Abstract
Colon carcinoma is one of the leading causes of death from cancer and is characterized by a heterogenic pool of cells with distinct differentiation patterns. Recently, it was reported that a population of undifferentiated cells from a primary tumor, so-called cancer stem cells (CSC), can reconstitute the original tumor on xenotransplantation. Here, we show that spheroid cultures of these colon CSCs contain expression of CD133, CD166, CD44, CD29, CD24, Lgr5, and nuclear β-catenin, which have all been suggested to mark the (cancer) stem cell population. More importantly, by using these spheroid cultures or freshly isolated tumor cells from multiple colon carcinomas, we now provide compelling evidence to indicate that the capacity to propagate a tumor with all differentiated progeny resides in a single CSC. Single-cell-cloned CSCs can form an adenocarcinoma on xenotransplantation but do not generate the stroma within these tumors. Moreover, they can self-renew and are capable of multilineage differentiation. Further analysis indicated that the lineage decision is dictated by phosphoinositide 3-kinase (PI3K) signaling in CSCs. These data support the hypothesis that tumor hierarchy can be traced back to a single CSC that contains multilineage differentiation capacity, and provides clues to the regulation of differentiation in colon cancers in vivo.
Keywords: colorectal cancer, tumor-initiating cell
Recent evidence suggests that various, if not all, tumors consist of heterogeneous populations of cells differing in marker expression and growth capacities (1, 2). The cancer stem cell (CSC) hypothesis suggests that this heterogeneity is because of ongoing differentiation within a tumor. In this model, only a small population of cells is clonogenic and contains tumor initiating potential whereas the majority of the tumor cells have undergone differentiation and lost this potential (1, 2). These clonogenic tumor cells are capable of inducing a new tumor in mice and are therefore defined as CSCs (3). In colorectal cancer (CRC), these cells have been reported to express CD133 and thus can be isolated by sorting out the CD133+ fraction of tumor cells (4–6). Xenotransplantation of this CD133+ enriched fraction results in a tumor that closely resembles the original malignancy in both morphology and marker expression. Although this suggests that CD133+ cells signify colon CSC, it has also been reported that only one in 262 CD133+ cells would be a true CSC (4). In addition, none of these studies actually used 100% pure CD133+ cells, thus allowing for alternative explanations (2, 7).
Besides CD133, other cell surface proteins have been reported to mark colon CSCs. For instance, CD166 combined with CD44 (8) or CD24 combined with CD29 (R. Fodde, unpublished data) define the colorectal CSC population. It is currently uncertain whether these markers overlap and define a similar population or designate different populations of cells. These data show the need for a better definition of CSCs and the markers used, but above all raise the question as to whether the capacity to regenerate a heterogenic tumor resides in a monoclonal cell population or depends on multiple different CSCs. This is especially relevant when considering the multiple differentiation patterns observed in colon carcinomas, which could be a sign of multilineage differentiation by a CSC, but could also exemplify a polyclonal CSC population in which different CSCs drive separate differentiation programs. Neither can be excluded at this point because heterogenic colon carcinomas have so far been generated by xenotransplantation of a purified, but not necessarily monoclonal, population of CSCs.
Therefore, we set out to analyze the multilineage differentiation patterns of colon CSCs and further characterize these cells by using multiple markers. We show that all currently reported CSC markers are coexpressed on cells that contain a tumor-initiating capacity. More importantly, by using single-cell-cloned colon CSCs, we show that CSCs contain multilineage differentiation potential both in vitro and in vivo. Our in vitro experiments furthermore revealed that PI3K is a crucial determinant in this cell fate decision.
Results
Colon Sheroid Cultures Show Tumor-Initiating Capacity and Are Heterogeneous.
Recent evidence indicates that spheroid cultures of primary cancer cells are superior to “regular” adherent grown cultures in medium containing serum (5, 9) because xenotransplanted tumors derived from such spheroid cultures more faithfully preserve the original gene expression profiles and tumor morphology (5, 9). In agreement, we have generated colon cancer spheroid cultures of primary colorectal cancers and liver metastases and these are consistently capable of inducing tumors upon xenotransplantation that resemble the original malignancies, both in morphology and marker expression (Fig. 1A) (6).
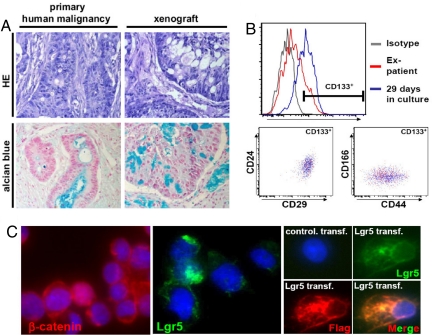
Fig. 1.
Colon spheroid cultures are heterogeneous for CSC marker expression. (A) Injection of colon cancer spheroid cells generate a tumor that closely resembles the original tumor in morphology, as judged by HE (Upper) and Alcian Blue staining (Lower) of paraffin-embedded sections. (B) Upper shows gradual enrichment for the CD133+ cells in a culture derived from a colon cancer liver metastasis. Lower shows that the marker expression of the CD133+ cells is conserved on expansion in vitro because the profiles for CD24/CD29 and CD44/CD166 expression are overlapping for the direct isolated CD133+ cells and the CD133+ cells in a spheroid culture. (C) Immunofluorescence staining of cytospins of a dissociated colon spheroid culture reveals heterogeneity in nuclear localization of β-catenin and Lgr5 expression. A control for the specificity of the Lgr5 antibody is shown in Right, which contains control transfected or Flag-tagged Lgr5 transfected 293T cells stained for anti-FLAG (red) or Lgr-5 (green). Merge shows both antibodies and indicates a complete overlap in antibody stainings.
It is important to note that these cultures are not homogeneous, but consist of heterogeneous populations of cells with respect to markers associated with CSCs. Although the majority of cells are negative for the differentiation marker cytokeratin-20, we observe heterogeneity for CD133, which is associated with the stem cell compartment in a variety of tissues (data not shown) [Fig. 1B and supporting information (SI) Fig. S1]. Moreover, CD24, CD29, CD44, and CD166, which have all been described to enrich for CSCs in CRCs (R. Fodde, unpublished data) (8), were also expressed on a subpopulation in those spheroid cultures (Fig. 1B and Fig. S1). Importantly, we observed that the small percentage of CD133+ cells present in the primary tumor before culture also show expression of these markers (Fig. 1B) and that culture under stem cell conditions selects for cells bearing the above described CSC markers (Fig. 1B). This suggests that the culture method allows for selective expansion of cells with an immature phenotype, without changing their marker expression profile. Importantly, staining for β-catenin revealed that only a minority of cells show clear nuclear localization of this protein (Fig. 1C), implying varying degrees of Wnt signaling activity. In normal colon tissue, active Wnt signaling is observed at the bottom of crypts and identifies colon stem cells. In apparent agreement, we also detected expression of the recently reported normal intestinal stem cell marker, Lgr5, in a defined subset of the spheroid cells in our cultures (Fig. 1C) (10). Combined, this illustrates that the spheroid cultures are heterogeneous with respect to marker expression and Wnt signaling activity. This heterogeneity could reflect the presence of multiple cell types within the cultures that may be responsible for the heterogeneity in the original malignancy and the xenotransplanted tumors derived from these spheroids. Alternatively, it could point to in vitro divergence from CSCs to more differentiated, less immature cells. Understanding this in further detail will provide important information on the origin of heterogeneity in solid tumors.
Clonogenicity of Colorectal CSCs.
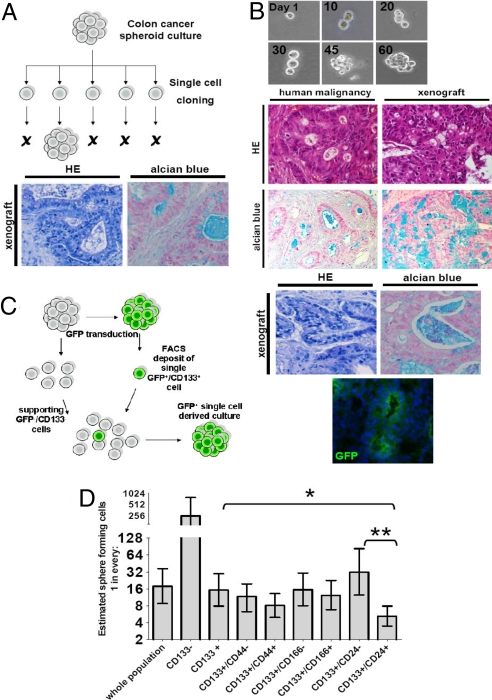
To elucidate whether a single colon cancer cell can undergo multilineage differentiation and has the capacity to generate a differentiated colon adenocarcinoma, we initiated a series of single-cell-cloning experiments. Using single-cell sorting of spheroid cells by flow cytometry we established that ≈1 in 20 cells has the capacity to induce a monoclonal culture as judged by the successful formation of spheroids (Fig. 2A). Moreover, we used a different CRC specimen and performed directly ex-patient single cell cloning by using single cell plating validated by microscopy (Fig. 2B).
Fig. 2.
Single-cell derived cultures give rise to differentiated adenocarcinomas on subcutaneous injection into nude mice. (A) Schematic representation of single-cell cloning of CSCs. Unstained single cells were sorted from a colon CSC culture into 96-well plates and after expansion formed single-cell-derived cultures. Twenty of 384 wells that received one single cell showed formation of a sphere. Lower shows formation of adenocarcinoma after subcutaneous injection of SCDCs in immunodeficient mice by using HE and Alcian Blue staining. (B) 384 wells were plated with bulk tumor cells from a T2N0M0 colorectal carcinoma. One hundred fifty-seven wells contained a single cell as judged by microscopy. Spheroids that arose were expanded and injected into immunodeficient mice where they formed an adenocarcinoma (Right) that resembles the original human malignancy (Left) as confirmed by HE and Alcian Blue staining. (C) Schematic representation of the mixing experiment by using CD133+ and CD133− cells. CD133+ GFP-transduced CSCs were single-cell deposited into different amounts (10, 50, 100, and 200) of CD133−/GFP− cells in 96-well plates and 4 spheres formed that were completely GFP+. Doublets were excluded by using stringent settings on FSC-width. Right shows formation of an adenocarcinoma that is GFP+ as confirmed by HE and Alcian Blue staining and GFP immunohistochemistry. (D) Limiting dilution analysis for different populations within the colon spheroid cultures show diverse clonogenic potential. 1, 2, 4, or 6 cells were deposited by FACS in wells from a 96-well plate and the outgrowth of spheres was monitored. Depicted is the calculated fraction of cells containing sphere initiating capacity. Error bars, 95% confidence interval (*, P < 0.05, **, P < 0.01).
Because CD133 is reportedly selective for CSCs in colon cancer, we tested whether clonogenicity was indeed present in the CD133+ subset of cells. We therefore FACS deposited a single GFP-transduced CD133+ spheroid cell into different amounts of GFP−CD133− cells from the same culture. This invariably resulted in GFP+ spheres (Fig. 2C). As transmission of the expression vector was excluded (Fig. S2), this indicated that the CD133+ and not the CD133− cells contain the clonogenic capacity. In agreement, limiting dilution experiments from a spheroid culture showed that ≈1 in 16 CD133+ cells has the capacity to generate spheroids, whereas a calculated 1 in 250 CD133− cells have this ability (Fig. 2D). To fully ascertain that this is also true for directly isolated tumor cells, we used a third primary CRC and initiated single cell cultures with purified CD133+ cells directly ex-patient. Also in this setting the CD133+ cell fraction generated single cell derived spheroids, whereas the CD133− cells were incapable of doing so (Fig. S3A and data not shown). This clearly confirmed that the clonogenic potential of our spheroid cultures resides in the CD133+ cells.
As stated above, the colon cancer spheroid cultures we obtained show heterogeneity for various markers related to the tumor initiating population in CRC even within the CD133+ population. We therefore determined whether any of the other CSC cell surface markers could improve the identification of the clonogenic population within the spheroid cultures. Coexpression of CD44, CD166, or CD29 with CD133 did not increase the selection of clonogenic cells. However, coexpression of CD133 and CD24 clearly identified the clonogenic cells with higher fidelity (≈1 in 5) (Fig. 2D), suggesting that the combination of these markers may provide a better selection of CSCs.
Single-Cell Propagation of Colorectal Cancer.
The single-cell-derived cultures (SCDCs) we obtained by FACS deposition or single cell plating displayed similar expansion rates as the original culture, indicating that we did not select for rapid proliferating cells (Fig. S4). To determine whether these isolated and monoclonal cells could still grow out to form an adenocarcinoma, we injected these cells s.c. into mice.
Irrespective of the method of subcloning, that is, either directly ex-patient or from spheroid culture, and either CD133-selected or unselected, all SCDCs formed tumors subcutaneously that had an adenocarcinoma appearance and resembled the primary human carcinoma from which they were derived (Fig. 2 A–C and Fig. S3B). Crypt-like structures surrounded with epithelial cells were detected in all cases, which are derived from injected cells as evidenced by GFP expression in GFP+ SCDC-derived tumors (Fig. 2C). In contrast, the stromal cells were GFP− and therefore mouse derived. This indeed confirms our idea that the capacity to generate a morphologically differentiated colon adenocarcinoma resides in a single cell. To determine whether this single cell also retains self-renewal capacity after in vivo expansion, we examined whether tumors derived from these single cells express the CSC marker CD133, and found that a small proportion of cells had preserved this expression in vivo (Fig. 3 A and B, and Fig. S3B). Importantly, when spheroid cultures were rederived from GFP+ SCDC-induced xenotransplants, these were again GFP+ (Fig. 3C and Fig. S5A). This supports the hypothesis that the original GFP+ single cell had undergone massive expansion and differentiation in vivo, but also preserved clonogenic potential in a small subpopulation of its offspring. In agreement, rederived cultures expressed CD133, but not CK20 (Fig. 3C) and, on injection, induced growth of an adenocarcinoma in which a minority of the cells is again CD133+ (Fig. 3D). These observations were corroborated with cells derived from xenotransplants that were originally derived from directly ex-patient single cell cloned CSCs (data not shown), and thus prove that in vivo self-renewal is retained in these single cell cloned colon CSCs. To bolster our claim that the tumors we analyzed are truly single- cell derived we examined the GFP expression of the GFP+ SCDCs and the cultures that were derived from their xenografts. In contrast to the original line, which contained a broad expression range of GFP, we found that the GFP expression levels were restricted to a very limited intensity range in SCDCs. Importantly, this was unchanged even after mouse passage (Fig. S5A). More importantly, Southern blot analysis was used to scrutinize GFP integration sites in the genome of the SCDCs and the cultures derived after xenotransplantation (SCDC.R1). The Southern blot profile of GFP integration sites shows complete similarity between the parental SCDCs and the cultures derived form the xenograft. Moreover, on a second round of single cell cloning of the GFP+ SCDCs, complete similarity was observed regarding the integration sites (Fig. S5B). This confirms the clonal origin of the GFP+ SCDC-derived tumors studied here.
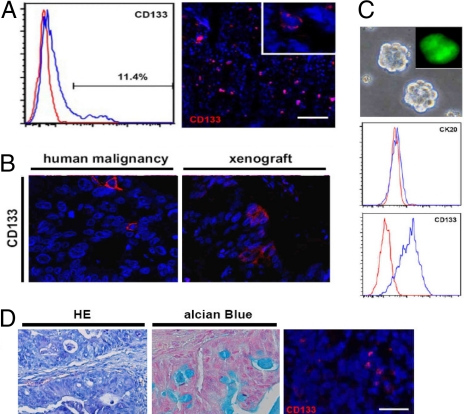
Fig. 3.
Functional CSCs can be reisolated from single cell-derived tumors. (A) Xenografts derived from SCDCs show CD133+ cells either detected by FACS analysis (Left) or by immuno-fluorescence staining (Right). (Inset) Higher magnification and confirms membrane localization of CD133. (Scale bar, 200 μm.) (B) SCDC-induced xenografts as generated in Fig. 2B, show a distinct CD133+ fraction, comparable to the primary human malignancy. (C) Spheroid cultures can be isolated from tumors derived from SCDCs, show the same spheroid morphology and phenotype (CK-20 and CD133), and are GFP+ in the case of GFP+ SCDC-derived tumors (Inset). (D) Subcutaneous injection of reisolated spheroid cultures induces a tumor with the same differentiated morphology. HE staining (Left), and Alcian Blue staining (Center) on paraffin sections. Right shows tumor immunofluorescent staining for CD133. (Scale bar, 40 μm.)
SCDCs Show Multilineage Differentiation Potential.
Colon carcinomas show a wide variety of differentiated cell types, often resembling the various lineages present in normal colon epithelium (11–15). This heterogeneity has been attributed to genetically distinct clones present in a tumor. In contrast, the CSC theory would rather argue for a remnant differentiation response resulting in multilineage differentiation. To date it has not been possible to prove this concept, but our SCDCs now provide us with the opportunity to confirm either of these models both in vitro and in vivo.
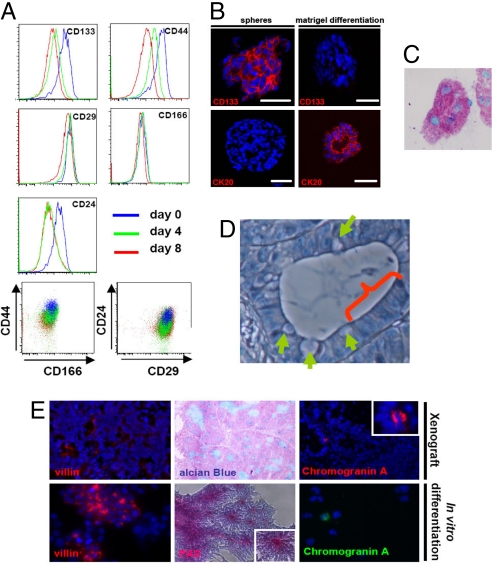
We tested the in vitro differentiation of CSCs by plating them on tissue culture treated plastics and applying medium containing serum. This procedure results in loss of tumor initiating capacity of the cultures (6). Upon the onset of differentiation, CD133 and CD24 are rapidly down regulated followed by CD44 (Fig. 4A). CD29 and CD166 show very limited change in expression in this setting. This corroborates our finding that CD133 and CD24 enrich for the most immature and highest clonogenic population in colon spheroid cultures (Fig. 2D).We recently demonstrated that differentiation can also be induced in matrigel in the presence of serum (6). This results in tubular, crypt-like structures that consist of cells that have lost CD133 and gained CK20 expression, reflecting a differentiated phenotype. In agreement, matrigel differentiation resulted in goblet-like cells because mucin is clearly produced in the crypt-like structures (Fig. 4C and Fig. S6A). Importantly, when we subject SCDCs to this differentiation scheme, a comparable morphology and marker expression is observed for all clones (Fig. 4B and Fig. S6 A and B), indicating that they maintain the capacity to differentiate.
Fig. 4.
Differentiation of SCDCs reveals multilineage differentiation potential both in vitro and in vivo. (A) Cells from a colon cancer spheroid culture show a gradual decrease in colon CSC marker expression on differentiated on an adherent plate in serum containing medium. Differences are observed in the speed at which the markers are lost. Here, 4 and 8 days of differentiation are shown. CD24 and CD133 show most rapid down regulation, followed by CD44. CD29 and CD166 show very limited change in cell surface expression levels. (B) Undifferentiated spheres were embedded in matrigel and were immediately snap frozen or allowed to differentiate for 10 days and then snap frozen. Sections were stained for CD133 (Upper) and CK20 (Lower). (Scale bars, 40 μm.) (A) Cells differentiated in matrigel show evidence for mucin production as shown here by Alcian Blue stain. (A and C) Data are representative for all clones analyzed (see also Fig. S6). (D) High-magnification microscopy reveals heterogeneous cell morphology in crypt-like structures of SCDCs. Both goblet cell-like (arrows) and enterocyte-like differentiation (arch) can be detected. (E) Staining for markers associated with different cell lineages in the colon epithelium of SCDC-derived xenografts (Upper) or in vitro-differentiated SCDCs (Lower). Villin indicates enterocyte-like differentiation. Alcian Blue and PAS staining reveal mucin production associated with goblet-like cell differentiation. Neuroendocrine differentiation is detected by Chromogranin A staining.
High-magnification microscopy of xenografts generated by SCDCs revealed heterogeneous cellular morphology (Fig. 4D). In analogy to normal colon crypts, goblet-like cells were detected next to enterocyte-like cells (Fig. 4D).
In vivo differentiation in SCDC-derived tumors was also analyzed by staining for markers associated with the variety of differentiation lineages present in colon epithelium. Besides the readily detectable Alcian Blue staining, which points to goblet-like cell differentiation, membranous localized Villin- and Chromogranin A-immunoreactive cells, respectively, reveal that enterocyte-like and neuroendocrine-like cells are also present (Fig. 4E). Luminal surface staining for Villin was present in low amounts throughout the tumors, and Chromogranin A expression was present in only a small fraction of these tumor cells.
Intriguingly, when SCDCs are subjected to differentiation induced by adherent plates and serum containing medium, we also observed only a small number of Chromogranin A+ cells (Fig. 4E and Fig. 5D). Similarly, enterocyte-like differentiation as determined by membrane-localized Villin expression (Fig. 4E and Fig. 5 C and D) or intestinal fatty acid binding protein (I-FABP) was only detected in a fraction of cells (Fig. 5 C and D, and Fig. S7). Combined, our observations prove that CSCs from human colon cancer possess multilineage differentiation capacity. We therefore conclude that the distinct differentiation patterns detected within tumors are not because of the presence of multiple clones, but because of remnant differentiation patterns. As these SCDC-derived cells can also self-renew in vivo they fulfill the theoretical criteria of CSCs.
Fig. 5.
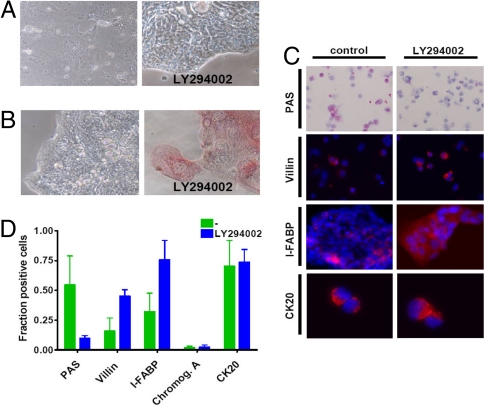
PI-3 kinase inhibition results in an enterocyte-like differentiation pattern. (A) Cell morphology of a differentiated colon spheroid culture on adherent plate with serum containing medium is changed in the presence of Ly294002. (B) Intestinal alkaline phosphatase (IAP) activity reveals induction of IAP activity in presence of Ly294002. (C) Staining of cytospins of differentiated colon spheroid cultures in the absence (Left) or presence (Right) of Ly294002 for PAS, Villin, and CK20. For I-FABP staining of an adherent culture is shown (for cytospins see Fig. S8C). (D) Quantification of PAS, Villin, I-FABP, CK20, and Chromogranin A positivity in differentiated cells.
SCDC Culture Differentiation can be Directed with PI3K Inhibition in Vitro.
The above data settle an important point in the origin of multiple distinct differentiation types within a tumor, but give no hint as to how this is orchestrated. In normal colon crypts and in adenomas, Notch signaling has been implicated in the decision between goblet- and enterocyte-like programs (16). To elucidate how differentiation of colon CSCs is coordinated and to gain insight into the nature of differentiation modulating signals, we initiated a screen of inhibitors that were applied during in vitro differentiation. We determined that the highest dose of the inhibitors that did not result in apparent cell death or severe proliferation inhibition of the spheroid cultures (Fig. S8). Subsequently, we applied those concentrations of inhibitors to a colon spheroid cell culture that was subjected to adherent plate differentiation. For most inhibitors we observed no gross morphological changes in the differentiated cells (Fig. S7B). However, in the case of the PI3K inhibitor Ly294002, we observed a clear difference in morphology of the differentiated cells (Fig. 5A). Cells were much more flattened and showed polarisation on the edges of cell aggregates. We confirmed that overall differentiation, as judged by loss of CD133 and gain of CK20 expression, was identical in the presence of PI3K inhibition (Fig. 5D and data not shown). Similarly, the low number of chromogranin A+ cells was unaltered in the presence of PI3K inhibition (Fig. 5D). However, cells treated with Ly294002 during differentiation showed increased intestinal alkaline phosphatase (IAP) activity (Fig. 5B and Fig. S8 A and B), which is associated with the brush border membrane in enterocytic cells. Combined with the 4-fold increase in cells positive for membrane localized Villin, a doubling of I-FABP+ cells and a concomitant decrease in mucin producing-cells, as demonstrated by periodic acid Shiff (PAS) reagent, pointed to a clear shift in the differentiation program (Fig. 5 C and D and Fig. S8C). This change in lineage decision was also detected during matrigel differentiation in the presence of Ly294002 (data not shown). We therefore concluded that the dominant goblet cell-like differentiation program is replaced by a more enterocyte-like differentiation.
Discussion
In this study, we provide evidence to support an important aspect of the cancer stem cell hypothesis, which claims that a single CSC can self-renew and reconstitute a complete and differentiated carcinoma. This was not only observed when the starting material for the single cell sort was a spheroid culture, but was also apparent when CSCs were single cell cloned directly from a primary tumor. Importantly, we now formally confirm that the CD133+/CD24+ population contains the clonogenic potential and that multilineage differentiation, which is, in part, dictated by PI3K signaling, is intrinsic to CSCs.
CSCs are relatively resistant to therapy and are suggested to be responsible for disease recurrence and possibly even metastasis. Defining CSCs within a tumor is, therefore, of great importance and currently depends on expression of cell surface markers, such as CD133, CD166/CD44, or CD24/CD29 (4, 8). Using an approach to culture CSCs, which has previously been described for tumorigenic mammaspheres, and gliospheres (9, 17), we now show that spheroid cultures from primary colon carcinoma selects for cells that coexpress all of these CSC markers. This provides strong support for the power of this culture method, which is further underlined by the expression of the colon stem cell marker Lgr5 (10).
Nevertheless, it is also clear from our observations that not all cells in these spheres are true CSCs. First of all, surface expression of CSC markers is heterogeneous, suggesting that multiple cell types, either from a different origin or at a different stage of differentiation, are present. Subsequent single-cell cloning confirmed that the latter hypothesis is most likely to be true because spheres derived from these single cells have similar heterogeneity. More importantly, this also occurs in vivo where full differentiation in multiple lineages can be detected. A second observation that argues against cancer stemness in all spheroid cells is the observation that nuclear β-catenin is high only in a minority of the cells. This appears to be similar to the β-catenin paradox in colon carcinomas, which describes that colon carcinomas display only a limited number of cells with nuclear β-catenin despite APC mutations (18). Apparently, regulation of β-catenin in APC deficient cells still occurs in vivo and we now show that this is also true in these cultures in vitro. Whether this signifies the onset of differentiation is not clear but it could potentially help in defining CSCs within tumors as suggested by Fodde and Brabletz (18). Finally, the third argument for limited stemness is the fact that single-cell cloning is only effective for ≈5% of the CD133+ cells and ≈20% of the CD133+/CD24+ cells. This suggests that the remainder may have lost this capacity because of initial differentiation steps. A hypothesis supported by our data because we show a striking correlation between clonogenic potential of subpopulations in our cultures and the time at which the markers that define those populations are lost during differentiation. Both CD133 and CD24, for instance, are rapidly down regulated on differentiation, whereas their coexpression is the best designation of the clonogenic population (Fig. 2D).
Although this is an appealing model, it poses an interesting dilemma. It is currently completely unclear why these spheroid cells, under apparent identical conditions, would retain stemness in some cells and turn on differentiation programs in others. Not only did we observe this in vitro, but in vivo we detected that a single CSC could yield progeny displaying markers associated with goblet-like, enterocyte-like, and neuroendocrine-like cells. This characteristic is not shared with colon carcinoma cell lines which can be cloned, but have the tendency to form a tumor in which all typical morphology and differentiation is lost (19). Previous observations have suggested that Notch and PI3K signaling are involved in lineage determination in normal crypts and homeostasis (16, 20). Our data support the idea that PI3K activity is crucial to lineage decision in CRCs and previous observations suggested a role for Notch in goblet cell fate in adenomas (16). However, neither observation provides a clue as to the actual signals that will differentially activate PI3K or Notch or, more importantly, the signals that will actually initiate differentiation of CSC offspring. In vitro, differentiation can be induced by changing growth factors and conditions for adherence (Fig. 4 and Fig. S6), but this appears to be an all-or-nothing signal and does not preserve self-renewal of CSCs. Intriguingly, PI3K activity, measured by pPKB, is observed in the spheroid cells and in differentiating cells and is prevented by Ly294002 (data not shown). This suggests that PI3K is active during differentiation but is unlikely to be solely responsible for the onset of differentiation even though PI3K inhibition dramatically changes the outcome (Fig. 5 and Fig. S8). In vivo, differentiation occurs alongside self-renewal, which is clear from the fact that CSCs are present in the xenotransplant (Fig. 3), suggesting that CSCs receive multiple signals that regulate their fate. When regarding normal stem cells as the paradigm, it seems logical to assume that this is regulated by a niche that provides coordinated signals (21, 22). In vitro, this niche can only be made up from neighboring tumor cells. Whether such a CSC niche really exists and which cells constitute this niche in vivo remains to be established (23), but proving its existence or identification of the signals that regulate CSC proliferation and differentiation could be of vital importance for therapeutic strategies to prevent tumor regeneration (24, 25).
Combined, our data reveal that heterogeneity in colorectal carcinomas, with respect to both differentiation grade and differentiation phenotype, is a clonal trait. This is in contrast to the more classical genetic model in which ongoing accumulation of mutations is thought to result in the presence of multiple genetically distinct clones within a tumor (26). This process, which is referred to as tumor Darwinism, fuels the phenotypical heterogeneity present in a malignancy (19). However, our data now reveal that a large part of these assorted phenotypes can be explained by the CSC hypothesis that proposes a hierarchical organization of a malignant clone in addition to remnant responses to differentiation guiding signals from the microenvironment. This implicates a model in which carcinomas can be viewed as atypical organs, including a functional stem cell compartment in which crucial mechanisms for homeostatic control are lost but other characteristics are consistently present.
Materials and Methods
Isolation of CSCs.
Colon CSC cultures were derived as described previously (6). CSCs were cultured in modified neurobasal A medium (27) containing N2 supplement (Invitrogen), Lipid Mixture-1 (Sigma), and high levels of bFGF (20 ng/ml) and EGF (50 ng/ml). A GFP+ subculture was obtained by lentiviral transduction.
Generation of SCDCs.
The FACSaria (BD Biosciences) was used for single-cell plating in 96-well, ultra low-adhesion plates (Corning) containing stem cell medium. We stringently gated on single, PI-negative cells. For the GFP+/CD133+ and GFP−/CD133− mixing experiment, AC133-PE (Miltenyi Biotec; 1:100) staining was used to select positive cells. After visible spheres arose, they were transferred into ultra low-adhesion flasks (Corning) and expanded.
Direct Single-Cell Isolation.
Tumor tissue was dissociated as described in ref. 6. CD45+ cells were depleted by using double magnetic bead depletion (MACS). Cells were plated on ultra low-adhesion 96-well plates at a concentration of a single cell per well, which was confirmed visually. Wells containing either none or more than one cell were excluded for further analysis. For enrichment of the CD133+ cells, we used microbeads conjugated with a CD133 antibody (AC133, cell isolation kit; Miltenyi Biotec).
Limiting Dilution Assay.
Cells forming different subpopulations of a colon spheroid cultures were deposited as 1, 2, 4, and 6 cells per well. Results were statistically evaluated by using the ‘limdil' function of the R software package.
In Vitro Differentiation Assay.
For in vitro differentiation, small spheres were plated in matrigel (GF reduced) and 2% FCS containing medium was layered on top. After 10 days, matrigel was snap frozen and processed for staining. Adherent plate differentiation was performed as described (5, 6) for 10 days. Cells were then either stained directly, or cytospins were generated after trypsinization.
Immunohistochemistry.
Immunofluorescence was performed on formaldehyde-fixed cryosections. The following antibodies were used: CD133 (Miltenyi Biotec; AC133), anti-CK20 (Genetex), anti-Villin C-19 (Santa Cruz), anti- I-FABP (Abcam), anti-Chromogranin A (Genetex), anti-β-catenin (Novacostra), and Lgr5 (Genetex, epitope C-terminus). Lgr5 staining was performed on formaldehyde-fixed cytospins of a trypsin-dissociated CSC culture. Validation of Lgr-5 staining was performed by using 293T cells transfected with an Lgr5 expression vector (kindly provided by H. Clevers). Alcian Blue staining was performed with Alcian Blue 8GX (Sigma) and counterstained with Nuclear fast Red (Lab Vision, Inc.) on cryosections. HE staining was performed with Ehrlich HE solution (Sigma) on paraffin-embedded sections. For periodic acid-Schiff (PAS) staining, standard histological techniques were used.
Flow Cytometry.
Flow cytometry was performed on trypsin-dissociated CSC cultures with AC133 (Miltenyi Biotec), anti-CK20 (Genetex), CD44 (BD PharMingen), CD166 (R&D Systems, clone 105901), CD24 (BD PharMingen), and CD29 (BD PharMingen). For intracellular CK20 staining, 7-AAD (eBioscience) preincubation was used to exclude dead cells.
In Vivo Tumor Propagation.
For transplantation of cancer cells, we injected 30 spheres (≈100 cells/sphere) suspended in 100 μl of PBS/BSA subcutaneously into nude mice. After 3–6 weeks, visible tumors arose. When tumor size reached 1 cm3, mice were killed, and tumors processed for either analysis or in vitro culture. For generation of tumors after direct single cell plating and short term in vitro expansion, 500 cells were injected all derived from the originally plated cell (Fig. 2B and Fig. S3).
IAP Activity Assay.
To measure IAP activity, we used Alkaline Phosphatase Substrate Kit 1 (SK-5100) from Vector Laboratories Inc. according to the manufacturer's instructions. Quantification was performed with an inverted fluorescence microscope (Zeiss).
Inhibitors.
Inhibitors were diluted in DMSO and used in adherent differentiation assays. Medium and inhibitors were refreshed every 48 h for 10 days.
Supplementary Material
Acknowledgments.
We thank B. Hooibrink for FACS sorting, Maartje van der Heijden for technical assistance, and the animal care takers for their support. This work was supported by Stichting Vanderes, Academic Medical Center (AMC), and the European Molecular Biology Organization (EMBO) fellowship (M.R.S.), and by Grants from AIRC (M.T. and G.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805706105/DCSupplemental.
References
- 1.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 2.Vermeulen L, et al. Cancer stem cells: Old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MF, et al. Cancer stem cells: Perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 5.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 6.Todaro M, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Kern SE, Shibata D. The fuzzy math of solid tumor stem cells: A perspective. Cancer Res. 2007;67:8985–8988. doi: 10.1158/0008-5472.CAN-07-1971. [DOI] [PubMed] [Google Scholar]
- 8.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 11.Blank M, et al. Expression of MUC2-mucin in colorectal adenomas and carcinomas of different histological types. Int J Cancer. 1994;59:301–306. doi: 10.1002/ijc.2910590302. [DOI] [PubMed] [Google Scholar]
- 12.Grabowski P, et al. Heterogeneous expression of neuroendocrine marker proteins in human undifferentiated carcinoma of the colon and rectum. Ann NY Acad Sci. 2004;1014:270–274. doi: 10.1196/annals.1294.030. [DOI] [PubMed] [Google Scholar]
- 13.Marsh KA, Stamp GW, Kirkland SC. Isolation and characterization of multiple cell types from a single human colonic carcinoma: tumourigenicity of these cell types in a xenograft system. J Pathol. 1993;170:441–450. doi: 10.1002/path.1711700407. [DOI] [PubMed] [Google Scholar]
- 14.Pierce GB, Nakane PK, Martinez-Hernandez A, Ward JM. Ultrastructural comparison of differentiation of stem cells of murine adenocarcinomas of colon and breast with their normal counterparts. J Natl Cancer Inst. 1977;58:1329–1345. doi: 10.1093/jnci/58.5.1329. [DOI] [PubMed] [Google Scholar]
- 15.West AB, et al. Localization of villin, a cytoskeletal protein specific to microvilli, in human ileum and colon and in colonic neoplasms. Gastroenterology. 1988;94:343–352. doi: 10.1016/0016-5085(88)90421-0. [DOI] [PubMed] [Google Scholar]
- 16.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 17.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 18.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg RA. The Biology of Cancer. New York: Garland Science; 2007. pp. 538–539. [Google Scholar]
- 20.Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 22.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Neaves WB. Normal stem cells and cancer stem cells: The niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 24.Al Hajj M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 26.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.