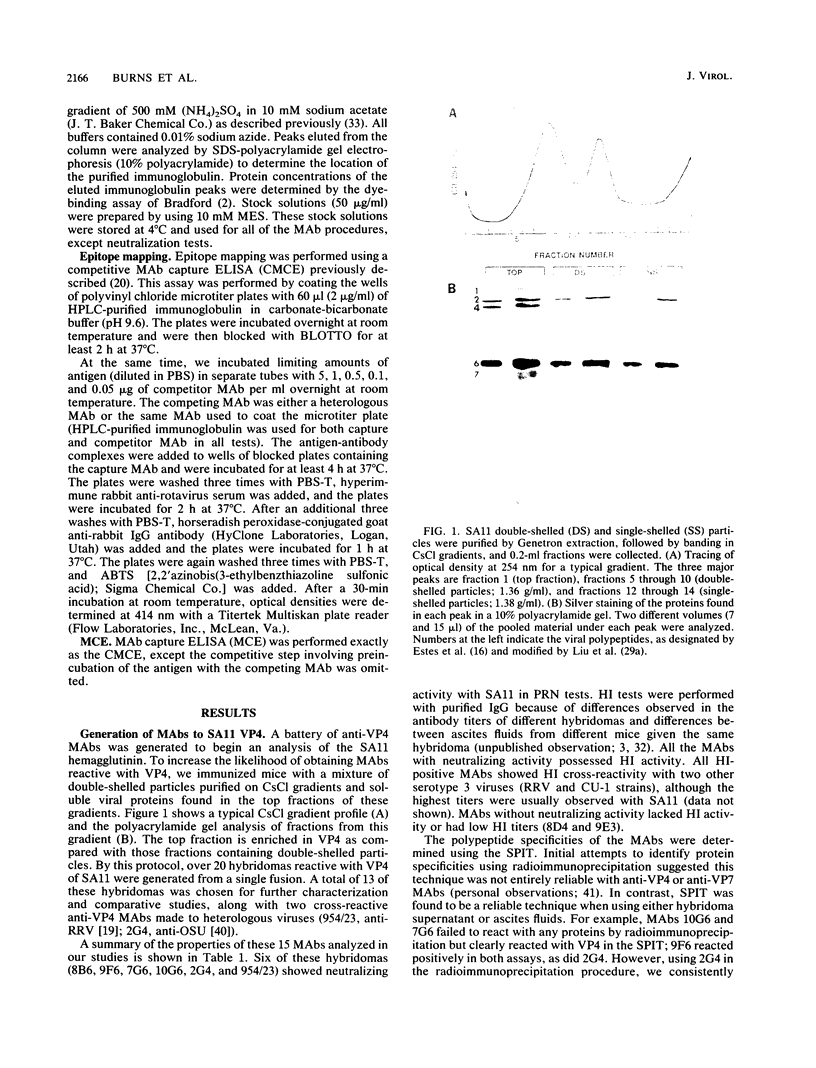
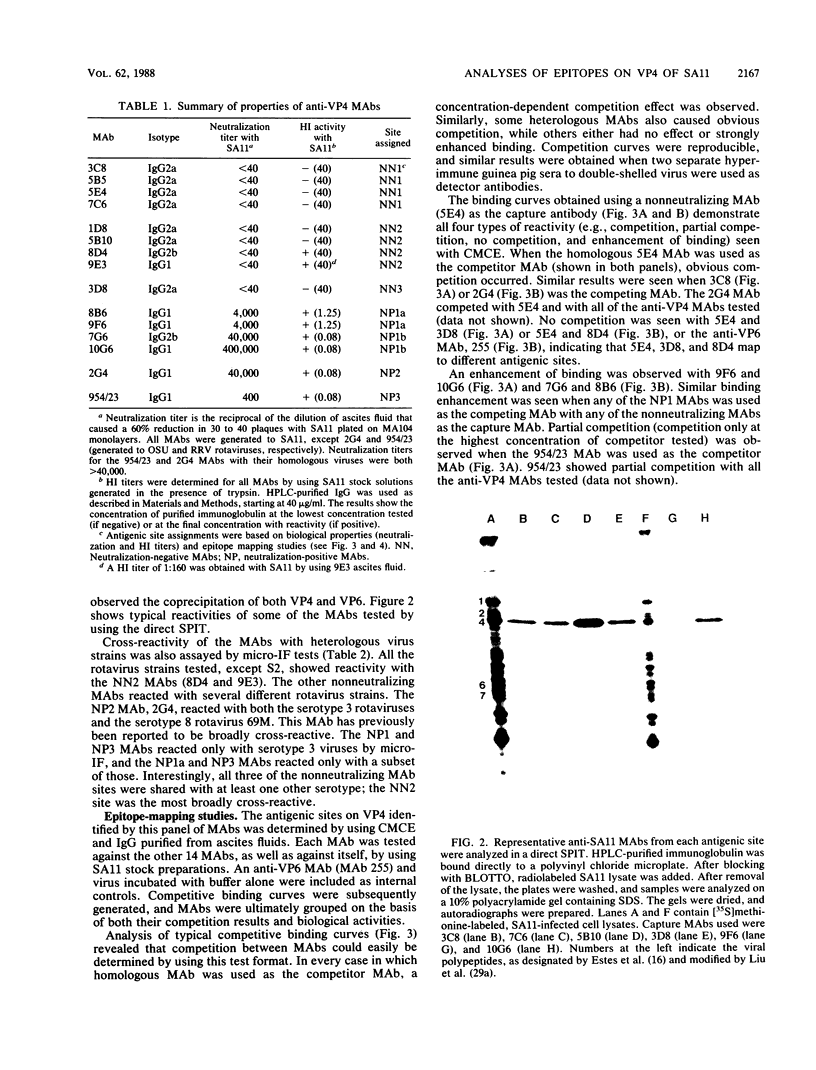
An immunochemical analysis of the hemagglutinin (VP4) of the simian rotavirus SA11 was performed to better understand the structure and function of this molecule. Following immunization of mice with double-shelled virus particles and VP4-enriched fractions from CsCl gradients, a battery of anti-SA11 hybridomas was generated. A total of 13 clones secreting high levels of anti-VP4 monoclonal antibody (MAb) was characterized and compared with two cross-reactive anti-VP4 MAbs generated against heterologous rhesus (RRV) and porcine (OSU) rotavirus strains. These cross-reactive MAbs effectively neutralized SA11 infectivity in vitro. The epitopes recognized by these 15 MAbs were grouped into six antigenic sites on the SA11 hemagglutinin. These sites were identified following analysis of the MAbs by using a simple competitive binding enzyme-linked immunosorbent assay (ELISA) and biological assays. Three of the antigenic sites were involved in neutralization of virus infectivity in vitro. All the MAbs with neutralization activity and two nonneutralizing MAbs were able to inhibit viral hemagglutination of human erythrocytes. Competitive binding ELISA data showed a positive cooperative binding effect with some pairs of the anti-VP4 MAbs, apparently due to a conformational change induced by the binding of the first MAb. Some of the MAbs also bound better to trypsin-treated virus than to non-trypsin-treated virus. A topographic map for VP4 is proposed on the basis of the observed properties of each antigenic site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C. F., Lizano M., López S. Synthesis in Escherichia coli and immunological characterization of a polypeptide containing the cleavage sites associated with trypsin enhancement of rotavirus SA11 infectivity. J Gen Virol. 1987 Mar;68(Pt 3):633–642. doi: 10.1099/0022-1317-68-3-633. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Tsang P., Larose Y. Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immunol Methods. 1984 Jul 6;71(2):265–272. doi: 10.1016/0022-1759(84)90073-5. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Penaranda M. E., Crawford S. E., Estes M. K. Two glycoproteins are produced from the rotavirus neutralization gene. Virology. 1986 Jun;151(2):243–252. doi: 10.1016/0042-6822(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., White J. R., Cotton R. G. Non-neutralizing monoclonal antibodies to a trypsin-sensitive site on the major glycoprotein of rotavirus which discriminate between virus serotypes. Arch Virol. 1987;93(3-4):199–211. doi: 10.1007/BF01310974. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Blacklow N. R., Cukor G. G., Vibulbandhitkit S., Changchawalit S., Boonthai P. Rotavirus as a cause of severe gastroenteritis in adults. J Clin Microbiol. 1983 Sep;18(3):663–667. doi: 10.1128/jcm.18.3.663-667.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y. Identification of rotaviruses of different origins by the plaque-reduction test. Am J Vet Res. 1980 Jan;41(1):151–152. [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry R. M., Fernie B. F., Anderson L. J., Godfrey E., McIntosh K. Monoclonal capture antibody ELISA for respiratory syncytial virus: detection of individual viral antigens and determination of monoclonal antibody specificities. J Immunol Methods. 1985 Mar 18;77(2):247–258. doi: 10.1016/0022-1759(85)90037-7. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. R., Lewicki H., Allison L., Salter M., Buchmeier M. J. Properties and characterization of monoclonal antibodies to Tacaribe virus. J Gen Virol. 1985 Jul;66(Pt 7):1383–1395. doi: 10.1099/0022-1317-66-7-1383. [DOI] [PubMed] [Google Scholar]
- Hrdy D. B. Epidemiology of rotaviral infection in adults. Rev Infect Dis. 1987 May-Jun;9(3):461–469. doi: 10.1093/clinids/9.3.461. [DOI] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- Kitaoka S., Fukuhara N., Tazawa F., Suzuki H., Sato T., Konno T., Ebina T., Ishida N. Characterization of monoclonal antibodies against human rotavirus hemagglutinin. J Med Virol. 1986 Aug;19(4):313–323. doi: 10.1002/jmv.1890190404. [DOI] [PubMed] [Google Scholar]
- Lane R. D., Crissman R. S., Lachman M. F. Comparison of polyethylene glycols as fusogens for producing lymphocyte-myeloma hybrids. J Immunol Methods. 1984 Aug 3;72(1):71–76. doi: 10.1016/0022-1759(84)90434-4. [DOI] [PubMed] [Google Scholar]
- Liu M., Offit P. A., Estes M. K. Identification of the simian rotavirus SA11 genome segment 3 product. Virology. 1988 Mar;163(1):26–32. doi: 10.1016/0042-6822(88)90230-9. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Gerhard W. Topological mapping antigenic sites on the influenza A/PR/8/34 virus hemagglutinin using monoclonal antibodies. Virology. 1981 Aug;113(1):64–72. doi: 10.1016/0042-6822(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar J. A., Gentsch J. R., Nathanson N., Gonzalez-Scarano F. Epitopes of the G1 glycoprotein of La Crosse virus form overlapping clusters within a single antigenic site. Virology. 1985 Jul 30;144(2):426–432. doi: 10.1016/0042-6822(85)90283-1. [DOI] [PubMed] [Google Scholar]
- Novo E., Esparza J. Composition and topography of structural polypeptides of bovine rotavirus. J Gen Virol. 1981 Oct;56(Pt 2):325–335. doi: 10.1099/0022-1317-56-2-325. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseto A., Scherrer R., Cohen J., Guillemin M. C., Charpilienne A., Feynerol C., Peries J. Isolation and characterization of anti-rotavirus immunoglobulins secreted by cloned hybridoma cell lines. J Gen Virol. 1983 Jan;64(Pt 1):237–240. doi: 10.1099/0022-1317-64-1-237. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Sonza S., Breschkin A. M., Holmes I. H. The major surface glycoprotein of simian rotavirus (SA11) contains distinct epitopes. Virology. 1984 Apr 30;134(2):318–327. doi: 10.1016/0042-6822(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Tamura G. S., Dailey M. O., Gallatin W. M., McGrath M. S., Weissman I. L., Pillemer E. A. Isolation of molecules recognized by monoclonal antibodies and antisera: the solid phase immunoisolation technique. Anal Biochem. 1984 Feb;136(2):458–464. doi: 10.1016/0003-2697(84)90244-6. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]