Abstract
Amyloid polymorphism underlies the prion strain phenomenon where a single protein polypeptide adopts different chain-folding patterns to form self-propagating cross-β structures. Three strains of the yeast prion [PSI], namely [VH], [VK], and [VL], have been previously characterized and are amyloid conformers of the yeast translation termination factor Sup35. Here we define specific sequences of the Sup35 protein that are necessary for in vivo propagation of each of these prion strains. By sequential substitution of residues 5–55 of Sup35 by proline and insertion of glycine at alternate sites in this segment, specific mutations have been identified that interfere selectively with the propagation of each of the three prion strains in yeast: the [VH] strain requires amino acid residues 7–21; [VK] requires residues 9–37; and [VL] requires residues 5 to at least 52. Minimal polypeptide segments capable of encoding prion conformations were defined by assembly of recombinant Sup35 fragments on purified prion nuclei to form amyloid fibers in vitro, whose infectivity was assayed in yeast. For the [VK] and [VL] strains, the minimal fragments approximately coincide with the strain-specific sequences defined by mutations of the N-terminal portion of the intact Sup35 (1–685); and for the [VH] strain, a longer Sup (1–53) fragment is required. Polymorphic structures of other amyloids might similarly involve different stretches of polypeptides to form cross-β amyloid cores with distinct molecular recognition surfaces.
Keywords: [PSI] strains, amyloid, Sup35
Amyloid is a generic class of ordered protein aggregates self-assembled by many unrelated proteins (1). X-ray diffraction studies reveal that amyloid fibers are rich in β-strands, which run perpendicular to the fiber axis, forming the characteristic cross-β pattern (2). One of the most intriguing features of the amyloid is structural polymorphism, whereby the same protein polypeptide can adopt distinct chain-folding patterns to give rise to a variety of cross-β structures (3). Structural plasticity of the amyloid underlies the prion strain phenomenon. Self-nucleating amyloid conformers faithfully maintain their distinct folds in the host but sometimes can adjust their structures when encountering different sequences (such as during interspecies transmission), thus giving rise to novel prion strains (4, 5). It is not well understood how distinct cross-β folding patterns are determined and what is the mechanism for their interconversion.
The yeast prion [PSI] is a self-propagating amyloid aggregate of Sup35, the yeast translation-termination factor whose aggregation in the [PSI+] state results in enhanced read-through of nonsense mutations (6, 7). Structural polymorphism of Sup35 amyloids gives rise to [PSI] strains that exhibit distinctive nonsense-suppression efficiencies and differential compatibility with Sup35 mutations (8–11). With the ease of biochemical and genetic manipulation, [PSI] strains provide excellent experimental systems to study amyloid polymorphism. However, because in vitro assembled amyloid samples often contain multiple fiber morphologies, great care must be taken to ensure correct interpretation of experimental results. Attributing genetic observations to pure structural causes, however, is complicated by the interplay between amyloid structures and cellular machineries. Here, we design a synthesized approach to avoid these difficulties, combining biochemistry and yeast genetics to examine the amyloid structures of three [PSI] strains: [VH], [VK], and [VL] (11). By individually substituting residues 5–55 of Sup35 with proline and inserting glycine in front of every other residue in this segment, we identify strain-specific Sup35 sequences in vivo. The necessity of these sequences for encoding infectious prion structures is then investigated by infecting yeast with amyloid fibers of Sup35 fragments assembled in vitro. Our goal is to define the minimally required Sup35 sequences that form core structures of the three [PSI] strains to understand how the same polypeptides are recruited in different ways to assemble polymorphic amyloid structures.
Results
Proline Substitutions.
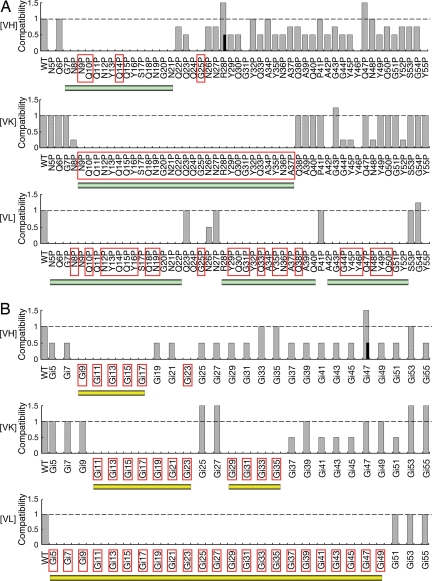
We employ proline substitutions to map possible β-structures along the polypeptide chain. Proline residues with the unique cyclic structure generate bulges in β-strands and destabilize periodic amyloid structures. A panel of 51 [psi−] haploid mutants, each having a single proline substitution in the amino acid residues 5–55 of the SUP35 allele, was created by homologous recombination. The proline substitution mutants were mated with WT cells carrying different prion strains. We first used GFP labeling to examine whether [PSI] was maintained in the resulting heterozygotes (12). [PSI+] heterozygotes were then sporulated to determine whether mutant haploids alone could support the propagation of the prion strain (i.e., preserve the infectious structure of the Sup35 amyloid). Our data revealed that each prion strain required a unique continuous Sup35 segment to propagate in vivo, which was destroyed by proline substitutions (Fig. 1A). For the [VH] strain, this segment extended from residues 7–21. Proline substitutions at most of the residues afterward weakened the nonsense suppression, but still maintained the characteristics of the [VH] strain. The [VK] segment was composed of amino acid residues 9–37. The required segment of the [VL] strain started from our first mutated residue (residue 5) and ran until the end (residue 52). Biochemical experiments below (see Table 1) indicate the [VL] segment might run even longer to at least residue 61.
Fig. 1.
Strain-specific sequences of [PSI]. (A) Proline substitutions. (B) Glycine insertions (in every other amino acid position, Gi5 indicates insertion of a glycine residue before residue 5 of the native sequence). [PSI] strains are labeled on the left. The compatibilities of Sup35 single mutations (labeled on bottom) are judged by nonsense read-through of the ade2–1(UAA) allele (1 = WT nonsense suppression efficiency, indicating efficient prion propagation; 0 = no suppression, indicating loss of prion). Red enclosing rectangles indicate prion loss in pro/WT or Gi/WT heterozygotes. Black bars at [VH] R28P and [VH] Gi47 indicate two populations of the mutant colonies exist, one with reduced nonsense suppression (0.5). [PSI] strain types remain unchanged in all mutations with altered (≠ 1, 0) nonsense suppression. Continuous sequence segments consisting of incompatible mutants are underlined with green (A) and yellow bars (B), respectively.
Table 1.
Infectivity of [VL] fibers
| Experiment | Sup(1–47) | Sup(1–53) | Sup(1–61) | Sup(1–70) | Seeds alone* |
|---|---|---|---|---|---|
| I | 1K/38 | 2L, 3K/112 | 7L, 2K/93 | 8L/97 | 0/83 |
| II | 3K/152 | 1K/156 | 4L, 4K, 1H/228 | 4L, 1K/228 | 0/228 |
*Equivalent amount of [VL] (Q23P) seeds added.
Glycine Insertions.
To further support the above findings, we constructed 26 single glycine insertions at alternate positions along the N-terminal portion (5–55) of the entire Sup35 protein. Insertion of a glycine residue in a β-strand would either alter the orientation of amino acid side chains after the insertion site or generate a bulge at the insertion. Both destabilize the amyloid structure. The strain-specific segments independently revealed by glycine insertions were in striking accordance with the proline substitution data (Fig. 1B). We further observed that glycine insertions in front of amino acids 25 and 27 strengthened the nonsense suppression efficiency of [VK] without affecting other [VK] characteristics. The data suggest relaxation of the [VK] structure at positions 25–27, thus dividing the [VK] segment into two “arms.”
Specific Thioflavin T (ThT) Affinity.
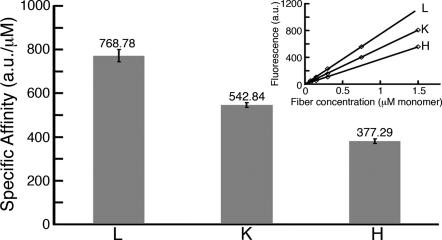
To provide independent evidence for the size difference of strain-specific sequences, we measured specific ThT binding affinities to amyloid fibers of the three prion strains. Infectious prion particles were affinity-purified from yeast by labeling with Sup (1–61)-GFP containing an attached C-terminal purification tag (8). Amyloid fibers were then prepared by adding the yeast particles to recombinant Sup (1–61)-GFP solutions that were shown previously to support in vitro propagation of prion infectivity (8). ThT binding to the fibers resulted in red shift of the fluorescence emission maximum, as expected for amyloids (13). The normalized maximal intensity at 485 nm (442 nm excitation) was used to indicate surface-exposed amyloid content. [VH] fibers had the least ThT affinity per Sup (1–61) monomer, [VK] had intermediate affinity, and [VL] had the strongest binding (Fig. 2).
Fig. 2.
Specific ThT affinities. The affinity value (on top of the bars) is the average of four to six independent measurements, each with an independent sample (n = 6 for [VH] and [VK]; n = 4 for [VL]). The inset shows single measurements. A sample was directly diluted to four concentrations. Affinity (α) was measured in excess of ThT (12.5 mM). Data are least squares fitted by using the linear equation y = αx. (R2 > 0.99 for all measurements). Error bars represent SD.
N-Terminal Truncations.
We next investigated prion propagation in heterozygous backgrounds coexpressing the WT Sup35 and an N-terminally truncated mutant. The proline substitution experiments suggest that deletion of the first 20 amino acid residues of Sup35 removes the [VH] core segment and, thus, the Δ (5–20) protein would not interfere strongly with the propagation of [VH]. In contrast, the truncated protein would probably still have enough residual interactive surface to affect the faithful replication of [VK] and [VL], and, thus, cause prion curing. We replaced the WT SUP35 allele with Sup35Δ (5–20) in a [psi−] haploid genetic background and then mated the deletion with the WT [PSI+] cells. Exactly as predicted, the resulting heterozygotes lost [VK] and [VL] but still allowed [VH] (eight of eight independent heterozygotes tested individually) [supporting information (SI) Fig. S1]. We sporulated the [VH] heterozygotes. Sup35Δ (5–20) spores were invariably [psi−] (12/12), and the WT spores all inherited [VH] (12/12).
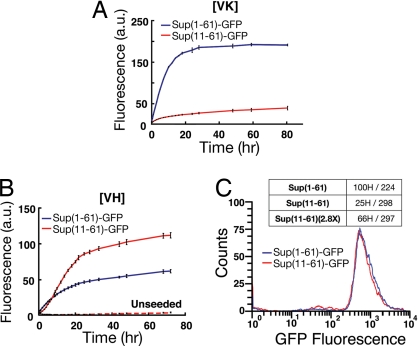
Similar experiments were performed with Δ (5–10) deletion. The resulting heterozygotes lost [VH] and [VL] (eight of eight each) but maintained [VK] moderately (11 [VK] cells of 20 randomly selected heterozygotes) (Fig. S1). When [VK] heterozygotes were randomly sporulated, the prion could only be unambiguously detected in 3 of 11 freshly obtained Sup35Δ (5–10) haploid colonies (see SI Methods). The three haploids exhibited diminished nonsense suppression and tended to lose [VK] in mitosis (see SI Methods). In contrast, most WT spores, obtained at the same time, stably inherited [VK] (14/16). The low stability of [VK] in the Δ (5–10) haploid background correlated well with the slow seeding kinetics of Sup (1–61)Δ (5–10)-GFP fibers (renamed Sup (11–61)-GFP hereinafter) (Fig. 3A). The in vitro assembled Sup (11–61)-GFP fibers nevertheless maintained robust [VK] infectivity when introduced into the WT [psi−] yeast (71K/223 randomly picked colonies; [VK] seeds alone: 1K/223).
Fig. 3.
Seeding of Sup (1–61)-GFP and Sup (11–61)-GFP fibers. (A) [VK] seeds. (B) [VH] seeds. The curves represent measured ThT intensity. The protein concentration is 4 mM for all experiments. Experiments were performed in duplicates. Error bars indicate SD. The ratio of specific ThT affinities [Sup(1–61)/Sup (11–61)] was determined to be 2.1 for [VK] fibers and 0.4 for [VH] fibers (72 h after seeding). (C) Diminished infectivity of [VH] Sup (11–61)-GFP fibers. Flow cytometry is used to normalize fiber length distribution. The Sup (11–61) sample has lower specific infectivity (25H/298: 25 [VH] of 298 randomly picked colonies).
In contrast to [VK], Sup (11–61)-GFP fibers nucleated by [VH] particles exhibited sigmoidal growth kinetics with robust growth rates (Fig. 3B). The sigmoidal kinetics suggested that a fast-growing fiber conformation built up in the solution and dominated the population. The new conformation had diminished infectivity because the Sup (11–61)-GFP specimen was determined to have lower specific infectivity in comparison with a Sup (1–61)-GFP specimen prepared in parallel (Fig. 3C).
Minimal Fragments to Encode Prion Infectivity.
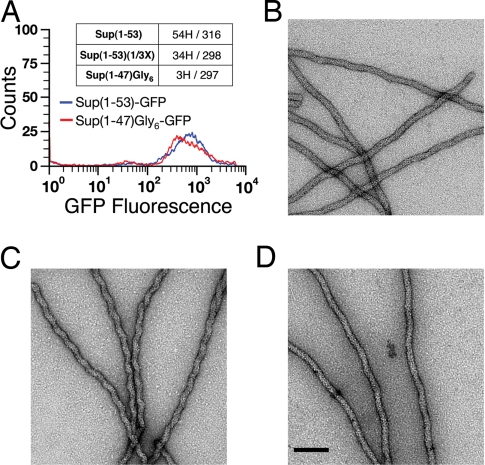
We next determine whether the strain-specific sequences revealed by proline substitution and glycine insertion experiments in vivo are necessary to encode infectious prion strain conformations. We used purified Sup (1–61)-GFP decorated yeast prion particles to nucleate amyloid fibers in vitro from recombinant Sup35 fragments, and then determined the infectivity of the fibers. Sup35 fragments were C-terminally tagged with GFP to enhance solubility and to facilitate real-time monitoring of the seeding process. For the [VH] strain, Sup (1–53) was required to nucleate infectious fibers. Sup (1–30)-GFP and Sup (1–40)-GFP did not form fibers, and Sup (1–47)-GFP fibers were not infectious. We demonstrated that amino acid residues 47–53 of Sup35 were required for the structure of [VH]. Six glycine residues were added to Sup (1–47) to assemble Sup(1–47) Gly6-GFP fibers. Equal amounts of Sup (1–53)-GFP and Sup(1–47) Gly6-GFP fibers with similar length distributions were then used for yeast transformation. Only the Sup (1–53)-GFP sample could transmit [VH] infectivity (Fig. 4A). The requirement of Sup (1–53) to propagate [VH], although not obvious from proline substitution and glycine insertion experiments (Fig. 1), is consistent with the observation that point mutations outside the Sup (1–21) region can modulate the nonsense-suppression efficiency of [VH] (11). It would be of great interest to determine whether the structure of the infectious [VH] fiber exhibits certain adaptability after amino acid residue 21.
Fig. 4.
Minimal Sup35 fragments for the [VH] and [VK] strains. (A) Infectious [VH] fibers require Sup (1–53). Flow cytometry is used to normalize fiber length distribution of Sup (1–53)-GFP and Sup(1–47)Gly6-GFP. Only Sup (1–53)-GFP fibers exhibit significant infectivity (54H/316: 54 [VH] of 316 randomly picked colonies). (B–D) Sup (1–40) transmits the [VK] structure faithfully. Sup (1–61)-GFP fibers of the [VK] strain type are wavy (B). Sup (1–40)-GFP fibers nucleated by [VK] exhibit jagged morphology (C), which reverts to the wavy shape when used as seeds to nucleate Sup (1–61)-GFP (D). (Scale bar, 100 nm.)
The minimal infectious fragment for [VK] is Sup (1–40) (18K/224; seeds alone: 1K/223), in good accordance with the [VK] segment defined above (Fig. 1). The Sup (1–40)-GFP fibers had a jagged shape (Fig. 4C). When used to seed Sup (1–61)-GFP, the resulting fibers returned to the normal wavy morphology (Fig. 4 B and D), indicating that the intrinsic structural order was faithfully maintained through the passages. Sup(1–27)Gly13-GFP, lacking one arm of the [VK] surface, did not form fibers from [VK] seeds.
We next study the [VL] strain. [VL] yeast cultures are unstable when Sup (1–61) is overexpressed, giving rise to 10% [VK] cells (8). To prepare pure [VL] particles from yeast, we took advantage of the observation that, in the Sup (1–61)-GFP context, the Q23P mutation had diminished affinity for [VH] and [VK] prion particles but preserved adequate binding to [VL] particles (12). We used the Sup (1–61)(Q23P)-GFP construct with an attached affinity-purification tag to label and purify [VL] particles. The prion particles obtained only imparted the [VL] strain phenotype when reintroduced into yeast cells (62L/207). The Q23P particles could nucleate fiber growth of Sup (1–47), (1–53), (1–61), and (1–70) fusion proteins, but only the Sup (1–61) and Sup (1–70) fibers could transmit the [VL] phenotype (Table 1). The Sup (1–47) and Sup (1–53) fibers nevertheless exhibited [VK] infectivity (Table 1). This result could only be explained by the growth of the [VK] structure from the nucleating [VL] particles because unseeded controls exhibited no infectivity. In addition to the [VL] infectivity, the Sup (1–61) sample also exhibited [VK] infectivity, consistent with the cross-seeding activity observed for the shorter fragments (Table 1). Nucleation of the [VK] conformation from [VL] seeds likely involves part of the extended [VL] surface that overlaps with the surface of [VK] (Fig. 1). “Conformational transmutation” thus provides a plausible explanation for the instability of [VL] culture overexpressing Sup (1–61)-GFP (8). Polypeptide segments following Sup (1–61) in the full-length protein might further stabilize the [VL] core structure and protect part of the accessible surface, thus allowing faithful [VL] inheritance in the WT yeast cell.
Strain Dominance and Genetic Backgrounds.
Prion strains are in metastable states. The efficiency of their cellular propagation is kinetically determined where the fastest multiplicating, true-breeding structures dominate. We previously suggested that the cellular frangibility of prion fibers played an important role in determining the strength of a prion strain (14). This notion was born out by a beautiful full treatment in Tanaka et al. (15) that included additional considerations regarding different rates of fiber growth in a dividing cell. It is nevertheless not clear whether the intrinsic fiber properties estimated in vitro can represent cellular parameters in vivo. For instance, we do not find a link between the length of strain-specific sequences and the efficiency of cellular propagation of the prion strains. Strain competition experiments with the yeast 5V-H19 genetic background established that the dominant relationship among [PSI] strains is [VH] > [VK] > [VL] (8, 11). However, with the 74-D-694 background, the dominant relationship is [VH] > [VL] > [VK] (Fig. S2). This genetic background effect makes clear that the competitive effectiveness of a prion strain is modulated by the host's genetic environment in addition to the intrinsic physical properties of its fiber. The molecular mechanism of host modulation remains to be determined.
Discussion
The minimal polypeptide lengths revealed here reconfirm our previous results that the first 61 residues of Sup35 are sufficient to encode the three prion strain conformations in vitro (8). The strain-specific segment of our strong [VH] strain defined in vivo (residues 7–21) differs from the strong strain amyloid core proposed by Toyama et al. (16). The discrepancy could result from structural variability of in vitro assembled fibers used in the study of Toyama et al. (16). Sup (1–61)-GFP fiber specimens that impart [VH] phenotypes contain two major morphologies, lanky and hunky, whose mass-per-fiber-length measurements correspond to ≈1 and 1.17 prion molecules per 4.7 Å cross-β repeat distance, respectively (14). The biphasic hydrogen exchange kinetics, reported by Toyama et al. (16) for the strong strain, could reflect similar dimorphism in their samples. In addition, the biphasic kinetics might indicate multiple structural environments within the same fiber as suggested by Toyama et al. (16). Tacking of extra prion molecules along a periodic cross-β core was proposed to cause the hunky fiber morphology, which would give rise to multiple environments for identical amino acid residues (14). It is not yet known whether the lanky and hunky fibers are equally infectious and interconvertible.
Nelson et al. (17) demonstrated Sup (7–13) peptide was able to form amyloid-like “steric-zipper” structures (18). In such structures, extended Sup (7–13) peptides stack parallel to the one below along the fiber axis to form a β-sheet. Two identical sheet surfaces then “zip-up” face-to-face by side chain interdigitation. In a [PSI] strain, the strain-specific segment (Fig. 1) might adopt a sinuous folding pattern consisting of few straight β-strands (14, 19–22). Each β-strand aligns with its counterpart in the polypeptide unit below via hydrogen bonds. β-sheets composed of neighboring strands then zip together to give the strain structure. Alternatively, assembly of three neighboring β-sheets to form a triangular β solenoid is possible, as it was observed in a recently determined structure of the HET-s prion (23). The models predict that mass-per-fiber-length measurement yields a value close to one Sup35 polypeptide mass per ≈4.7 Å repeat distance. This was indeed observed for the lanky fibers of the [VH] and [VL] strains (14). The wavy [VK] fiber has a measurement of 1.15 polypeptide mass per 4.7 Å. Tilting of β-sheets by ≈30° to make a helix would require systemic rotation of vertically aligned β-segments, disrupting the tight steric-zipper configuration (14).
In this study, we focus on minimally required Sup35 fragments that are sufficient to encode infectious amyloid structures in vitro. It is known from genetic experiments that the minimal length to impart infectivity (defined here by yeast transformation experiments) is not sufficient to maintain cellular propagation of [PSI] strains (24, 25). There is additional evidence to indicate that amino acid residues following Sup (1–61) contribute to fiber structures (11, 16, 20, 26). The extra polypeptide length might be required either to facilitate interactions with cellular machinery for prion propagation or to further stabilize and protect the minimal core structures in a cell. Regardless of the cellular complexity, our study of amyloid structures of three yeast prion strains reveals that the same Sup35 protein uses different stretches of amino acid to assemble distinctive self-propagating structures. The [VK] structure perhaps contains two interacting arms, with structural relaxation in the middle near a charged residue (R28) and termination before a native proline residue (P41). The [VL] structure appears to possess surface bits resembling that of [VK], and thus can seed the [VK] conformation when Sup35 is overexpressed. [VH] requires longer polypeptide fragments than the strain-specific core segment defined by proline substitution mutagenesis to stabilize the infectious conformation, which otherwise has propensity to transmute to noninfectious structures [revealed here by seeding Sup (11–61) and Sup (1–47)]. This study provides a general experimental strategy for focusing on the structures of biologically relevant amyloid species and avoiding complications in data interpretation caused by structural heterogeneity in the sample. The approach can be applied to other natural and artificial yeast prions to establish general principles of amyloid structural polymorphism. Such knowledge would help the determination of prion strain structures at atomic resolution and further aid the design of interesting amyloid-like structures for novel applications (27, 28).
Materials and Methods
Yeast Strains, General Methods, and PCR Primers.
All genetic experiments were performed in the 5V-H19 genetic background (MATa SUQ5 ade2–1(UAA) can1–100 leu2–3,112 ura3–52) (29) with one exception. For the determination of dominant relationships among [PSI] strains, the 74-D-694 background (MATa ade1–14(UGA) leu2 ura3 his3 trp1) (30) was also used. gα5V-H19-PrpΔSF is a MATα, [psi−], [PIN+] derivative of 5V-H19 in which the Sup35 (5–55) coding sequence is replaced with the mouse Prpa (94–230) sequence. Colonies of gα5V-H19-PrpΔSF are white. Standard protocols were used for media preparation and yeast genetic manipulation (31). Escherichia coli strains BL21(DE3)/pLysS and BLR (DE3)/pLysS (32) were purchased from Novagen. PCR primers are listed in Table S1.
Proline Substitutions and Glycine Insertions.
YCp-I-SUPF and YIp-I-SUPN (11) containing the 1243-bp SUP35 promoter, followed by a BamHI restriction site and then the SUP35 coding sequence, were subjected to site-directed mutagenesis by using the QuikChange II kit (Stratagene) to generate proline substitution and glycine insertion mutations. Mutant plasmids were used as templates for PCR amplification of SUP35 sequences, using primers CYK-43 and CYK-2. The PCR products were cotransformed with YCplac111 (33) into yeast gα5V-H19-PrpΔSF to select red colonies on SC-LEU agar plates. For each mutation, two independent red colonies were isolated and verified by BamHI/BstXI digestion of genomic PCR products, by using primers CYK-43 and CYK-15 and sequencing the first 372 bp of the SUP35 coding sequence.
Compatibility with [PSI] Strains.
The mutants were mated with the 5VH19 ΔHIS4 [YCplac111(LEU2)] background bearing prion strains [VH], [VK], and [VL], respectively. Resulting diploid cells were selected and purified by complementation of nutritional markers. Red diploids were further transformed with YEp-CUP1-Sup35 (1–61)-GFP-Strep(II)-T (8) to grow at 30°C overnight in SC-URA media and then observed by fluorescence microscopy. A mutant is classified as PNM (no longer PSI) when <5% of the cells (>100 cells observed) exhibited the characteristic particulate labeling pattern of [PSI+]. Otherwise, it was classified as antisuppression (ASN). For each mutation, three (for [VH] and [VK]) or five (for [VL]) independent red diploids were transformed and observed, and PNM was assigned only when all of the diploids showed <5% labeling.
Red ASN diploids as well as white and pink diploids, were subjected to random sporulation to select his− spores for further analysis (eliminating surviving diploid cells). Spores of each mutation were grouped as white, pink, dark pink, and red, according to the colony color. We determined which color group was specifically associated with the mutation. Four colonies of each color group were analyzed for allotype by BamHI digestion of a SUP35(−1243–372) genomic PCR fragment (primers CYK-43 and CYK-15). Mutant alleles were distinguished from the WT by the unique BamHI restriction site located before the first codon. The [PSI] compatibility of each mutant was then scored by the colony color (the WT color = 1; red = 0; whiter than WT > 1; darker than WT < 1, [ρ+] cells), which was indicative of [PSI] strength in the 5V-H19 genetic background (11). Mutations with compatibility scores that were not 0 or 1 were further strain-typed by strain-specific GFP labeling using Sup (1–61)-GFP, Sup (1–61)(G20D)-GFP, and Sup (1–61)(Q23P)-GFP (12) (two independent colonies). We did not observe strain type changes in all occasions. For each mutation, the whole experiment was performed for two independently isolated colonies. Consistent results were obtained.
ThT Assays.
Twenty microliters of [PSI] particles [0.3–1.0 μM monomer concentration in Buffer E (100 mM Tris·HCl, 1 mM EDTA, 2.5 mM desthiobiotin, pH 8.0)] were added to 1-ml solutions of 10 μM His5-Sup35(1–61)-GFP(Y67L)-Strep(II) in Buffer W (100 mM Tris·HCl, 1 mM EDTA, pH 8.0) to nucleate amyloid fibers at room temperature quiescently. His5-Sup35(1–61)-GFP(Y67L)-Strep(II) was prepared from the BLR21(DE3)/pLysS E. coli strain, purified by Ni-NTA affinity columns (Qiagen) as described in ref. 8, and then desalted with PD-10 columns (GE) equilibrated in Buffer W. The colorless Y67L mutant did not interfere with ThT fluorescence but still maintained the correct folding of GFP (35). After 15 h, [VK] and [VL] fibers were sonicated on ice (6 W power output, three times, 10-s duration each with 30-s cooling between each pulsing), and then collected by centrifugation at 200,000 × g on top of a 30% sucrose cushion (g ml−1 in Buffer W) for 2 h. Fiber pellets were suspended, recollected, and resuspended in Buffer W plus 0.5 M Trehalose (Sigma T9531). [VH] fibers were collected 6 h after nucleation. Before centrifugation, they were sonicated on ice at 4 W power output for 10 short pulses, each lasting <0.5 s. The gentler sonication condition for [VH] fibers was because of their low stability (8), where hefty treatment might result in loss of fibers. Prolonging [VH] seeding resulted in corresponding decrease of specific infectivity and concomitant increase of specific ThT affinity. Unseeded controls did not yield fiber pellets in 15 h.
Specific ThT affinities were measured at 25°C in freshly prepared ThT solutions (12.5 μM ThT, 50 mM Tris·HCl, 25 mM Glycine, 0.5 mM EDTA, and 250 mM Trehalose; pH 8.0). Fiber concentrations (monomer equivalent) were determined by UV absorption at 280 nm in 7 M guanidine hydrochloride (36) (molar extinction coefficient = 38,500). ThT fluorescence was measured with excitation at 442 nm and emission at 485 nm (10-nm windows) in a Varian Cary Eclipse fluorescence spectrophotometer. Yeast seeds alone were determined to contribute negligibly to both concentration and fluorescence measurements. The stability of the fluorescence spectrophotometer was monitored with Compound 610 (Starna Scientific) before each use.
To monitor the time course of fiber formation, recombinant proteins were diluted in 3 ml of buffer containing 12.5 μM ThT, 50 mM Tris·HCl, 25 mM Glycine, and 0.5 mM EDTA (pH 8.0). Twenty microliters of yeast seeds were added to the solution and mixed gently. Seeding reactions were monitored at 25°C and undisturbed for 72 h, except that, before each measurement, sealed cuvettes were gently reverted once to redistribute the fibers. Protein solutions mock-seeded with Buffer E were included as controls.
Sup35 N-Terminal Deletions.
The BamHI/BstXI fragment of YIp-I-SUPN (11) was replaced with the BamHI/BstXI fragments of Sup (1–61) Δ (5–10) and Sup (1–61) Δ (5–20) PCR products (Table S1) to obtain the YIp-I-SUPNΔ (5–10) and YIp-I-SUPNΔ (5–20) plasmids, respectively. The plasmids were then used as templates for PCR amplification of SUP35 sequences, using primers CYK-43 and CYK-2. The PCR products were cotransformed with YCplac111 (33) into yeast gα5V-H19-PrpΔSF to select red colonies on SC-LEU agar plates. For each derivative, two independent red colonies were isolated and verified by sequencing genomic PCR products (primers CYK-43 and CYK-15).
To determine the [PSI] status, Δ(5–10)/WT and Δ(5–20)/WT heterozygotes, as well as their haploid spores, were transformed with YEp-CUP1-Sup35 (1–61)-GFP-Strep(II)-T (8) to observe the [PSI]-specific particulate labeling pattern (11). Haploid spores were allotyped by BamHI/BstXI digestion of genomic PCR products (primers CYK-43 and CYK-15).
Sup35 Fragments.
DNA encoded Sup35 fragments were amplified by PCR using YCp-I-SUPF as the template and oligonucleotides listed in Table S1. PCR products were digested with BamHI/XmaI to replace the Sup (1–61) fragment of pHIS- Sup35 (1–61)-GFP-Strep(II) and YEp-CUP1-Sup35 (1–61)-GFP-Strep(II)-T (8) to obtain E. coli expression and yeast labeling vectors, respectively. Sup35 fragments were sequenced.
Proteins were overexpressed as GFP fusions in BL21(DE3)/pLysS and purified by Ni-NTA and StrepTactin affinity chromatography as described in refs. 8 and 34. Purified proteins were seeded immediately.
Infectivity Assay.
Purified yeast prion particles (10 μl in Buffer E, 0.3–1.0 μM monomer concentration) were added to 200 μl of purified Sup35 fragments (30–100 μM) to nucleate fiber growth at room temperature for 48 h quiescently. The fibers were sonicated on ice for 10 seconds with 6-W power output and then used to transform yeast by spheroplast fusion as described in ref. 8. Transformed cells were subjected to [PSI] strain-typing as described in ref. 12. Concentrations of different Sup35 fragments were normalized by GFP auto-fluorescence (excitation: 488 nm; emission: 507 nm). Three types of controls were included for yeast transformation: (i) Sup35 fragments alone, mock-seeded by 10 μl of buffer E for 48 h and then sonicated; (ii) 10/210 dilution of the yeast seeds; and (iii) Buffer E alone. Fibers were subjected to SDS-polyacrylamide gel electrophoresis analysis to ensure correct monomer sizes.
For the measurement of specific infectivity, fibers were collected by centrifugation at 200,000 × g on top of a 30% sucrose cushion (g ml−1 in Buffer W) for 2 h. The pellets were washed and resuspended in Buffer W and agitated by vortex and/or sonication to give similar fiber length distributions as measured by flow cytometry (10,000 events counted in a Becton-Dickinson FACS Calibur analyzer, using GFP auto-fluorescence; excitation: 488 nm; emission: 530 nm; 30-nm window). More than 95% of the fibers were single-stranded, as observed by electron microscopy. Equal amounts of fibers (normalized by total GFP fluorescence) were then used for yeast transformation.
Electron Microscopy.
Samples were negatively stained with 3.5% (w/V) uranyl acetate. Images were collected at ×30,000 magnification.
Dominance Relationship in the 74-D-694 Background.
[PSI] strains were introduced to the 74-D-694 background by spheroplast transformation with purified yeast particles (12). Haploids bearing different [PSI] strains were mated. Four independent mating reactions were performed for each [PSI] strain combination. For example, for [VK]X[VL], there were two independent (MATa, [VK]) × (MATα, [VL]) and two independent (MATa, [VL]) × (MATα, [VK]) matings. Diploids were selected and purified by complementation of nutritional markers (two diploids from each mating reaction). The [PSI] strain type of the diploids (eight for each strain combination) were determined by strain-specific GFP labeling using Sup (1–61)-GFP, Sup (1–61)(G20D)-GFP, and Sup (1–61)(Q23P)-GFP (12).
Supplementary Material
Acknowledgments.
We thank Ms. C.-I. Yu for preparing 74-D694 cells and Dr. R. Diaz-Avalos for preliminary EM analysis. This work was supported by the National Science Council, Taiwan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 13191.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802215105/DCSupplemental.
References
- 1.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Eanes ED, Glenner GG. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968;16:673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- 3.Petkova AT, et al. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 4.Vanik DL, Surewicz KA, Surewicz WK. Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol Cell. 2004;14:139–145. doi: 10.1016/s1097-2765(04)00155-8. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Chien P, Yonekura K, Weissman JS. Mechanism of cross-species prion transmission: An infectious conformation compatible with two highly divergent yeast prion proteins. Cell. 2005;121:49–62. doi: 10.1016/j.cell.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Tuite MF, Cox BS. The [PSI+] prion of yeast: A problem of inheritance. Methods. 2006;39:9–22. doi: 10.1016/j.ymeth.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: Inherited structures and biological roles. Nat Rev Microbiol. 2007;5:611–618. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King C-Y, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 10.Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King C-Y. Supporting the structural basis of prion strains: induction and identification of [PSI] variants. J Mol Biol. 2001;307:1247–1260. doi: 10.1006/jmbi.2001.4542. [DOI] [PubMed] [Google Scholar]
- 12.King C-Y, Wang H-L, Chang H-Y. Transformation of yeast by infectious prion particles. Methods. 2006;39:68–71. doi: 10.1016/j.ymeth.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.LeVine H. Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Avalos R, King C-Y, Wall J, Simon M, Caspar DLD. Strain-specific morphologies of yeast prion amyloid fibrils. Proc Natl Acad Sci USA. 2005;102:10165–10170. doi: 10.1073/pnas.0504599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka M, Collins SR, Toyama BH, Weissman JS. The physical basis of how prion conformations determine strain phenotypes. Nature. 2006;442:585–589. doi: 10.1038/nature04922. [DOI] [PubMed] [Google Scholar]
- 16.Toyama BH, Kelly MJS, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449:233–237. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- 17.Nelson R, et al. Structure of the cross-β spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawaya MR, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 19.Kajava AV, Baxa U, Wickner RB, Steven AC. A model for Ure2p prion filaments and other amyloids: The parallel superpleated β-structure. Proc Natl Acad Sci USA. 2004;101:7885–7890. doi: 10.1073/pnas.0402427101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shewmaker F, Wickner RB, Tycko R. Amyloid of the prion domain of Sup35p has an in-register parallel β-sheet structure. Proc Natl Acad Sci USA. 2006;103:19754–19759. doi: 10.1073/pnas.0609638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baxa U, et al. Characterization of β-sheet structure in Ure2p1–89 yeast prion fibrils by solid-state nuclear magnetic resonance. Biochemistry. 2007;46:13149–13162. doi: 10.1021/bi700826b. [DOI] [PubMed] [Google Scholar]
- 22.Chan JCC, Oyler NA, Yau W, Tycko R. Parallel-sheets and polar zippers in amyloid fibrils formed by residues 10–39 of the yeast prion protein Ure2p. Biochemistry. 2005;44:10669–10680. doi: 10.1021/bi050724t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasmer C, et al. Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 24.Bradley ME, Liebman SW. The Sup35 domains required for maintenance of weak, strong or undifferentiated yeast [PSI+] prions. Mol Microbiol. 2004;51:1649–1659. doi: 10.1111/j.1365-2958.2003.03955.x. [DOI] [PubMed] [Google Scholar]
- 25.Shkundina IS, Kushnirov VV, Tuite MF, Ter-Avanesyan MD. The role of the N-terminal oligopeptide repeats of the yeast Sup35 prion protein in propagation and transmission of prion variants. Genetics. 2006;172:827–835. doi: 10.1534/genetics.105.048660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tessier PM, Lindquist S. Prion recognition elements govern nucleation, strain specificity and species barriers. Nature. 2007;447:556–561. doi: 10.1038/nature05848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheibel T, et al. Conducting nanowires built by controlled self-assembly of amyloid fibers and selective metal deposition. Proc Natl Acad Sci USA. 2003;100:4527–4532. doi: 10.1073/pnas.0431081100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles TP, et al. Role of intermolecular forces in defining material properties of protein nanofibrils. Science. 2007;318:1900–1903. doi: 10.1126/science.1150057. [DOI] [PubMed] [Google Scholar]
- 29.Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [PSI+] in the yeast Saccharomyces cerevisiae. Genetics. 1994;137:671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- 31.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 32.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 33.Gietz RD, Akio S. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 34.Skerra A, Schmidt TG. Use of the Strep- tag and streptavidin for detection and purification of recombinant proteins. Methods Enzymol. 2000;326:271–304. doi: 10.1016/s0076-6879(00)26060-6. [DOI] [PubMed] [Google Scholar]
- 35.Rosenow MA, Huffman HA, Phail ME, Wachter RM. The crystal structure of the Y66L variant of green fluorescent protein supports a cyclization-oxidation-dehydration mechanism for chromophore maturation. Biochemistry. 2004;43:4464–4472. doi: 10.1021/bi0361315. [DOI] [PubMed] [Google Scholar]
- 36.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.