Abstract
The molecular mechanism of hepatic cell growth and differentiation is ill defined. In the present study, we examined the putative role of tyrosine phosphorylation in normal rat liver development and in an in vitro model, the α-fetoprotein-producing (AFP+) and AFP-nonproducing (AFP−) clones of the McA-RH 7777 rat hepatoma. We demonstrated in vivo and in vitro that the AFP+ phenotype is clearly associated with enhanced tyrosine phosphorylation, as assessed by immunoblotting and flow cytometry. Moreover, immunoprecipitation of proteins with anti-phosphotyrosine antibody showed that normal fetal hepatocytes expressed the same phosphorylation pattern as stable AFP+ clones and likewise for adult hepatocytes and AFP− clones. The tyrosine phosphorylation of several proteins, including the β-subunit of the insulin receptor, insulin receptor substrate-1, p85 regulatory subunit of phosphatidylinositol-3-kinase, and ras-guanosine triphosphatase-activating protein, was observed in AFP+ clones, whereas the same proteins were not phosphorylated in AFP− clones. We also observed that fetal hepatocytes and the AFP+ clones express 4 times more of the insulin receptor β-subunit compared with adult hepatocytes and AFP− clones and, accordingly, that these AFP+ clones were more responsive to exogenous insulin in terms of protein tyrosine phosphorylation. Finally, growth rate in cells of AFP+ clones was higher than that measured in cells of AFP− clones, and inhibition of phosphatidylinositol-3-kinase by LY294002 and Wortmannin blocked insulin- and serum-stimulated DNA synthesis only in cells of AFP+ clones. These studies provide evidences in support of the hypothesis that signaling via insulin prevents hepatocyte differentiation by promoting fetal hepatocyte growth.
INTRODUCTION
Cell growth and differentiation are regulated by complex and highly coordinated networks of extracellular signaling molecules including hormones and growth factors (Heldin and Westermark, 1984; Pawson and Bernstein, 1990; Cross and Dexter, 1991). The development of adequate experimental models is critical to the characterization of growth-related signaling pathways involved in tissue-specific cell maturation. The production by malignant cells of certain transient proteins, generally expressed only during the early stages of fetal development, has been extensively employed as a tool in in vitro studies of the regulatory mechanisms by which cells switch between stages of differentiation and by which neoplastic cells return to earlier stages of differentiation (Uriel, 1979). α-Fetoprotein (AFP) is the most extensively studied cell differentiation and tumor marker. It is expressed by fetal or malignant hepatocytes and repressed in normal mature hepatocytes (Abelev et al., 1963; de Néchaud and Uriel, 1971; Rouslahti and Seppala, 1971; Hirai et al., 1973; Sell et al., 1976).
We have previously demonstrated that McA-RH 7777 rat hepatoma cells are heterogeneous in terms of the expression of AFP (Khamzina, 1987; Eraiser and Khamzina, 1988). A panel of both stable AFP-producing (AFP+) and AFP-nonproducing (AFP−) and unstable clones was isolated from this cell population on the basis of the level of expression of AFP (Khamzina et al., 1995). Analysis of these clones showed that the phenotypes of stable AFP+ and AFP− clones correspond, respectively, to the fetal and adult phenotypes in the normal hepatocyte development, while the phenotypes of unstable clones correspond to intermediate stages. The hepatocyte-specific marker, albumin, normally expressed through all stages of hepatocyte development, was detected in all clones. Thus, our hepatic cell lines constitute a useful system for in vitro analysis of regulatory pathways involved in the control of cellular growth and differentiation.
Many growth factors exert their effects through binding to and activation of cell surface receptors with intrinsic protein tyrosine kinase activity (Yarden and Ullrich, 1988; Schlessinger and Ullrich, 1992; van der Geer et al., 1994). Growth factor regulation of hepatocyte growth and differentiation remain ill defined. Insulin, a well-known hepatotrophic and growth-promoting factor for a wide variety of cell types, may be a candidate (Rosen, 1987; Cheatham and Kahn, 1995). Insulin action is mediated through the insulin receptor (IR), a transmembrane glycoprotein possessing intrinsic tyrosine kinase activity. Upon insulin binding to the α-subunit of the IR, the β-subunit becomes autophosphorylated on tyrosine residues, an event resulting in enhanced receptor tyrosine kinase activity toward intracellular substrates (Kasuga et al., 1982; Myers and White, 1996).
In the present study, we investigated the possible implication of tyrosine phosphorylation events in hepatic cell growth and differentiation using an in vivo model, the fetal, newborn, and adult rat liver, and an in vitro model, the AFP+ and AFP− clones of the McA-RH 7777 rat hepatoma. We demonstrated that at least 12 phosphoproteins observed in fetal hepatocytes and AFP+ clones were not phosphorylated in adult hepatocytes and AFP− clones. We also identified in vivo and in vitro that the AFP+ phenotype is associated with the IR overexpression. Moreover, we showed that the cells of AFP+ clones expressed enhanced tyrosine phosphorylation of the IR and were clearly more responsive to the action of exogenous insulin as assessed by the levels of IR β-subunit and insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation. Finally, we demonstrated that inhibition of phosphatidylinositol-3-kinase (PIK) activity affects growth of AFP+ cells.
MATERIALS AND METHODS
Antibodies
Unconjugated and FITC-conjugated anti-phosphotyrosine monoclonal antibodies (anti-PY mAb and FITC-anti-PY) (mouse IgG2bk, clone 4G10), and rabbit polyclonal antibodies to the p85 regulatory subunit of rat PIK and to ras-guanosine triphosphatase-activating protein (ras-GAP) were purchased from UBI (Lake Placid, NY). Rabbit polyclonal antibodies to the β-subunit of IR, IRS-1, and mAb to ras-GAP (mouse IgG2a, clone B4F8) were from Santa Cruz (Santa Cruz, CA). TRITC-conjugated goat anti-mouse IgG, FITC-conjugated mouse IgG2, and mouse IgG were from Sigma (Oakville, Canada). Rabbit antisera against AFP and albumin were kindly provided by Dr. L. Belanger (Belanger et al., 1978); antibodies were purified from these antisera by affinity chromatography on protein A-Sepharose CL-4B (Pharmacia, Baie d’Urfe, Canada).
Cell Cultures
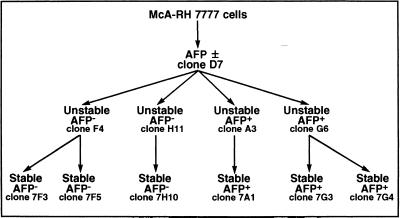
McA-RH 7777 rat hepatoma cell lines were grown in DMEM/L15 (50:50) medium supplemented with 10% FCS, 2 mM glutamine, and 1 mM sodium pyruvate. We previously established a method of stabilizing cloning (7-step selection) allowing the isolation of AFP+ and AFP− clones with different levels of AFP phenotype stability (Eraiser and Khamzina, 1988). Among the stable and unstable clones previously isolated (Khamzina et al., 1995), 11 clones were selected and used in the present study: mixed (AFP±) clone D7; unstable AFP− clones H11, F4; stable AFP− clones 7H10, 7F3, and 7F5; unstable AFP+ clones G6 and A3; and stable AFP+ clones 7A1, 7G3, and 7G4 (Figure 1).
Figure 1.
The filiation of clones isolated by stabilizing cloning from the McA-RH 7777 rat hepatoma cell line.
Northern Blot Analysis
Total RNA was extracted from the cells as described by Chomczynski and Sacchi (1987), electrophoresed on 1% formaldehyde-agarose gel, transferred to Hybond-N membranes, and then hybridized with either random primer 32P-labeled cDNA of AFP gene or a 28S rRNA probe (Khamzina et al., 1995).
Fixation-Permeabilization-Staining Procedures
Indirect AFP and albumin staining were performed on methanol-fixed cells, and visualized using the avidin-biotin complex and 3,3′- diaminobenzidine-H2O2 procedure (Khamzina et al., 1995). Immunofluorescent staining was performed on 2 × 106 cells fixed with 0.8% formaldehyde in buffer A (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 2 mM sodium orthovanadate, 1 mM PMSF, 10 μM leupeptin, 10 μg/ml aprotinin) for 5 min at 25°C and permeabilized with methanol for 5 min at 25°C. These fixation-permeabilization conditions were found to be optimal for the McA-RH 7777 cells. Indeed, in these conditions, minimal morphological cell damage was observed in comparison with standard fixation procedures in 4% formaldehyde or 4% paraformaldehyde at 4°C. It has been reported that saponin permeabilization of cells is reversible, which implies that the detergent must be present in the washing and antibody dilution buffers (Willingham, 1980). Since similar staining patterns were obtained in cells permeabilized with methanol or saponin, the former was used in the present studies. The standard antibody dilution buffer, PBS, was replaced by a Tris buffer because the presence of phosphate ions led to artifactual fluctuations in the measurements of the cellular PY level.
In double immunofluorescence analysis, indirect AFP staining was visualized with TRITC-conjugated goat anti-mouse IgG. Unoccupied reactive sites of the second antibody were blocked with excess of mouse IgG and 2.5 μg/ml of FITC-anti-PY mAb were then applied. Buffer A containing 0.05% gelatin and 0.1% (vol/vol) Tween 20 was used to block nonspecific binding during washing and antibody incubation steps. DNA was stained with 0.5 μg/ml of Hoechst 33258, and cell cycle phases were determined using DNA histogram analysis based on the mathematical model of Dean and Jett (1974).
Flow Cytometric Analysis
Flow cytometry was performed on an EPICS ELITE ESP Cell Sorter (Coulter, Hialeah, FL) fitted with three lasers (Ar, HeCd, and HeNe) for excitation of the three fluorochromes used: FITC, TRITC, and Hoechst 33258. Forward light scatter and 90° light scatter were used to gate out debris, damaged cells, and aggregates. Nonspecific antibody binding was controlled using the isotypic control IgG to set cursors. At least 10,000 cells were analyzed for each sample, and data were collected in listmode files.
Protein Determination
Protein was determined with the Coomassie blue protein assay reagent of Pierce Chemical (Rockford, IL) using crystalline serum albumin as standard.
Western Blot Analysis
Cell and tissue extracts were prepared according to Laemmli (1970). The solubilized proteins were then resolved by SDS-PAGE and electrophoretically transferred onto polyvinyldifluoride membranes (Towbin et al., 1979; Burnette, 1981). The membranes were incubated with appropriate antibodies, and the immune complexes were visualized with the ECL detection system (Amersham, Oakville, Canada). Prestained standard proteins (Sigma) were used to calculate the approximative molecular weights of phosphorylated proteins. The calibration curve was generated from the log of the molecular weights of the standard proteins and their relative mobility values.
Immunoprecipitations
Cells were lysed by boiling in Laemmli buffer, and lysate supernatants (2 mg of protein) were passed through a Sephadex G-10 column (Pharmacia, Baie d’Urfe, Canada) for removal of SDS. The resulting SDS-free lysates were diluted fivefold in buffer B (62.5 mM Tris-HCl, pH 6.8, 150 mM NaCl, 10% glycerol, 0.15% [vol/vol] Tween 20, 2 mM sodium orthovanadate, 1 mM PMSF, 10 μM leupeptin, 10 μg/ml aprotinin) and were precleared with rabbit IgG-coupled protein A-Sepharose, mouse IgG-coupled protein A-Sepharose, and protein A-Sepharose, successively (each step 30 min at 4°C, end-over-end rotation). Immunoprecipitation with appropriate antibodies was carried out during 3 h at 4°C. Immune complexes were precipitated by addition of 50 μl of protein A-Sepharose for 1 h at 4°C. The immunoprecipitates were washed five times with buffer B, and 100 μl of SDS-stop buffer containing 5% β-mercaptoethanol were added. After boiling, the resulting samples were divided into two equal parts, which were subjected to SDS-PAGE and Western blotted as described above.
DNA Synthesis
For serum- and insulin-stimulated DNA synthesis, 2 × 105 cells/ml were grown in 35-mm poly-l-lysine (0.01%)–treated Petri dishes for 30 h and serum starved for 48 h in DMEM containing 0.02% BSA. The cells were incubated with or without inhibitors (5 μM LY294002 or 5 μM Wortmannin) in the presence or absence of insulin (1 μM) or calf serum (10%) for 6 h and then incubated with [3H]thymidine (1 μCi per dish) for the next 10 h. Radioactivity was determined by liquid scintillation counting of 200-μl aliquots of medium from each Petri dish.
RESULTS
Analysis of AFP Phenotypes in McA-RH 7777 Hepatoma Clones
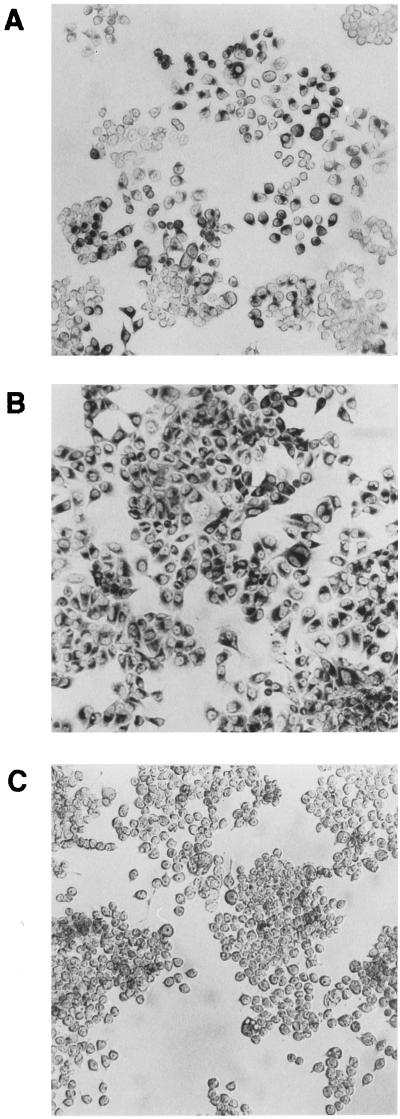
The phenotypes of previously isolated stable and unstable clones (Figure 1) were determined on the basis of expression of AFP and albumin, as described previously (Khamzina et al., 1995). The AFP expression was characterized by two independent and complementary methods: immunocytochemical staining, which localizes the intracellular protein and assesses the population homogeneity (Figure 2), and Northern blot analysis, which measures the AFP mRNA and estimates the actual biosynthesis of AFP (Figure 3A). The various patterns of AFP expression observed are referred to as the AFP+, AFP±, and AFP− phenotypes of clones (Figure 2). Staining using anti-rat albumin antibodies demonstrated the presence in all clones of the hepatocyte-specific marker, albumin (our unpublished results). The remarkable differences in the morphology of the stable AFP+ and AFP− clones were described previously (Khamzina et al., 1995). Figure 2 shows that the stable AFP− clone 7H10 was composed of round cells, whereas the stable AFP+ clone 7G4 consisted mainly of elongated bipolar cells.
Figure 2.
AFP phenotype expression in cells of the McA-RH 7777 rat hepatoma cell line and its clones as determined by immunohistochemical staining. (A) McA-RH 7777 cell monolayer with typical heterogeneity of AFP expression. (B) Stable AFP+ clone 7G4. (C) Stable AFP− clone 7H10.
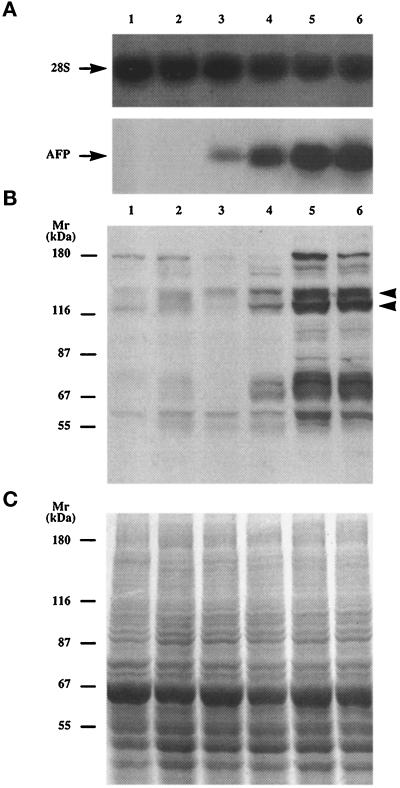
Figure 3.
Northern and Western blot analysis of clones of the McA-RH 7777 rat hepatoma cell line. (A) Northern blot analysis of AFP mRNA levels in total cellular RNA isolated from the stable AFP− clone 7H10 (lane 1), the unstable AFP− clone H11 (lane 2), the AFP± clone D7 (lane 3), the unstable AFP+ clone A3 (lane 4), the stable AFP+ clones 7A1 (lane 5), and 7G4 (lane 6). Integrity of the RNA and equal loading were verified by hybridization with 28S ribosomal RNA probe. (B) Anti-PY immunobloting of whole-cell proteins from these clones. The strongly phosphorylated proteins around 120 kDa and 135 kDa are indicated by arrowheads. (C) Equal protein loading (50 μg/lane) was verified by Coomassie brilliant blue staining of a gel duplicate. Molecular weight markers are indicated.
Tyrosine Phosphorylation of Proteins in Clones of the McA-RH 7777 Hepatoma
To assess the tyrosine phosphorylation status, cells of each clone were cultured in Petri dishes under the conditions used for the AFP phenotype analysis. The cells were lysed by boiling in Laemmli buffer, and total proteins were subjected to SDS-PAGE and Western blotted with anti-PY mAb. Cell numbers and total protein concentrations were monitored to obtain equal protein loading, which was assessed by Coomassie brilliant blue staining (Figure 3C). As shown in Figure 3B, strong phosphorylation of several proteins was observed in AFP+ clones, while the same proteins were much less apparent in either AFP− and AFP± clones and in the parental 7777 cell line (not shown). Eight strongly phosphorylated proteins, p185, p140, p135, p125, p120, p75, p70, and p62, were detected in stable AFP+ clones; four weaker bands, p165, p95, p85, and p55, were also observed in AFP+ clones (Figure 3B). These phosphorylated proteins were consistently detected in our stable AFP+ clones independently of passage number or culture conditions (such as the serum lot or the type of medium used). The same phosphorylation pattern was observed upon immunoprecipitation of proteins with anti-PY-agarose beads followed by blotting with anti-PY mAb (our unpublished results). In unstable AFP+ clones, only four proteins (p140, p125, p70, and p62) were lightly phosphorylated, whereas p185, p165, p135, p120, p95, p85, p75, and p55 were not detected (Figure 3B).
Tyrosine Phosphorylation Status of Clones of the McA-RH 7777 Hepatoma at the Single Cell Level
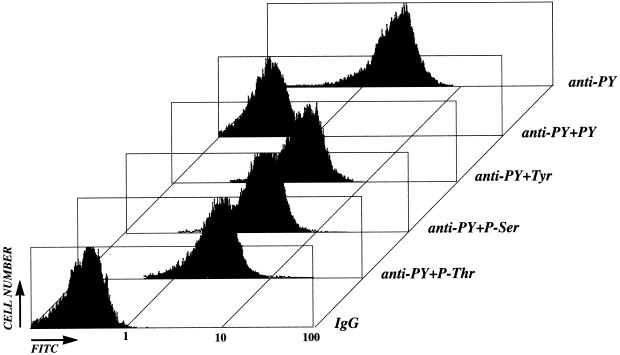
Flow cytometry was used to quantify the levels of protein tyrosine phosphorylation and AFP in individual cells of the parental line and its clones. As described in MATERIALS AND METHODS, different fixation-permeabilization conditions were tested, and optimal procedures for the McA-RH 7777 cells were established. The specificity of the anti-PY antibody used was then assessed. Cells of AFP+ clones, pretreated (or not) with different competitors, were stained with FITC-anti-PY mAb, and the staining patterns were analyzed by flow cytometry (Figure 4). PY (1–5 mM) completely shifted the fluorescence level to that of a control antibody. In contrast, tyrosine (Tyr), phosphoserine (P-Ser), or phosphothreonine (P-Thr) in excess had no effect on the staining pattern obtained with the FITC-anti-PY mAb. Similar results were obtained with another FITC-conjugated anti-PY mAb (mouse IgG1, clone PT66 from Sigma).
Figure 4.
Cytofluorometric assessment of the specificity of cell (stable AFP+ clone 7G4) labeling with anti-PY mAb. Competitors phosphothreonine (P-Thr), phosphoserine (P-Ser), tyrosine (Tyr), and PY were added at the concentration of 1 mM to permeabilized cells before incubation with anti-PY mAb. The results of nonspecific labeling obtained using a control IgG (mouse IgG2 isotype) are also shown.
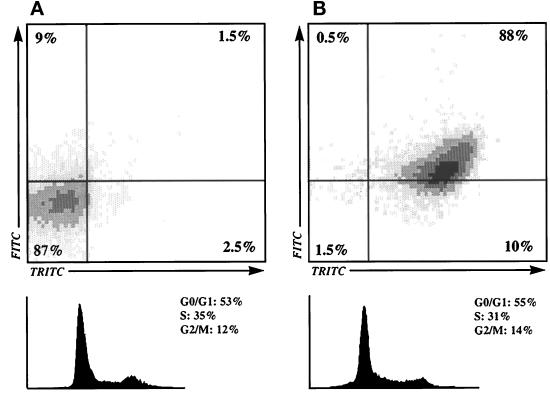
Multiple color flow cytometry was then used to determine the levels of PY and AFP in cells of the parental line and its clones. The phosphorylation intensities in cells of the various clones were similar to those observed by Western blotting, ranging from almost undetectable PY levels in AFP− clones to high levels in AFP+ clones. The low PY level in cells of AFP− clones was taken as the reference. By comparison, the PY level in cells of the parental line and the AFP± clone was about 1.5-fold higher, while the increase was twofold in cells of unstable AFP+ clones and almost fivefold in cells of stable AFP+ clones (Table 1). This correlation between AFP+ phenotype and high PY content was investigated at the single cell level using double fluorescence (FITC-anti-PY and TRITC-anti-AFP) analysis. As shown in Table 1 and Figure 5, the percentage of cells with PY and AFP increased from 1.5–3% in AFP− to 21% in AFP±, 56% in unstable AFP+, and 88% in stable AFP+ populations. Accordingly, the percentage of cells not presenting the association of PY and AFP decreased from 81–87% in AFP− to 39% in AFP±, 9% in unstable AFP+, and 1.5% in stable AFP+ populations. Thus, the AFP+ phenotype was found to be clearly associated with enhanced tyrosine phosphorylation. Analyses were considered only when at least 40% of the cells analyzed were in S + G2/M phases (Figure 5).
Table 1.
Flow cytometric analysis of PY and AFP levels in cells of McA-RH 7777 cell line and its clones
| Clone | AFP phenotype | PY staining | AFP staining | PY and AFP staining (percentage of cells)
|
|||
|---|---|---|---|---|---|---|---|
| (mean fluorescence) | PY+AFP− | PY+AFP+ | PY−AFP− | PY−AFP+ | |||
| 7F3 | Stable | 1.1 | 0.4 | 9 | 1.5 | 87 | 2.5 |
| 7H10 | AFP− | ||||||
| F4 | Unstable | 1.3 | 1.1 | 11 | 3 | 81 | 5 |
| H11 | AFP− | ||||||
| 7777a | AFP± | 1.9 | 9.5 | 7 | 21 | 39 | 33 |
| D7 | |||||||
| A3 | Unstable | 2.7 | 15 | 3 | 56 | 9 | 32 |
| G6 | AFP+ | ||||||
| 7A1 | Stable | 5.4 | 22 | 0.5 | 88 | 1.5 | 10 |
| 7G3 | AFP+ | ||||||
| 7G4 | |||||||
McA-RH 7777 rat hepatoma cell line.
Figure 5.
Upper panels, Comparative analysis of PY-FITC and AFP-TRITC levels in individual cells of AFP+ and AFP− clones. (A) The stable AFP− clone 7H10. (B) The stable AFP+ clone 7G4. The percentage of cells in each quadrant is indicated. Lower panels, DNA histograms from Hoechst 33258-labeled cells. The percentage of cells in G0/G1, S, and M phases is shown on the right.
Identification of Tyrosine Phosphorylated IR β-Subunit, IRS-1, p85 Subunit of PIK, and ras-GAP in AFP+ Clones
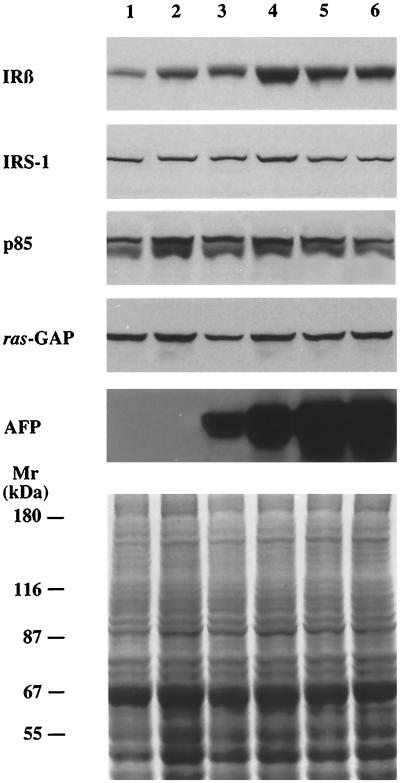
Attempts to identify some of the proteins undergoing phosphorylation in stable AFP+ clones were focused on the IR tyrosine kinase and some its downstream effectors. Total proteins from McA-RH 7777 hepatoma cells and its clones were extracted and analyzed by Western blotting with antibodies to IR β-subunit, IRS-1, p85 subunit of PIK, and ras-GAP. As shown in Figure 6, except for the IR β-subunit, equal amounts of these proteins were detected in all cell populations analyzed. Densitometric analysis of the bands of the IR β-subunit, corrected for the protein loading, showed that the level of this protein in stable AFP+ clones was fourfold higher than that in stable AFP− clones (Figures 6 and 8).
Figure 6.
The levels of IR β-subunit, IRS-1, p85, ras-GAP, and AFP expression in AFP+, AFP±, and AFP− clones of the McA-RH 7777 rat hepatoma cell line. Total proteins were isolated from cells of the stable AFP− clone 7H10 (lane 1), the unstable AFP− clone H11 (lane 2), the AFP± clone D7 (lane 3), the unstable AFP+ clone A3 (lane 4), the stable AFP+ clones 7A1 (lane 5), and 7G4 (lane 6), separated by SDS-PAGE and Western blotted with antibodies to IR β-subunit (IRβ), IRS-1, p85 regulatory subunit of PIK (p85), ras-GAP, and AFP. Equal protein loading (50 μg/lane) was verified by Coomassie brilliant blue staining of a gel duplicate. Molecular weight markers are indicated.
Figure 8.
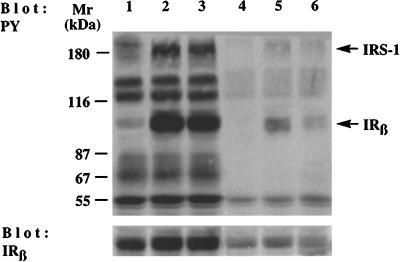
Effect of insulin on expression and tyrosine phosphorylation of IR β-subunit in cells of AFP+ and AFP− clones of the McA-RH 7777 hepatoma cell line. Cells of the stable AFP+ clone 7G3 were incubated at 37°C without insulin (lane 1) or with 100 nM insulin for 20 min (lane 2) or 3 h (lane 3). Cells of the stable AFP− clone 7F3 were incubated at 37°C without insulin (lane 4) or with 100 nM insulin for 20 min (lane 5) or 3 h (lane 6). Proteins from control and insulin-stimulated cells were Western blotted with antibodies to PY or IR β-subunit (IRβ).
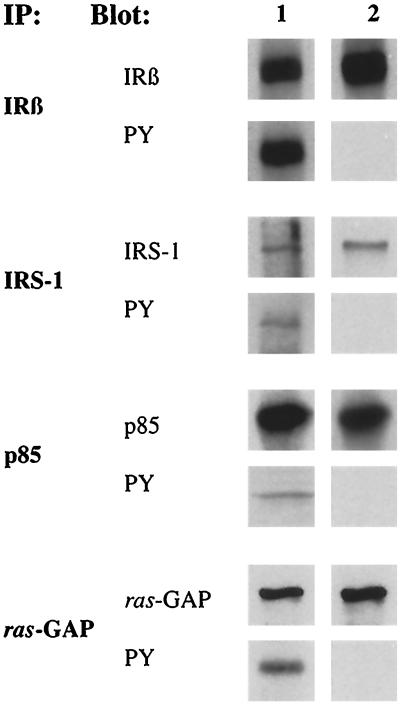
We next investigated by immunoprecipitation whether these IR signaling proteins show enhanced tyrosine phosphorylation in stable AFP+ clones. Cell lysates from both stable AFP+ and stable AFP− clones were immunoprecipitated with the appropriate antibodies (see MATERIALS AND METHODS) and blotted with either the antibody used for immunoprecipitation or anti-PY antibody. An equal quantity of the IR β-subunit was precipitated from both types of clones when an excess of AFP− cell lysates was used (Figure 7). As shown in Figure 7, the β-subunit of IR, IRS-1, p85 of PIK, and ras-GAP were specifically precipitated from both AFP+ and AFP− clones. However, only in cells of AFP+ clones were these proteins phosphorylated on tyrosine residue. Proteins purified with beads of anti-PY-agarose were then blotted with the specific antibody. Bands corresponding (molecular weights) to IR β-subunit, IRS-1, p85, and ras-GAP were detected (our unpublished results). Nonimmune mouse or rabbit serum failed to immunoprecipitate these proteins (our unpublished results). It was concluded that the p165, p120, p95, and p85 recognized by anti-PY antibody in stable AFP+ cells corresponded respectively to IRS-1, ras-GAP, β-subunit of IR, and p85 subunit of PIK.
Figure 7.
Analysis of IR β-subunit, IRS-1, p85, and ras-GAP tyrosine phosphorylation in AFP+ (lane 1) and AFP− clones (lane 2) by immunoprecipitation. The cell lysates from the stable AFP+ clone 7G4 and the stable AFP− clone 7H10 were immunoprecipitated (IP) with antibodies to IR β-subunit (IRβ), IRS-1, p85 regulatory subunit of PIK (p85), and ras-GAP. Each of these immunoprecipitates was divided into two equal parts for Western blotting (BLOT) with either antibodies used for the immunoprecipitations or anti-PY mAb (PY). The two lanes of each blot were on the same filter. In analysis of IR β-subunit, 5 × 106 and 20 × 106 cells were used in lanes 1 and 2, respectively. In analysis of IRS-1, p85, and ras-GAP, 5 × 106 and 8 × 106 cells were used in lanes 1 and 2, respectively.
Effect of Insulin on Expression and Tyrosine Phosphorylation of the IR in AFP+ and AFP− Clones
Cells of stable AFP+ and AFP− clones were stimulated with 100 nM insulin for 20 min or 3 h. Proteins from control and insulin-stimulated cells were analyzed by SDS-PAGE followed by immunoblotting with anti-PY and anti-IR β-subunit antibodies. As shown in Figure 8, insulin caused a significant increase in IR β-subunit (∼30-fold) and IRS-1 (∼5-fold) phosphorylation in AFP+ cells at both stimulation times. In AFP− cells, the stimulation of phosphorylation induced by insulin at 20 min was of lower magnitude for both IR β-subunit and IRS-1. After 3 h of insulin stimulation, the phosphorylation of the IR β-subunit had significantly decreased (75%), whereas that of the IRS-1 had nearly returned to basal levels. Immunoblotting with anti-IR β-subunit antibody demonstrated that the level of IR expression is fourfold higher in the AFP+ clone than that in the AFP− clone. Insulin stimulation (the short and long periods) induced slight increases in the levels of IR expression in the AFP+ and AFP− clones (80% and 35%, respectively; Figure 8). Similar data were obtained with other stable AFP+ and AFP− clones (our unpublished results). It is likely that the enhanced expression of the IR β-subunit in AFP+ clones results in increased sensitivity and/or responsiveness to insulin and thereby accounts for the enhanced tyrosine phosphorylation events in these cells.
Effect of Inhibition of PIK Activity on DNA Synthesis in AFP+ and AFP− Clones
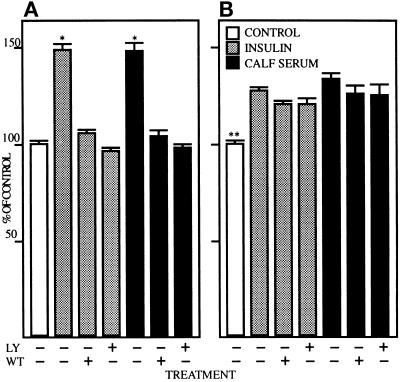
IR signaling involves the stimulation of PIK, an important event in cell growth regulation (Cheatham et al., 1994; Toker and Cantley, 1997). To determine the consequence of PIK pathway inhibition on DNA synthesis, cells of stable AFP+ and AFP− clones were serum starved and incubated 6 h in serum-free medium or medium containing 1 μM insulin or 10% calf serum in the presence of 5 μM LY294002 or 5 μM Wortmannin, two well characterized PIK inhibitors. Thymidine incorporation was determined after an additional 10 h incubation and the data are presented as percent increase over control (cells growing in serum-free medium without treatment). As shown in Figure 9A, insulin stimulated an approximately 50% increase in thymidine incorporation into DNA in cells of the stable AFP+ clone; a similar increase was observed in cells stimulated with 10% calf serum. In the presence of LY294002 or Wortmannin, both insulin- and serum-stimulated effects on DNA synthesis were blocked. At the concentration (5 μM) and incubation time (16 h) used, neither inhibitor was cytotoxic on McA-RH 7777 hepatoma cells (our unpublished results). As shown in Figure 9B, insulin and serum caused a smaller but significant increase of thymidine incorporation (28% and 34%, respectively) in cells of the stable AFP− clone. Both insulin- and serum-stimulated thymidine incorporations were little affected (less than 10% inhibition) by LY294002 or Wortmannin. Moreover, growth rate in cells of the AFP− clone was 72% of that measured in cells of the AFP+ clone (our unpublished results). Similar data were obtained with other stable AFP+ and AFP− clones (our unpublished results). Thus, two chemically distinct inhibitors of PIK, LY294002 and Wortmannin, having different mechanisms of action, blocked growth of the faster growing population of AFP+ cells, but did not affect growth of the slower growing population of AFP− cells.
Figure 9.
[3H]Thymidine incorporation in cells of AFP+ and AFP− clones of the McA-RH 7777 hepatoma cell line after treatment with LY294002 and Wortmannin. Cells of the stable AFP+ clone 7G4 (A) and the stable AFP− clone 7H10 (B) were serum starved for 48 h, treated with 5 μM LY294002 (LY) or 5 μM Wortmannin (WT), and stimulated with 1 μM insulin or 10% calf serum for 6 h. Thymidine incorporation was determined after an additional 10-h incubation. The data are presented as percent increase over control (without treatment); control thymidine incorporation in the AFP− clone was 72% of that measured in the AFP+ clone. Values expressed as mean ± SEM of three separate experiments each performed in triplicate. *, Statistically different (p < 0.01) from all other treatments in panel A; **, statistically different (p < 0.01) from all other treatments in panel B. Statistical analysis was performed by analysis of variance using Fisher’s Protected Least Significant Difference method.
Tyrosine Phosphorylation of Proteins during Liver Development
To assess whether patterns of protein tyrosine phosphorylation of stable AFP+ and AFP− 7777 hepatoma clones correspond, respectively, to patterns of normal fetal and adult hepatocytes, total proteins were extracted from fetal, newborn, and adult rat liver cells. The extraction of proteins was carried out in triplicate from livers of Wistar and Sprague Dawley rats. Tissue samples were lysed by boiling in Laemmli buffer, and total proteins were subjected to SDS-PAGE and Western blotted with anti-PY mAb. Total protein concentrations were monitored to obtain equal protein loading, which was assessed by Coomassie brilliant blue staining (Figure 10C). As shown in Figure 10A, normal fetal hepatocytes expressed the protein pattern of the stable AFP+ 7777 hepatoma clones, and likewise for adult hepatocytes and the AFP− cells.
Figure 10.
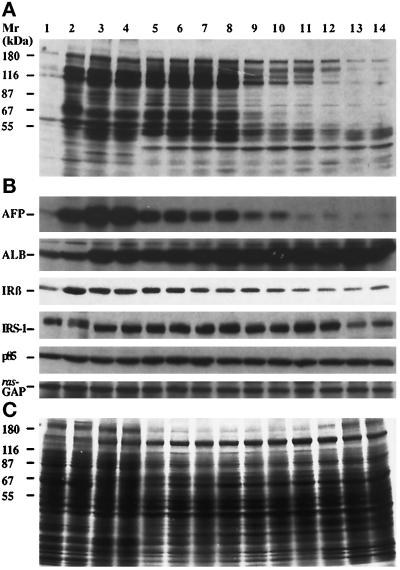
Western blot analysis of rat liver proteins during the course of normal development. (A) Anti-PY immunobloting of proteins from the stable AFP− clone 7H10 (lane 1), the stable AFP+ clones 7A1 (lane 2), the fetal liver of 19 and 20 d of gestation (lanes 3 and 4, respectively), the newborn liver of 3-, 7-, 11-, 14-, 16-, 18-, 21-, and 28-d-old (lanes 5–12, respectively), and the adult livers (lanes 13 and 14). (B) The same proteins as in panel A were blotted with antibodies to AFP, albumin (ALB), IR β-subunit (IRβ), IRS-1, p85 regulatory subunit of PIK (p85), and ras-GAP. (C) Equal protein loading (50 μg/lane) was verified by Coomassie brilliant blue staining of a gel duplicate. Molecular weight markers are indicated.
To assess changes in the level of protein tyrosine phosphorylation during the course of normal rat liver development, fetal livers obtained at 15–20 d of gestation and newborn livers from birth to 35 d were studied. Tyrosine phosphorylation was observed in 15-d fetal liver and reached a maximum at day 19 (our unpublished results). As shown in Figure 10A, this strong protein tyrosine phosphorylation of fetal hepatocytes persisted in newborn hepatocytes until day 14, and then rapidly declined. Indeed, the protein tyrosine phosphorylation was much less apparent in newborn hepatocytes of 16- to 28-d rats, and these patterns were similar to that observed in cells of unstable clones of McA-RH 7777 hepatoma (Figure 3B). The newborns were suckling and were not weaned from their mother until the end of the experiment (day 28); thus, dietary glucose levels were not changed through the experiment. We next investigated the specificity of the observed tyrosine phosphorylation events. Immunoprecipitation of proteins with anti-PY-agarose beads followed by blotting with anti-PY mAb showed that normal fetal hepatocytes expressed the same phosphorylation pattern as stable AFP+ clones, and likewise for adult hepatocytes and AFP− clones (our unpublished results).
We also investigated whether enhanced phosphorylation events in normal liver development correlate with the expression of AFP and albumin. Total proteins from fetal, newborn, and adult liver were analyzed by Western blotting with anti-PY mAb and then with antibodies to rat AFP or albumin. As shown in Figure 10B, albumin was present in high amounts during the course of liver development, and its expression increased about twofold in adult hepatocytes in comparison to fetal hepatocytes (densitometric analysis of the bands corresponding to albumin, corrected for the protein loading). However, the changes of albumin expression did not follow changes in the level of tyrosine phosphorylation. In contrast, the levels of AFP expression and tyrosine phosphorylation closely correlated (Figure 10B). From birth to day 14, high AFP and tyrosine phosphorylation levels were detected in rat liver; after day 15, the levels of AFP and tyrosine phosphorylation dropped gradually to the very low levels observed in adult hepatocytes. Thus, the correlation between AFP and tyrosine phosphorylation levels observed in vitro in clones of McA-RH 7777 hepatoma (Figure 3B) was also observed in vivo during normal hepatocyte development.
We next assessed by Western blotting the expression of IR signaling proteins (IR β-subunit, IRS-1, p85 subunit of PIK, and ras-GAP) at different stages of hepatocyte development. As shown in Figure 10B, equal amounts of the p85 subunit of PIK and ras-GAP were detected in fetal, newborn, and adult liver. The level of the IRS-1 varied but did not correlate with the level of tyrosine phosphorylation since the low level of the IRS-1 was detected only in the adult stage of hepatocyte development. In contrast, the analysis of IR β-subunit expression during hepatocyte development showed some correlation with the tyrosine phosphorylation level. Densitometric analysis of the bands of the IR β-subunit, corrected for the protein loading, showed that the level of this protein in fetal hepatocytes and in young newborns, i.e., from birth to day 11, was, respectively, about fourfold and twofold higher than that in normal adult hepatocytes (Figure 10B). Thus, the more undifferentiated phenotype of normal liver cells correlated with enhanced events of protein tyrosine phosphorylation and with overexpression of the IR β-subunit.
DISCUSSION
The present studies demonstrated that the AFP+ phenotype is clearly associated with enhanced protein tyrosine phosphorylation; at least 12 phosphoproteins observed in fetal hepatocytes and AFP+ clones were not phosphorylated in adult hepatocytes and AFP− clones. Immunoprecipitation studies of proteins with anti-PY antibody also showed that normal fetal hepatocytes expressed the same phosphorylation pattern as stable AFP+ clones and likewise for adult hepatocytes and AFP− clones. Thus, the correlation between AFP expression and increased protein tyrosine phosphorylation was cell autonomous and was observed in both hepatic cell lines and in normal hepatocytes during development. These data as well as the observation of elevated IR β-subunit levels solely in hepatic cells of the fetal stage led us to examine the hypothesis that insulin signaling varies in the fetal and adult stages of development and thereby could be involved in the control of hepatic cell growth and differentiation.
To verify this hypothesis, some functions of the IR such as the activity of its tyrosine kinase and the receptor responsiveness to exogenous insulin were investigated in vitro. The identification in cells of AFP+ clones of the 95-kDa phosphoprotein as the IR β-subunit using anti-PY mAb and polyclonal antibodies suggested that the intrinsic tyrosine kinases of the IR (Kasuga et al., 1982; White and Kahn, 1994) is involved in the ongoing phosphorylation processes. The prominent intracellular target of the kinase-activated IR is a 185-kDa protein, termed IRS-1 (Myers and White, 1996). Accordingly, IRS-1 was also found to be phosphorylated in AFP+ clones. However, in these in vitro studies the cell culture medium was not supplemented with exogenous insulin, making uncertain the nature of the IR activator. Indeed, it is possible that the low concentration of insulin present in serum is sufficient for receptor activation in AFP+ cells; alternatively, two structurally related polypeptides, insulin-like growth factors I and II, present in serum, can also act as IR activators since they are known as ligands and activators of the IR (Jones and Clemmons, 1995). The modulation of protein-tyrosine phosphatase activities may also play a role in the regulation of the IR kinase activity (Goldstein, 1993). Indeed, when protein levels of the leukocyte common antigen-related protein tyrosine phosphatase were suppressed by 63% in the McA-RH 7777 rat hepatoma cell line, insulin-dependent tyrosine phosphorylation and PIK activation were increased by 150% and 350%, respectively (Kulas et al, 1995).
It was also of interest to evaluate the responsiveness of cells of AFP+ and AFP− clones to exogenous insulin. We demonstrated that the cells of AFP+ clones were clearly more responsive to the action of exogenous insulin as assessed by the tyrosine phosphorylation levels of the IR β-subunit and IRS-1. Our observation of an increased level of the IR β-subunit in cells of AFP+ clones compared with cells of AFP− clones likely accounts for their increased responsiveness to insulin. However, it cannot be excluded that other alterations of the IR previously reported in hepatoma cells, such as an alternative splicing of the exon 11 of the IR ligand-binding domain resulting in increased affinity to insulin (Mosthaf et al., 1990; McClain, 1991; Yamaguchi et al., 1991) or altered kinetic properties of the IR tyrosine kinase, may be implicated (Takayama et al., 1984; Williams and Olefsky, 1990).
In the signal transduction cascade triggered by insulin, the phosphorylation of IRS-1 at multiple tyrosine residues creates docking sites for a number of signaling molecules, thereby providing additional links between the IR and other signaling events. Thus, PIK is activated through the binding of its p85 regulatory subunit to IRS-1 (White et al., 1985, 1987). In the present study, the demonstrated phosphorylation of p85 in AFP+ clones is consistent with previous observations of p85 tyrosine phosporylation by the IR in vivo and in vitro (Hayashi et al., 1991–1993). Moreover, PIK activity can be detected in anti-PY immunoprecipitates, which confirms that some component of the active PIK enzyme complex is tyrosine phosphorylated (Endemann et al., 1990; Ruderman et al., 1990; Giorgetti et al., 1993).
Although the exact roles of PIK products are unknown, several lines of evidence implicate them in cell growth regulation (Parker and Waterfield, 1992; Toker and Cantley, 1997). In our in vitro system, normal growth rate in cells of the AFP+ clones was higher than that measured in cells of the AFP− clones; moreover, both LY294002 and Wortmannin blocked growth only of AFP+ cells, supporting that higher insulin-responsive growth in hepatic cells is associated with the fetal stage of development. It is known that LY294002 behaves as a competitive reversible inhibitor of the ATP-binding site of PIK and abolishes PIK activity in vitro and in vivo (Vlahos et al., 1994). Wortmannin (a fungal metabolite) acts as a covalent, irreversible inhibitor of PIK, binding to the p110 catalytic subunit of the kinase (Yano et al., 1993). The utilization in the present studies of two PIK inhibitors of different structures and mechanisms of action makes it unlikely that the observed action of the inhibitors on cell growth is the consequence of unspecific effects of the compounds.
Ras is an important component of mitogenic signaling pathways; the interaction of ras with the phosphorylated IRS-1 in insulin signaling has been shown to result in ras activation (Baltensperger et al., 1993; Skolnik et al., 1993; Tobe et al., 1993). The herein demonstrated tyrosine phosphorylation of a ras-GAP in AFP+ clones is in agreement with previous observations that ras-GAP associates with the autophosphorylated IR and becomes tyrosine phosphorylated in response to insulin in cells overexpressing the IR and treated with an inhibitor of protein tyrosine phosphatases (Pronk et al., 1992). However, while ras-GAP is possibly involved as a negative regulator of ras (McCormick, 1989; Feig, 1993), the significance of tyrosine phosphorylation of a ras-GAP in insulin signaling remains unclear. Neither the phosphorylation of ras-GAP nor its putative, transient association with the insulin receptor appear to be required for insulin-stimulated ras activation (Porras et al., 1992). Additional in vivo studies will be required to clarify the significance of p85 regulatory subunit of PIK and ras-GAP tyrosine phosphorylation in AFP+ clones.
Thus, the strong association of elevated IR β-subunit levels, insulin responsiveness in terms of cell growth, and tyrosine phosphorylation solely in hepatic cells of the fetal stage supports the hypothesis that insulin signaling promote fetal hepatocyte growth and prevent hepatocyte differentiation. Furthermore, the decline in proliferative activity of hepatocytes in the developing rat liver between 14 and 18 d after birth (Sell et al., 1974; Guillouzo et al., 1979; Belanger et al., 1983) is clearly compatible with our observation of a transition to lower tyrosine phosphorylation and IR expression levels in liver cells at this time. Viewed in this context, our results also suggest that the loss of insulin receptor, coupled with lower insulin-responsive growth, might help promote or maintain adult hepatocyte differentiation. Accordingly, liver regeneration, a process accompanied by intense proliferative activity and expression of the fetal AFP+ phenotype in liver cells (Tamaoki and Fausto, 1984; Petropoulos et al., 1985; Sell and Dunsford, 1989), has been shown to be associated with the increased IR function as overexpression of the IRS-1 and enhanced tyrosine phosphorylation of the IR and IRS-1 (Sasaki et al., 1993; Diehl and Rai, 1996). Finally, the regenerative response to partial hepatectomy is significantly impaired in rats pretreated with anti-insulin antisera (Bucher and Swaffield, 1973). In summary, the present studies on insulin signaling in cells of hepatic origin open new perspectives in developmental and cancer biology of the liver.
ACKNOWLEDGMENTS
We acknowledge Dr. R. Al-Daccak, Dr. S. Bourgoin, Dr. C. Léveillé, Dr. N. Marceau, and Dr. P. H. Naccache for helpful discussion and critical review of the manuscript. We also thank M. Dufour, C. Gilbert, and S. Lille for expert technical assistance. We gratefully acknowledge the referees for their very pertinent and insightful comments. L.K. was supported in part by a postdoctoral fellowship from Le Centre Hospitalier de l’Université Laval Research Center. This work was supported by a grant from the Medical Research Council of Canada.
Footnotes
Abbreviations: AFP, α-fetoprotein; AFP+, α-fetoprotein-producing phenotype; AFP±, phenotype with mixed α-fetoprotein production; AFP−, α-fetoprotein-nonproducing phenotype; GAP, guanosine triphosphatase-activating protein; IR, insulin receptor; IRS-1, insulin receptor substrate-1; mAb, monoclonal antibody; PIK, phosphatidylinositol-3-kinase; PY, phosphotyrosine.
REFERENCES
- Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal α-globulin by transplantable mouse hepatoma. Transplantation. 1963;1:174–180. doi: 10.1097/00007890-196301020-00004. [DOI] [PubMed] [Google Scholar]
- Baltensperger K, Kozma LM, Cherniack AD, Klarlund JK, Chawla A, Banerjee U, Czech MP. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993;260:1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Belanger L, Hamel D, Dufour D, Guillouzo A, Chiu JF. Hormonal control and putative cell cycle dependency of AFP production: further observations in vivo and in vitro. Scand J Immunol. 1978;8:239–246. [Google Scholar]
- Belanger L, Baril P, Guertin M, Gingras M-C, Gourdeau H, Anderson A, Hamel D, Boucher J-M. Advances in Enzyme Regulation. Vol. 21. G. Weber, New York: Pergamon Press; 1983. Oncodevelopmental and hormonal regulation of α-fetoprotein gene expression; pp. 73–98. [DOI] [PubMed] [Google Scholar]
- Bucher NLR, Swaffield MN. Regeneration of liver in rats in the absence of portal splanchnic organs and a portal blood supply. Cancer Res. 1973;33:3189–3194. [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- Cheatham B, Vlahos CJ, Cheatham L, Wang L, Wang L, Blenis J, Kahn CR. Phosphotidylinositol 3-kinase activation is required for insulin stimulation of Pp 70S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell. 1991;64:271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- de Néchaud B, Uriel J. Antigènes cellulaires transitories du fois de rat. I. Secretion et synthese des proteines seriques foetospecifiques au cours du developpement et de la regeneration hepatiques. Int J Cancer. 1971;8:71–80. doi: 10.1002/ijc.2910080110. [DOI] [PubMed] [Google Scholar]
- Dean PN, Jett JH. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974;60:523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AM, Rai RM. Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215–227. doi: 10.1096/fasebj.10.2.8641555. [DOI] [PubMed] [Google Scholar]
- Endemann G, Yonezawa K, Roth RA. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990;265:396–400. [PubMed] [Google Scholar]
- Eraiser TL, Khamzina L. Phenotypic variability of cultured rat hepatoma cell population in respect to alpha-fetoprotein synthesis. Int J Cancer. 1988;42:633–637. doi: 10.1002/ijc.2910420427. [DOI] [PubMed] [Google Scholar]
- Feig LA. The many roads that lead to Ras. Science. 1993;260:767–768. doi: 10.1126/science.8484117. [DOI] [PubMed] [Google Scholar]
- Giorgetti S, Ballotti R, Kowalski-Chauvel A, Tartare S, van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993;268:7358–7364. [PubMed] [Google Scholar]
- Goldstein BJ. Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation. Receptor. 1993;3:1–15. [PubMed] [Google Scholar]
- Guillouzo A, Boisnard-Rissel M, Belanger L, Bourel M. α-Fetoprotein production during the hepatocyte growth cycle of developing rat liver. Biochem Biophys Res Commun. 1979;91:327–331. doi: 10.1016/0006-291x(79)90621-1. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Kamohara S, Nishioka Y, Kanai F, Miyake N, Fukui Y, Shibasaki F, Takenawa T, Ebina Y. Insulin treatment stimulates the tyrosine phosphorylation of the α-type 85-kDa subunit of phosphatidylinositol 3-kinase in vivo. J Biol Chem. 1992;267:22575–22580. [PubMed] [Google Scholar]
- Hayashi H, Miyake N, Nishioka Y, Kanai F, Shibasaki F, Takenawa T, Ebina Y. Phosphorylation in vitro of the 85-kDa subunit of phosphatidylinositol 3-kinase and its possible activation by the insulin receptor tyrosine kinase. Biochem J. 1991;280:769–775. doi: 10.1042/bj2800769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Nishioka Y, Kamohara S, Kanai F, Ishii K, Fukui Y, Shibasaki F, Takenawa T, Kido H, Katsunuma N, Ebina Y. The α-type 85-kDa subunit of phosphatidylinositol 3-kinase is phosphorylated at tyrosines 368, 580, and 607 by the insulin receptor. J Biol Chem. 1993;268:7107–7117. [PubMed] [Google Scholar]
- Heldin C-H, Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984;37:9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hirai H, Nishi S, Watabe H, Tsukada Y. Some chemical, experimental, and clinical investigations of α-fetoprotein. Gann. 1973;14:19–34. [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Karlsson FA, Kahn CR. Insulin stimulates the phosphorylation of the 95,000 dalton subunit of its own receptor. Science. 1982;215:185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Khamzina L. Heterogeneity of alpha-fetoprotein expression in hepatomas. Annu Rev Med USSR. 1987;18:32–37. [Google Scholar]
- Khamzina L, Eraiser TL, Borgeat P. Alpha-fetoprotein (AFP) expression in clones of McA-RH 7777 rat hepatoma: correlation with the occurrence of homogeneously staining regions on chromosome 14. Cancer Res. 1995;55:3615–3622. [PubMed] [Google Scholar]
- Kulas DT, Zhang WR, Goldstein BJ, Furlanetto RW, Mooney RA. Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J Biol Chem. 1995;270:2435–2438. doi: 10.1074/jbc.270.6.2435. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClain DA. Different ligand affinities of the two human insulin receptor splice variants are reflected in parallel changes in sensitivity for insulin action. Mol Endocrinol. 1991;5:734–739. doi: 10.1210/mend-5-5-734. [DOI] [PubMed] [Google Scholar]
- McCormick F. ras GTPase activating protein: signal transmitter and signal terminator. Cell. 1989;56:5–8. doi: 10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990;9:2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, White MF. Insulin signal-transduction and the IRS-1 proteins. Annu Rev Pharmacol. 1996;36:615–658. doi: 10.1146/annurev.pa.36.040196.003151. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Waterfield MD. Phosphatidylinositol 3-kinase: a novel effector. Cell Growth Differ. 1992;3:747–752. [PubMed] [Google Scholar]
- Pawson T, Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990;6:350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- Petropoulos CJ, Yaswen P, Panzica M, Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of α-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985;45:5762–5768. [PubMed] [Google Scholar]
- Porras A, Nebreda AR, Benito M, Santos E. Activation of Ras by insulin in 3T3 L1 cells does not involve GTPase-activating protein phosphorylation. J Biol Chem. 1992;267:21124–21131. [PubMed] [Google Scholar]
- Pronk GJ, Medema RH, Burgering BM, Clark R, McCormick F, Bos JL. Interaction between p21ras GTPase activating protein and the insulin receptor. J Biol Chem. 1992;267:24058–24063. [PubMed] [Google Scholar]
- Rosen OM. After insulin bind. Science. 1987;237:1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rouslahti E, Seppala M. Studies of carcinofetal proteins: physical and chemical properties of human α-fetoprotein. Int J Cancer. 1971;7:218–225. doi: 10.1002/ijc.2910070205. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Kapeller R, White MF, Cantley LC. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci USA. 1990;87:1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Zhang XF, Nishiyama M, Avruch J, Wands JR. Expression and phosphorylation of insulin receptor substrate 1 during rat liver regeneration. J Biol Chem. 1993;268:3805–3808. [PubMed] [Google Scholar]
- Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Sell S, Becker FF, Leffert H, Watabe H. Expression of an oncodevelopmental gene product (α-fetoprotein) during fetal development and adult oncogenesis. Cancer Res. 1976;36:4239–4249. [PubMed] [Google Scholar]
- Sell S, Dunsford H. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989;134:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sell S, Nichols M, Becker FF, Leffert L. Hepatocyte proliferation and α-fetoprotein in pregnant, neonatal, and partially hepatectomized rats. Cancer Res. 1974;34:865–871. [PubMed] [Google Scholar]
- Skolnik EY, Lee C-H, Batzer A, Vincentini LM, Zhou M, Daly R, Myers MJ, Backer JM, Ullrich A, White MF, Schlessinger J. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signaling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, White MF, Lauris V, Kahn CR. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci USA. 1984;81:7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T, Fausto N. Recombinant DNA and Cell Proliferation. G. Stein and J. Stein, New York: Academic Press Inc.; 1984. Expression of the α-fetoprotein gene during development, regeneration, and carcinogenesis; pp. 145–168. [Google Scholar]
- Tobe K, Matuoka K, Tamemoto H, Ueki K, Kaburagi Y, Asai S, Noguchi T, Matsuda M, Tanaka S, Hattori S, Fukui Y, Akanuma Y, Yazaki Y, Takenawa T, Kadowaki T. Insulin stimulates association of insulin receptor substrate-1 with the protein abundant Src homology/growth factor receptor-bound protein 2. J Biol Chem. 1993;268:11167–11171. [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin J, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriel J. Retrodifferentiation and the fetal patterns of gene expression in cancer. Adv Cancer Res. 1979;29:127–174. doi: 10.1016/s0065-230x(08)60847-7. [DOI] [PubMed] [Google Scholar]
- van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- White MF, Maron R, Kahn CR. Insulin rapidly stimulates tyrosine phosphorylation of a Mr 185, 000 protein in intact cells. Nature. 1985;318:183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- White MF, Stegmann EW, Dull TJ, Ullrich A, Kahn CR. Characterization of an endogenous substrate of the insulin receptor in cultured cells. J Biol Chem. 1987;262:9769–9777. [PubMed] [Google Scholar]
- Williams JF, Olefsky MJ. Defective insulin receptor function in down-regulated HepG2 cells. Endocrinology. 1990;127:1706–1717. doi: 10.1210/endo-127-4-1706. [DOI] [PubMed] [Google Scholar]
- Willingham MC. Electron microscopic immunocytochemical localization of intracellular antigens in cultured cells: the EGS and ferritin bridge procedures. Histochem J. 1980;12:419–434. doi: 10.1007/BF01011958. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Flier JS, Yokota A, Benecke H, Backer M, Moller DE. Functional properties of two naturally occurring isoforms of the human insulin receptor in Chinese hamster ovary cells. Endocrinology. 1991;129:2058–2066. doi: 10.1210/endo-129-4-2058. [DOI] [PubMed] [Google Scholar]
- Yano HS, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]