Abstract
Sperm competition has classically been thought to maintain anisogamy (large eggs and smaller sperm) because males are thought to maximize their chance of winning fertilizations by trading sperm size for number. More recently it has been recognized that sperm quality (e.g., size, velocity) can also influence sperm competition, although studies have yielded conflicting results. Because sex evolved in the sea, debate has continued over the role of sperm competition and sperm environment in determining both sperm and egg size in externally fertilizing broadcast spawners. Remarkably, however, there have been no direct tests of whether broadcast spawners change the traits of their gametes depending on the likelihood of sperm competition. We manipulated the density (and thus, sperm environment) of a broadcast spawning ascidian (Styela plicata) in the field and then determined whether the phenotype of eggs and sperm changed. We found that sperm from adults kept at high density were larger and more motile than sperm from low-density adults. In vitro fertilizations revealed that sperm from high-density adults also lived longer and induced less polyspermy. Adult density also affected egg traits: eggs from high-density adults were smaller targets for sperm overall but produced larger ovicells than eggs from low-density adults. This suggests that broadcast spawning mothers balance (potentially conflicting) pre- and postzygotic selection pressures on egg size. Overall, our results suggest that sperm competition does not represent a strong force maintaining anisogamy in broadcast spawners. Instead, sperm limitation seems to select for large eggs and smaller, more numerous sperm.
Keywords: anisogamy, sperm competition, adaptive maternal effect, transgenerational plasticity
The fundamental difference between the sexes is that males produce numerous, tiny sperm, and females produce relatively fewer, large eggs (anisogamy). Classic theory suggests that anisogamy most likely evolved by, and is maintained through, sperm competition (whereby sperm from 2 or more males compete to fertilize an egg) because males producing a greater number of sperm have a competitive advantage (refs. 1 and 2; but see ref. 3 for an alternative). In species with internal fertilization, competition for fertilizations can therefore be viewed as a “raffle,” whereby males that have the highest representation of sperm in the pool have the greatest chance of fertilizing eggs (4, 5). More recently, however, it has been proposed that sperm quality (e.g., sperm size, velocity, and longevity) can also influence the outcome of sperm competition (6, 7). Furthermore, because a male's sperm supply is limited (8, 9) and each mating opportunity may carry a variable risk of sperm competition (10), males must make decisions on the optimal allocation of both sperm number and quality for each mating event. The optimal allocation of sperm size and number within an ejaculate remains a source of intense debate (reviewed in refs. 7 and 9).
The prediction that males should increase the number of sperm within an ejaculate under increased risk of sperm competition is generally supported by empirical evidence from internal fertilizers (9, 11–13). In contrast, tests of predicted changes in sperm quality have yielded mixed results (7, 14). Interspecific comparisons show that sperm size can increase (15–17), decrease (18), or have no relationship (19–22) with increased risk of sperm competition—with some studies of the same taxonomic groups producing conflicting results (reviewed in ref. 7). Results from intraspecific studies are also equivocal, with advantages shown alternatively for larger sperm (23, 24), smaller sperm (11), or for sperm of no particular size at all (25, 26). Much of this variability may arise from the fact that sperm size may covary with velocity and longevity, and the benefits of these traits may be highly context dependent. The relationship between sperm competition and sperm longevity may be positive (21), negative (27, 28), or absent (29), and fertilization success may increase for sperm that are faster (26) or longer lived (27). Hence, it remains unclear whether males should adaptively adjust sperm traits in response to changes in the risk of sperm competition.
Recently, manipulative experiments on internal fertilizers suggest that males may indeed adjust their sperm quality in response to the perceived risk of sperm competition (29–32). However, although our understanding of the role of sperm competition in shaping selection on sperm size and number in internal fertilizers has increased, it is difficult to relate these findings back to the ancestral condition of broadcast spawning. Broadcast spawning is the main mode of reproduction in the sea and involves the release of both eggs and sperm into the water column, whereupon gamete contact and fertilization occur externally. External fertilization is therefore a more risky process than internal fertilization: at one end of the spectrum, fertilization success can be limited by insufficient sperm due to rapid dilution, and at the other extreme, very high local concentrations of sperm can induce lethal polyspermy (33). Thus, the selective forces acting on broadcast sperm are very different from those acting on internal fertilizers (3, 34, 35), and theoretical predictions developed for internal fertilizers are unlikely to apply to external fertilizers. For example, Bode and Marshall (3) show that selection should favor males that release fewer, rather than more, sperm when faced with competition. This is because the proportion of eggs that are likely to suffer polyspermy increases with the number of competing males. Thus, a more comprehensive understanding of the evolution and maintenance of anisogamy requires broadcast spawners to be examined directly. Because the effect of sperm traits on fertilization success can be observed during in vitro fertilization in these systems (36, 37), broadcast spawners provide a unique opportunity to directly assess the functional consequences of changes in sperm traits. Moreover, because sexual selection in this group mainly manifests through the interaction of gametes in the water column, we would expect gamete responses to sexual selection to be particularly strong (38).
Selection on sperm size is only half of the anisogamy equation, and for broadcast spawners in particular the local sperm environment represents a selective pressure on egg size (reviewed in ref. 37). Larger eggs provide larger targets for sperm, so at low sperm concentrations larger eggs are more likely to be hit by sperm and fertilized (39–41). At high sperm concentrations, however, larger eggs are more likely to come into contact with multiple, coincident sperm and become fatally polyspermic (41–43). The local sperm environment may represent such a strong selection pressure that differences in egg size among species of sea urchin may be driven by interspecific differences in the local sperm environment (44–46). However, although the sperm environment probably represents a significant selection pressure on egg size, postzygotic pressures will also determine the optimal size of eggs mothers should make (47). Egg size affects development rate, settlement behavior, and postmetamorphic performance in marine invertebrates (47). Hence, mothers may face the challenge of optimizing investment to balance potentially opposing pre- and postzygotic selection pressures. The recognition of these conflicting selection pressures has led to an intense debate over the role of the sperm environment in the evolution of egg size in broadcast spawners (37).
Increasing egg size is not the only way in which mothers can increase the chances that their eggs will be fertilized. Many marine invertebrate eggs have accessory structures, such as follicle cells or jelly coats, that increase the overall target size for searching sperm (48, 49). Furthermore, the eggs of some species release sperm chemoattractants that increase the virtual size of eggs, increasing their chances of being contacted by sperm (50, 51). Such egg accessory devices are thought to be energetically inexpensive relative to the actual ovicell itself (52), and so some have argued that selection due to the sperm environment will act more strongly on accessory structures than ovicell size per se (49). One potential means of resolving this debate is to manipulate the local sperm environment that mothers face and determine whether mothers change the relative size of their eggs in response. Previous studies on brooding marine invertebrates show that mothers adaptively adjust the size of their offspring in response to changes in local competition (53), but none have examined the effect of manipulating the sperm environment. By manipulating the density of adults in the field (and thus the likely sperm environment), we examined whether gamete size is a plastic trait in broadcast spawners, providing insight into the role of sperm competition in the maintenance of anisogamy.
Results
Effect of Adult Density on Egg Properties.
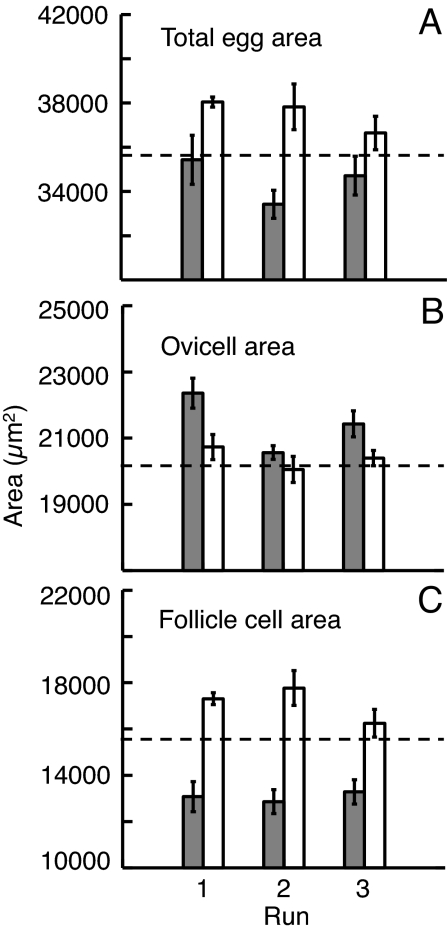
Adult density had a significant effect on egg size after 1 month in the field (Table 1). Adults at low densities produced eggs with a total egg area (i.e., sperm target size) that was 9% larger than in eggs produced from adults at higher densities (Fig. 1a). However, different components of the egg responded differently to adult density. Eggs from low-density adults had a 31% larger follicle cell area (Fig. 1c), but the ovicell area was on average 5% smaller (Fig. 1b). Thus, the increase in overall size was due to the increase in follicle cell area. In simple terms, eggs from low-density adults were larger targets for sperm (owing to their larger follicle cells), whereas eggs from high-density adults had larger ovicells (thereby yielding larger embryos) yet were smaller targets for sperm overall (owing to their smaller follicle cells).
Table 1.
Two-factor ANOVAs (reduced model) showing the effect on egg traits of manipulating adult density for 1 month in the field
| Source | df | Mean squares | F | P |
|---|---|---|---|---|
| Total egg area | ||||
| Treatment | 1 | 96,923,300 | 21.933 | 0.000* |
| Run | 2 | 4,509,384 | 1.020 | 0.370 |
| Error | 38 | 4,419,012 | ||
| Ovicell area | ||||
| Treatment | 1 | 9,977,287 | 14.151 | 0.001* |
| Run | 2 | 4,793,281 | 6.798 | 0.003* |
| Error | 38 | 705,067 | ||
| Follicle cell area | ||||
| Treatment | 1 | 169,095,000 | 74.378 | 0.000* |
| Run | 2 | 1,283,477 | 0.565 | 0.573 |
| Error | 38 | 2,273,446 |
Treatment = high density (15 individuals per cage), low density (1 individual per cage).
*, P < 0.05.
Fig. 1.
Effect of manipulating adult density for 1 month in the field on egg traits of S. plicata (high adult density shown in dark bars; low adult density shown in white bars). Dashed horizontal line shows mean egg traits of unmanipulated “wild” specimens collected from the same site. Bars show the mean (± SE) area of the “total egg area” (which includes both the ovicell and follicle cell area and therefore reflects the target size of the egg) (a), the “ovicell area” (the principle energetic investment in the egg) (b), and the “follicle cell area” (the accessory structures of the egg) (c).
Effect of Adult Density on Sperm Properties.
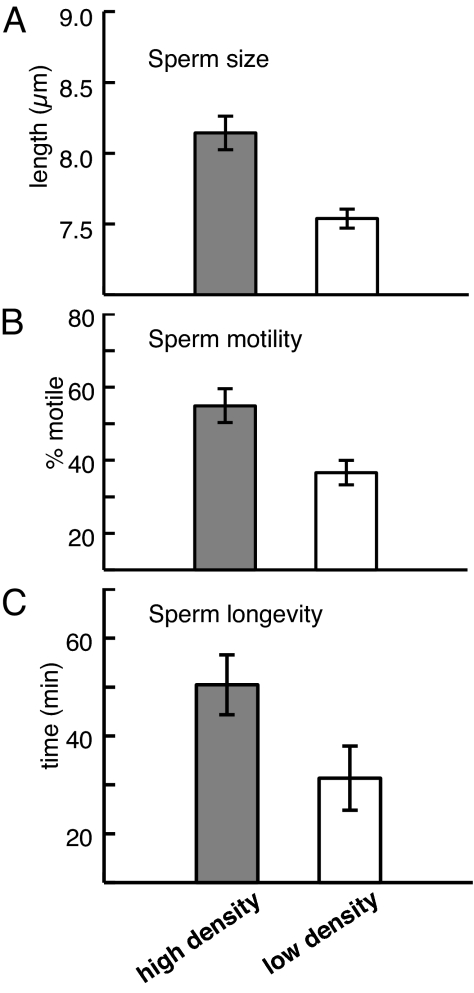
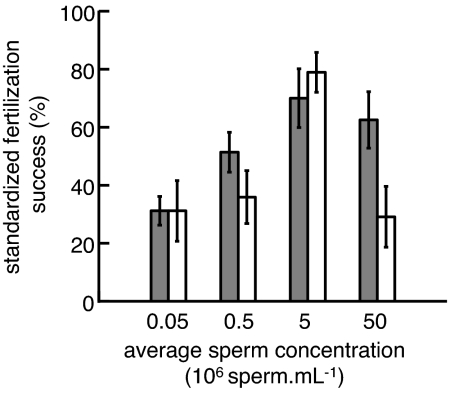
Adult density also had a significant effect on sperm size, sperm motility, and sperm longevity (Table 2). Sperm from high-density adults were larger (Fig. 2a), more motile (Fig. 2b), and remained capable of fertilizing eggs for longer (Fig. 2c) than sperm from low-density adults. These changes in sperm characteristics also had functional consequences when we conducted in vitro fertilizations. Whereas sperm from adults kept at different densities in the field did not differ significantly in the level of fertilization success they achieved at low to moderate sperm concentrations, they differed at higher sperm concentrations. Sperm from high-density adults achieved higher levels of fertilization success at high sperm concentrations than sperm from low-density adults (Fig. 3 and Table 2), suggesting that sperm from high-density adults induced lower levels of fatal polyspermy. Importantly, there was no difference in the concentrations of sperm extracted from individuals in either treatment (see “Data Analysis” in Methods).
Table 2.
ANOVAs (reduced model) showing the effect on sperm traits of manipulating adult density for 1 month in the field
| Source | df | Mean squares | F | P |
|---|---|---|---|---|
| Sperm size | ||||
| Treatment | 1 | 2.193 | 20.765 | 0.000* |
| Run | 1 | 0.010 | 0.094 | 0.763 |
| Error | 21 | 0.106 | ||
| Sperm motility | ||||
| Treatment | 1 | 2,684.446 | 10.648 | 0.003* |
| Run | 1 | 0.775 | 0.003 | 0.956 |
| Error | 29 | 252.097 | ||
| Sperm longevity | ||||
| Treatment | 1 | 2,004.545 | 5.537 | 0.040* |
| Run | 1 | 123.068 | 0.340 | 0.573 |
| Pair (Run) | 9 | 470.417 | 1.299 | 0.343 |
| Error | 10 | 362.045 | ||
| Sperm fertilization success | ||||
| Treatment | 1 | 506.262 | 0.842 | 0.363 |
| Run | 1 | 628.781 | 1.046 | 0.311 |
| Sperm dilution | 3 | 6,673.402 | 11.097 | 0.000 |
| Pair (Run) | 8 | 1,774.361 | 2.950 | 0.008 |
| Treatment × Sperm dilution | 3 | 2,191.091 | 3.643 | 0.018* |
| Error | 55 | 601.381 | ||
| Post hoc analyses | ||||
| High vs. low: 0.001 [sperm] | 0.915 | |||
| High vs. low: 0.010 [sperm] | 0.134 | |||
| High vs. low: 0.100 [sperm] | 0.248 | |||
| High vs. low: 1.000 [sperm] | 0.002* |
Treatment = high density (15 individuals per cage), low density (1 individual per cage).
*, P < 0.05.
Fig. 2.
Effect of manipulating adult density for 1 month in the field on sperm traits of S. plicata (high adult density shown in dark bars; low adult density shown in white bars). Bars show the mean (± SE) sperm size (measured as sperm head length) (a), motility (measured as the percentage of sperm showing a swimming motion) (b), and longevity (measured as the average time for fertilization to drop below 50% of the maximum) (c).
Fig. 3.
Effect on sperm fertilization potential of manipulating adult density (high adult density shown in dark bars; low adult density shown in white bars) of S. plicata for 1 month in the field. Bars show the mean (± SE) percent fertilization success for each serial dilution of sperm, standardized to the maximum fertilization observed within each trial.
Discussion
Changing the likely sperm environment seems to result in adaptive plasticity of gamete traits in the broadcast spawning ascidian, Styela plicata. Animals in high-density environments (and thus with high levels of sperm competition) produced sperm that were larger, more motile, and remained viable for longer than sperm collected from animals kept at low density. Moreover, we found support for the functional significance of these changes in sperm traits, because sperm from high-density individuals had a higher in vitro fertilization success in high sperm concentration conditions. Gamete plasticity was not restricted to sperm traits alone: animals in high-density environments produced eggs with a reduced overall size while simultaneously increasing the ovicell and thus embryonic size of each egg. Therefore, it seems that these broadcast spawners are adaptively adjusting the properties of their gametes in response to the risk of a combination of both pre- and postzygotic factors.
The functional benefits underlying changes in sperm traits seem to result from a reduction in the incidence of lethal polyspermy, given that fertilization success significantly dropped in high sperm concentration trials using sperm from low-density animals compared with high-density animals (Fig. 3). Interestingly, this result occurred despite the use of standard in vitro fertilization techniques designed to minimize the effects of polyspermy (see Methods). Because local density is a good indicator of the likely sperm environment, animals in high-density environments are most likely to face polyspermy-inducing sperm concentrations and seem to be producing sperm that are less likely to induce polyspermy. In external fertilizers with (relatively) fast blocks to polyspermy, faster sperm are predicted to have an advantage at finding unfertilized eggs (26, 28, 54, 55); however, high-velocity sperm may also be more likely to induce polyspermy (42). Although we were unable to directly measure sperm velocity, velocity is expected to trade off against longevity (6, 26, 55; but see 29, 56). Therefore, given that sperm from high-density animals lived longer, it is possible that the high-density sperm had a reduced velocity, which could lower collision rates and serve a similar function to reducing sperm numbers under high competition [as predicted by Bode and Marshall (3)]. Such an interpretation can only be speculative without direct measurements on sperm velocity, but it is interesting that sperm from high-density animals not only lived longer but achieved identical levels of fertilization at lower sperm concentrations—a result that would not be predicted on the basis of differences in sperm velocity alone (57). Regardless of the direct mechanism, it seems clear that S. plicata manipulate the qualities of their sperm in a way that is likely to increase their fertilization success when population densities are high and polyspermy is likely. Note that our results represent a very different situation to that usually seen in internal fertilizers, whereby a premium is placed on sperm competitive ability. In broadcast spawners, theory predicts that sperm competition will select for the release of fewer, less fiercely competing sperm, so that polyspermy does not destroy all of the available eggs (3). In our study at least, a shift to a sperm phenotype that is less likely to induce polyspermy does seem to be adaptive.
Plasticity in egg traits seemed to reflect selection pressures at both ends of the sperm environment spectrum and on multiple life-history stages—the threat of sperm limitation at low densities and the threat of both polyspermy and high postmetamorphic competition at high densities. Mothers facing sperm limitation can increase their chance of fertilization by increasing the target size of their eggs (37, 39–41). Here it seems that at low densities, mothers maximize their reproductive potential by increasing the target size of their eggs by increases in the area of relatively cheap follicle cells (49). Conversely, mothers in high-density environments can reduce the risk of polyspermy by reducing the overall size of their eggs (43). Among species, sea urchins that spawn in the most sperm-competitive conditions also produce eggs that are most resistant to polyspermy (46). Thus, it seems that there are multiple paths to avoiding, or at least minimizing, polyspermy in broadcast spawners. Nonmanipulated individuals (i.e., individuals from the same site not subjected to density manipulations, hereafter referred to as “wild” individuals) had an overall egg area that was intermediate between eggs from low- and high-density treatments (Fig. 1). This implies that the plastic response observed was indeed driven by the threat of both sperm limitation (at low densities) and polyspermy (at high densities).
Our results suggest that adults are also reacting to changes in population density by producing eggs with larger ovicells when exposed to high-density conditions. This may in turn have wider implications for postsettlement survival of offspring. Some ascidians spawn in conditions that promote gamete and larval retention (58–60) and are therefore unlikely to disperse far from their natal habitat. This means that in addition to being a good predictor of the sperm environment, local density may also be a good predictor of offspring environment. Hence, if larvae do not disperse far, offspring from mothers in high-density environments are likely to face high levels of postsettlement competition. In ascidians, egg size affects both dispersal time and postsettlement performance in the presence of competition (61). We cannot be certain whether mothers are increasing the ovicell size of eggs to increase offspring dispersal potential, competitive ability, or both. Nevertheless, it is interesting that an increase in population density (and thus an increase in intraspecific competition) in the maternal generation leads to an increase, rather than a decrease, in offspring size [as found by Allen et al. (53)].
It is possible that the differences in ovicell area observed between treatments resulted from a tradeoff in investment between the ovicell and follicle cells, rather than an adaptive response. However, this explanation seems unlikely because there was no significant difference between the ovicell area in low-density and wild animals, whereas the ovicell area of high-density animals was significantly larger than in both wild and low-density animals (Fig. 1; unpublished data, A.J.C.). More generally, if we assume that resources for reproduction are limited, then any increase in gamete size should result in the reduction of either the number of gametes in the current reproductive bout or a reduction in reproductive output in later bouts (62). Unfortunately, the use of the strip-spawning technique precludes a detailed quantitative measure of gamete number vs. size trade-offs. However, at higher densities (whereby resources available to reproduction are expected to be lower) there was an increase in the average size of both sperm and ovicells, implying an adaptive plastic response rather than a simple energetic constraint.
In summary, we found striking plasticity in the traits of both eggs and sperm in response to changes in adult density and the likely sperm environment. Increasing the threat of sperm competition resulted in an apparently adaptive shift in sperm traits, which had functional significance for the performance of sperm at high sperm concentrations. Surprisingly, the change in sperm size we observed was the converse of that predicted by classic sperm competition theory (2, 11). This suggests that the maintenance of small sperm size in broadcast spawners may be due to sperm limitation rather than sperm competition—males in isolation that fail to produce sufficient numbers of sperm may suffer reproductive failure due to the rapid dilution of gametes in the hydrodynamic environment of the sea (3). Thus, the selection pressures acting on males that reproduce by the ancestral strategy of broadcast spawning seem to be very different from those acting on internal fertilizers. Importantly, the nature of changes/plasticity in egg traits seems to reflect responses to both pre- and postzygotic selection pressures associated with an increase in the density of conspecifics. Our study adds to the growing list of studies that demonstrate transgenerational plasticity in offspring phenotype in response to changes in the conditions offspring are likely to face (reviewed in refs. 63 and 64). Interestingly, our results also indicate that mothers manipulate different elements of the offspring phenotype in response to differing pre- and postzygotic selection pressures. That is, mothers respond to increases in conspecific density by simultaneously reducing the target size of their eggs (presumably to decrease the risk of polyspermy) and increasing the size of their embryos (presumably making them better dispersers or competitors in the face of an increased risk of intraspecific competition). Overall, S. plicata shows a remarkable degree of gamete plasticity, with adults responding to a combination of risks from sperm competition, sperm limitation, and postzygotic competition.
Methods
Study Species and Site.
Styela plicata is a broadcast spawning, solitary ascidian and a simultaneous hermaphrodite (although self-sterile) (65). S. plicata is thought to be invasive to eastern Australia, is common on made-made structures (growing both in isolation and in clumps), and in vitro fertilization by strip-spawning techniques is straightforward (66). Adult S. plicata were collected and field manipulations conducted at Manly Boat Harbor (Brisbane, Australia; 27.467 E 153.183 S). This private access marina consists of floating docks and is protected from wave action by a large breakwater.
Field Manipulations.
We collected S. plicata from pier pilings during the reproductive season and immediately transferred them into 18 × 18 × 18-cm rigid plastic mesh cages (mesh size, 1 cm2) suspended 20 cm below the surface. We randomly allocated wild individuals to either a high-density (15 individuals) or low-density (1 individual) treatment. Each week we checked the treatment animals for survival, rotated positions within each cage, and cleaned the cages to maintain water flow. After 1 month in the field we collected all low-density and a randomly selected high-density individual from each cage, measured the animals, and transported them to the laboratory at the University of Queensland. We also randomly sampled wild individuals (i.e., those not involved in a density manipulation) at this time if required for fertilization experiments (see below). We held animals in aerated seawater for a maximum of 48 h before experimentation. Between January and April 2007 we ran 4 separate experimental runs, with 5 to 8 replicate cages per treatment in each run. One run was excluded from egg size analyses because the eggs of several low-density treatment animals were of poor quality. Sperm size and longevity were measured in runs 1 and 2, and sperm motility and fertilization potential were measured in runs 3 and 4.
Gamete Collection.
We harvested gametes using standard strip-spawning techniques (67). Briefly, we dissected the gonads from each individual into a Petri dish with a few drops of filtered sea water. We gently pressed the gonad extract with a rubber stopper to release the gametes and washed the extract through a 100-μm filter with filtered seawater into a small beaker. This procedure allowed the eggs to be retained inside the filter, whereas the sperm passed through into the beaker. Where eggs were required for fertilization experiments, we briefly soaked them in 0.5 M potassium chloride to kill any remaining sperm (66).
Effect of Adult Density on Egg Size.
We harvested eggs from treatment animals and recorded digital images on a dissecting microscope under 45× magnification using PixeLINK Capture SE software. Later we used these images to measure a random sample of 30 eggs per individual with Image-Pro Express (Media Cybernetics). We defined “total egg area” as the area of the egg including both the ovicell and follicle cells. Thus, total egg area represents the target size or physical size of the egg, and ovicell area represents the portion of the egg available for embryonic development. We estimated total egg area by digitally tracing around the perimeter of the follicle cells, ovicell area by tracing around the intersection of the follicle cells and ovicell, and follicle cell area by subtracting the ovicell area from the total egg area.
Effect of Adult Density on Sperm Size and Motility.
Sperm size.
We measured sperm size by capturing digital images of freshly extracted sperm solution on a compound microscope under 400× magnification. Because tail length could not be accurately measured from images, we measured the head length of 30 replicate sperm per animal using Image-Pro Express.
Sperm motility.
To measure sperm motility, we collected 10 ml of sperm solution from each treatment animal (i.e., 10 ml of seawater was used to wash the gonad extract through a 100-μm filter). We estimated sperm concentration with a Neubauer improved hemocytometer under 400× magnification on a compound microscope, with 3 replicate counts per sample. We then recorded 4 s of video using PixeLINK OEM software, with 5 replicate videos per individual. We scored all sperm in the field of view as motile (sperm moving from side to side in a swimming motion) or nonmotile and calculated the average percent motility per video. We recognize that this method yields only an approximate estimate of motility, but our attempts to use the specialized Sperm Quality Analyzer IIB (Medical Electronic Systems) were unsuccessful because we found the S. plicata sperm to be too large and slow to be recognized by software designed for mammalian sperm. Regardless, our results suggest that our technique, although not ideal, was sensitive enough to detect differences among the groups.
Effect of Adult Density on Sperm Longevity and Fertilization Potential.
We used in vitro fertilization trials to test the longevity and fertilization potential of sperm from treatment animals. Ideally we would have also examined the performance of eggs from our treatment groups at high and low sperm concentrations, but logistical constraints prevented us from running fertilization assays on eggs and sperm at the same time. Because the effect of differences in egg size on fertilization success under varying sperm concentrations has already been examined in ascidians (43), we decided to analyze the fertilization potential of sperm. We used a split-clutch/split-ejaculate technique whereby we tested both a high-density “male” (i.e., an individual used as a sperm source) and a low-density male simultaneously and assayed their sperm against a common pool of eggs (i.e., a paired design). For each pairing we created a common pool of eggs using eggs from 2 wild individuals (see “Field manipulations”). Where self-fertilization in an egg batch exceeded 10%, we excluded the trial from analyses (n = 2).
Sperm longevity.
To examine sperm longevity, we split eggs across 18 small Petri dishes (1 ml of egg solution per dish) and left them covered until needed. Eggs left for 2 h achieved high fertilization success when fertilized with fresh sperm (unpublished data, A.J.C.), indicating that eggs remained viable throughout the assay. We then extracted 5 ml of sperm solution from a low-density and high-density treatment animal and kept it in concentrated form in a constant temperature cabinet at 22°C between time periods. We initiated fertilization by adding 0.5 ml of sperm solution to an egg sample immediately after sperm was extracted and then to successive egg samples every 15 min for 2 h. For each egg sample we estimated fertilization success by scoring all eggs in the field of view (mean = 20) as cleaved or uncleaved when viewed at 30× magnification on a dissecting microscope. We took 5 replicate counts per dish. Fertilization success was calculated 1 h after the initiation of fertilization because more than 50% of cleaved eggs had progressed beyond the 2-cell stage at this time. Although this method may underestimate absolute levels of either fertilization or polyspermy, any bias should be constant across treatments. At the end of each assay we calculated the time period in which fertilization success dropped below 50% of the maximum for both high- and low-density treatments.
Sperm fertilization potential.
To estimate the fertilization potential of sperm, we first split eggs across 8 small Petri dishes (0.5 ml egg solution per dish). We then extracted 5 ml of sperm solution from both a low-density and a high-density treatment animal and estimated sperm concentration as described above. To create 4 sperm concentration treatments (per adult density treatment), we serially diluted the sperm extract to 100%, 10%, 1%, and 0.1% of the original extraction. We added each sperm treatment to the egg samples in 3 0.5-ml doses at 5-min intervals—a standard procedure to allow time for polyspermy blocks to form (42, 66). After 1 h we estimated fertilization success by scoring cleaved and uncleaved eggs (as described in “Sperm longevity”). To account for differences in egg quality, we standardized fertilization success among trials by converting all fertilization estimates to a percentage of the maximum fertilization success observed within each trial. Importantly, this standardization has no effect on the outcome of our analyses; rather, it represents a way of simplifying the analyses by omitting extra terms such as Male nested within Run.
Data Analysis.
To examine the effect of density treatment on egg size, sperm size, and sperm motility, we used a 2-factor ANOVA, with Treatment (high-density and low-density) as a fixed factor and Run as a random factor. Because the interaction term in all cases was nonsignificant [see supporting information (SI) Table S1], it was excluded. We included adult length as a covariate in egg size analyses; however, it was excluded from the final models because it explained little variance (Table S1) (68). We found no significant differences in adult sizes between treatments in any run (F1,70 = 0.065; P = 0.799); therefore, adult length was not included as a covariate. Importantly, we found no differences between sperm concentration estimates in either sperm motility trials (F1,29 = 2.009; P = 0.167) or sperm fertilization potential assays (F1,9 = 0.793; P = 0.396); therefore, we excluded this factor from our analyses. To test the effect of density treatment on sperm longevity and fertilization success, we included Pair nested within Run as an additional factor, to reflect the paired nature of these fertilization trials. We also included Sperm dilution as a fixed factor in the sperm fertilization potential model. Once again, we excluded interaction terms from the models where nonsignificant (Table S1). Because the interaction term Treatment × Sperm dilution was significant, we performed simple main effects tests to determine for which sperm dilution the treatments significantly differed (68).
Supplementary Material
Acknowledgments.
We thank B. Galletly, K. Baker, and M. Rius for assistance in the laboratory and field, East Coast Marina for access to the private docks, K. Harrison and the Queensland Fertility Group for loan of the SQAIIB, and J. C. Castilla, D. R. Levitan, P. J. Krug, and K. Monro for comments that greatly improved the manuscript. A.J.C. was supported by an Australian Postgraduate Award. D.J.M. was supported by grants from the Australian Research Council.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806590105/DCSupplemental.
References
- 1.Parker GA, Smith VGF, Baker RR. Origin and evolution of gamete dimorphism and male-female phenomenon. J Theor Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- 2.Parker GA. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J Theor Biol. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. [DOI] [PubMed] [Google Scholar]
- 3.Bode M, Marshall DJ. The quick and the dead? Sperm competition and sexual conflict in sea. Evolution. 2007;61:2693–2700. doi: 10.1111/j.1558-5646.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Parker GA. Sperm competition games—Raffles and roles. Proc R Soc B. 1990;242:120–126. [Google Scholar]
- 5.Parker GA. Sperm competition games—Sperm size and sperm number under adult control. Proc R Soc B. 1993;253:245–254. doi: 10.1098/rspb.1993.0110. [DOI] [PubMed] [Google Scholar]
- 6.Parker GA. In: Sperm Competition and Sexual Selection. Birkhead TR, Moller AP, editors. London: Academic; 1998. pp. 3–54. [Google Scholar]
- 7.Snook RR. Sperm in competition: Not playing by the numbers. Trends Ecol Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Dewsbury DA. Ejaculate cost and male choice. Am Nat. 1982;119:601–610. [Google Scholar]
- 9.Wedell N, Gage MJG, Parker GA. Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol. 2002;17:313–320. [Google Scholar]
- 10.Parker GA, Ball MA, Stockley P, Gage MJG. Sperm competition games: A prospective analysis of risk assessment. Proc R Soc London B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gage MJG, Morrow EH. Experimental evidence for the evolution of numerous, tiny sperm via sperm competition. Curr Biol. 2003;13:754–757. doi: 10.1016/s0960-9822(03)00282-3. [DOI] [PubMed] [Google Scholar]
- 12.Pizzari T, Cornwallis CK, Lovlie H, Jakobsson S, Birkhead TR. Sophisticated sperm allocation in male fowl. Nature. 2003;426:70–74. doi: 10.1038/nature02004. [DOI] [PubMed] [Google Scholar]
- 13.Pound N, Gage MJG. Prudent sperm allocation in Norway rats, Rattus norvegicus: A mammalian model of adaptive ejaculate adjustment. Anim Behav. 2004;68:819–823. [Google Scholar]
- 14.Hosken DJ. Sperm biology: Size indeed matters. Curr Biol. 2003;13:R355–R356. doi: 10.1016/s0960-9822(03)00275-6. [DOI] [PubMed] [Google Scholar]
- 15.Gomendio M, Roldan ERS. Sperm competition influences sperm size in mammals. Proc R Soc London B. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- 16.Gage MJG. Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc R Soc London B. 1994;258:247–254. [Google Scholar]
- 17.Byrne PG, Simmons LW, Roberts JD. Sperm competition and the evolution of gamete morphology in frogs. Proc R Soc London B. 2003;270:2079–2086. doi: 10.1098/rspb.2003.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockley P, Gage MJG, Parker GA, Moller AP. Sperm competition in fishes: The evolution of testis size and ejaculate characteristics. Am Nat. 1997;149:933–954. doi: 10.1086/286031. [DOI] [PubMed] [Google Scholar]
- 19.Harcourt AH. Sperm competition and the evolution of non-fertilizing sperm in mammals. Evolution. 1991;45:314–328. doi: 10.1111/j.1558-5646.1991.tb04406.x. [DOI] [PubMed] [Google Scholar]
- 20.Briskie JV, Montgomerie R. Sperm size and sperm competition in birds. Proc R Soc London B. 1992;247:89–95. doi: 10.1098/rspb.1992.0013. [DOI] [PubMed] [Google Scholar]
- 21.Gage MJG, Stockley P, Parker GA. Effects of alternative male mating strategies on characteristics of sperm production in the Atlantic salmon (Salmo salar): Theoretical and empirical investigations. Philos Trans R Soc B. 1995;350:391–399. [Google Scholar]
- 22.Gage MJG, Freckleton RP. Relative testis size and sperm morphometry across mammals: No evidence for an association between sperm competition and sperm length. Proc R Soc London B. 2003;270:625–632. doi: 10.1098/rspb.2002.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radwan J. Intraspecific variation in sperm competition success in the bulb mite: A role for sperm size. Proc R Soc London B. 1996;263:855–859. [Google Scholar]
- 24.LaMunyon CW, Ward S. Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc R Soc London B. 1998;265:1997–2002. doi: 10.1098/rspb.1998.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow EH, Gage MJG. Sperm competition experiments between lines of crickets producing different sperm lengths. Proc R Soc London B. 2001;268:2281–2286. doi: 10.1098/rspb.2001.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gage MJG, et al. Spermatozoal traits and sperm competition in Atlantic salmon: Relative sperm velocity is the primary determinant of fertilization success. Curr Biol. 2004;14:44–47. [PubMed] [Google Scholar]
- 27.Neff BD, Fu P, Gross MR. Sperm investment and alternative mating tactics in bluegill sunfish (Lepomis macrochirus) Behav Ecol. 2003;14:634–641. [Google Scholar]
- 28.Burness G, Casselman SJ, Schulte-Hostedde AI, Moyes CD, Montgomerie R. Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus) Behav Ecol Sociobiol. 2004;56:65–70. [Google Scholar]
- 29.Rudolfsen G, Figenschou L, Folstad I, Tveiten H, Figenschou M. Rapid adjustments of sperm characteristics in relation to social status. Proc R Soc London B. 2006;273:325–332. doi: 10.1098/rspb.2005.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilgallon SJ, Simmons LW. Image content influences men's semen quality. Biol Lett. 2005;1:253–255. doi: 10.1098/rsbl.2005.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornwallis CK, Birkhead TR. Changes in sperm quality and numbers in response to experimental manipulation of male social status and female attractiveness. Am Nat. 2007;170:758–770. doi: 10.1086/521955. [DOI] [PubMed] [Google Scholar]
- 32.Pizzari T, Cornwallis CK, Froman DP. Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc R Soc London B. 2007;274:853–860. doi: 10.1098/rspb.2006.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franke ES, Babcock RC, Styan CA. Sexual conflict and polyspermy under sperm-limited conditions: In situ evidence from field simulations with the free-spawning marine echinoid Evechinus chloroticus. Am Nat. 2002;160:485–496. doi: 10.1086/342075. [DOI] [PubMed] [Google Scholar]
- 34.Levitan DR. Effects of gamete traits on fertilization in the sea and the evolution of sexual dimorphism. Nature. 1996;382:153–155. [Google Scholar]
- 35.Parker GA, Ball MA, Stockley P, Gage MJG. Sperm competition games: Individual assessment of sperm competition intensity by group spawners. Proc R Soc London B. 1996;263:1291–1297. [Google Scholar]
- 36.Marshall DJ. Reliably estimating the effect of toxicants on fertilization success in marine broadcast spawners. Mar Pollut Bull. 2006;52:734–738. doi: 10.1016/j.marpolbul.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Levitan DR. The relationship between egg size and fertilization success in broadcast-spawning marine invertebrates. Integr Comp Biol. 2006;46:298–311. doi: 10.1093/icb/icj025. [DOI] [PubMed] [Google Scholar]
- 38.Levitan DR. In: Sperm Competiton and Sexual Selection. Birkhead TR, Moller AP, editors. London: Academic; 1998. pp. 175–218. [Google Scholar]
- 39.Levitan DR. The importance of sperm limitation to the evolution of egg size in marine invertebrates. Am Nat. 1993;141:517–536. doi: 10.1086/285489. [DOI] [PubMed] [Google Scholar]
- 40.Levitan DR. Predicting optimal and unique egg sizes in free-spawning marine invertebrates. Am Nat. 1996;148:174–188. [Google Scholar]
- 41.Levitan DR. Density-dependent sexual selection in external fertilizers: Variances in male and female fertilization success along the continuum from sperm limitation to sexual conflict in the sea urchin Strongylocentrotus franciscanus. Am Nat. 2004;164:298–309. doi: 10.1086/423150. [DOI] [PubMed] [Google Scholar]
- 42.Styan CA. Polyspermy, egg size, and the fertilization kinetics of free-spawning marine invertebrates. Am Nat. 1998;152:290–297. doi: 10.1086/286168. [DOI] [PubMed] [Google Scholar]
- 43.Marshall DJ, Styan CA, Keough MJ. Sperm environment affects offspring quality in broadcast spawning marine invertebrates. Ecol Lett. 2002;5:173–176. [Google Scholar]
- 44.Levitan DR. Does Bateman's principle apply to broadcast-spawning organisms? Egg traits influence in situ fertilization rates among congeneric sea urchins. Evolution. 1998;52:1043–1056. doi: 10.1111/j.1558-5646.1998.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 45.Levitan DR. Density-dependent selection on gamete traits in three congeneric sea urchins. Ecology. 2002;83:464–479. [Google Scholar]
- 46.Levitan DR, terHorst CP, Fogarty ND. The risk of polyspermy in three congeneric sea urchins and its implications for gametic incompatibility and reproductive isolation. Evolution. 2007;61:2007–2014. doi: 10.1111/j.1558-5646.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- 47.Marshall DJ, Keough MJ. The evolutionary ecology of offspring size in marine invertebrates. Adv Mar Biol. 2008;53:1–60. doi: 10.1016/S0065-2881(07)53001-4. [DOI] [PubMed] [Google Scholar]
- 48.Podolsky RD. Evolution of egg target size: An analysis of selection on correlated characters. Evolution. 2001;55:2470–2478. doi: 10.1111/j.0014-3820.2001.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 49.Podolsky RD. Life-history consequences of investment in free-spawned eggs and their accessory coats. Am Nat. 2004;163:735–753. doi: 10.1086/382791. [DOI] [PubMed] [Google Scholar]
- 50.Jantzen TM, de Nys R, Havenhand JN. Fertilization success and the effects of sperm chemoattractants on effective egg size in marine invertebrates. Mar Biol. 2001;138:1153–1161. [Google Scholar]
- 51.Riffell JA, Krug PJ, Zimmer RK. The ecological and evolutionary consequences of sperm chemoattraction. Proc Natl Acad Sci USA. 2004;101:4501–4506. doi: 10.1073/pnas.0304594101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolton TF, Thomas FIM, Leonard CN. Maternal energy investment in eggs and jelly coats surrounding eggs of the echinoid Arbacia punctulata. Biol Bull. 2000;199:1–5. doi: 10.2307/1542700. [DOI] [PubMed] [Google Scholar]
- 53.Allen RM, Buckley YM, Marshall DJ. Offspring size plasticity in response to intraspecific competition: An adaptive maternal effect across life-history stages. Am Nat. 2008;171:225–237. doi: 10.1086/524952. [DOI] [PubMed] [Google Scholar]
- 54.Ball MA, Parker GA. Sperm competition games: External fertilization and “adaptive” infertility. J Theor Biol. 1996;180:141–150. doi: 10.1006/jtbi.1996.0090. [DOI] [PubMed] [Google Scholar]
- 55.Levitan DR. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc R Soc London B. 2000;267:531–534. doi: 10.1098/rspb.2000.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gage MJG, MacFarlane C, Yeates S, Shackleton R, Parker GA. Relationships between sperm morphometry and sperm motility in the Atlantic salmon. J Fish Biol. 2002;61:1528–1539. [Google Scholar]
- 57.Vogel H, Czihak G, Chang P, Wolf W. Fertilization kinetics of sea urchin eggs. Math Biosci. 1982;58:189–216. [Google Scholar]
- 58.Havenhand JN, Svane I. Roles of hydrodynamics and larval behavior in determining spatial aggregation in the tunicate Ciona intestinalis. Mar Ecol Prog Ser. 1991;68:271–276. [Google Scholar]
- 59.Marshall DJ. In situ measures of spawning synchrony and fertilization success in an intertidal, free-spawning invertebrate. Mar Ecol Prog Ser. 2002;236:113–119. [Google Scholar]
- 60.Castilla JC, et al. Bio-foam enhances larval retention in a free-spawning marine tunicate. Proc Natl Acad Sci USA. 2007;104:18120–18122. doi: 10.1073/pnas.0708233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marshall DJ, Bolton TF. Effects of egg size on the development time of non-feeding larvae. Biol Bull. 2007;212:6–11. doi: 10.2307/25066575. [DOI] [PubMed] [Google Scholar]
- 62.Smith CC, Fretwell SD. Optimal balance between size and number of offspring. Am Nat. 1974;108:499–506. [Google Scholar]
- 63.Mousseau TA, Fox CW. Maternal Effects as Adaptations. Oxford: Oxford Univ Press; 1998. [Google Scholar]
- 64.Marshall DJ, Allen RM, Crean AJ. The ecological and evolutionary importance of maternal effects in the sea. Oceanogr Mar Biol Annu Rev. 2008;46:203–250. [Google Scholar]
- 65.Villa LA, Patricolo E. The follicle cells of Styela plicata (Ascidiacea, tunicata): A sem study. Zool Sci. 2000;17:1115–1121. doi: 10.2108/zsj.17.1115. [DOI] [PubMed] [Google Scholar]
- 66.Galletly BC, Blows MW, Marshall DJ. Genetic mechanisms of pollution resistance in a marine invertebrate. Ecol Appl. 2007;17:2290–2297. doi: 10.1890/06-2079.1. [DOI] [PubMed] [Google Scholar]
- 67.Marshall DJ, Styan CA, Keough MJ. Intraspecific co-variation between egg and body size affects fertilisation kinetics of free-spawning marine invertebrates. Mar Ecol Prog Ser. 2000;195:305–309. [Google Scholar]
- 68.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge Univ Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.