Abstract
The nuclear factor E2-related factor 2 (Nrf2) is a master transcriptional activator of genes encoding numerous cytoprotective enzymes that are induced in response to environmental and endogenously derived oxidative/electrophilic agents. Under normal, nonstressed circumstances, low cellular concentrations of Nrf2 are maintained by proteasomal degradation through a Keap1-Cul3-Roc1-dependent mechanism. A model for Nrf2 activation has been proposed in which two amino-terminal motifs, DLG and ETGE, promote efficient ubiquitination and rapid turnover; known as the two-site substrate recognition/hinge and latch model. Here, we show that in human cancer, somatic mutations occur in the coding region of NRF2, especially among patients with a history of smoking or suffering from squamous cell carcinoma; in the latter case, this leads to poor prognosis. These mutations specifically alter amino acids in the DLG or ETGE motifs, resulting in aberrant cellular accumulation of Nrf2. Mutant Nrf2 cells display constitutive induction of cytoprotective enzymes and drug efflux pumps, which are insensitive to Keap1-mediated regulation. Suppression of Nrf2 protein levels by siRNA knockdown sensitized cancer cells to oxidative stress and chemotherapeutic reagents. Our results strongly support the contention that constitutive Nrf2 activation affords cancer cells with undue protection from their inherently stressed microenvironment and anti-cancer treatments. Hence, inactivation of the Nrf2 pathway may represent a therapeutic strategy to reinforce current treatments for malignancy. Congruously, the present study also provides in vivo validation of the two-site substrate recognition model for Nrf2 activation by the Keap1-Cul3-based E3 ligase.
Keywords: cancer cell microenvironment, multidrug resistant-associated protein, oxidative stress, somatic mutation, ubiquitin-proteasome system
Oxidative and mutagenic damage to DNA, proteins, and lipids occurs continuously as a result of exposure to environmental pollutants, ionizing radiation, endogenous reactive oxygen species (ROS), electrophilic intermediates of xenobiotic metabolism, and inflammation. Damage from these exogenous and endogenous insults, if not repaired, can contribute to various pathologies including cancer (1, 2). The transcription factor Nrf2 mediates gene induction of protective phase-2 detoxifying enzymes and its activation by thiol-active compounds, found in a variety of natural foods and dietary supplements, has been advocated as a means to prevent cancer (3, 4). By contrast, Nrf2-deficient mice are highly susceptible to chemically induced carcinogenesis of multiple organs (5, 6). Under normal, nonstressed circumstances, low cellular concentrations of Nrf2 are maintained by proteasomal degradation, through a Keap1-Cullin 3-Roc1-dependent mechanism in which Keap1 serves as the substrate adaptor subunit in the E3 holoenzyme. Activation of Nrf2 allows it to escape proteolysis and to rapidly accumulate in the nucleus where it stimulates gene activation (7–9). Hence, experimental strategies to target Keap1, including siRNA knockdown and small molecule modulation, have also been used as a means to activate Nrf2 (10, 11).
Enzymes that are encoded by Nrf2 target genes, such as NAD(P)H quinine oxidoreductase-1 (NQO1) and peroxiredoxins (PRDX), are cytoprotective under normal conditions. Paradoxically, these enzymes are increased in many cancers, thereby affording these cells with protective capabilities to environmental stresses, which is a hallmark of malignancy (12, 13). This observation implicates aberrant Nrf2 activation in the process of human cancer development. In this regard, recent studies have revealed genetic alterations in KEAP1 that can lead to uncontrolled Nrf2 activation in cancers of the lung (14–16). Although polymorphisms have been identified in the promoter region of the Nrf2 gene (17, 18), which are thought to predispose individuals to lung injury and oxidative related diseases, no conclusive findings on the occurrence of genetic variations in the coding region have yet been published. Given that the intermolecular association between Nrf2 and Keap1 is mandatory for Nrf2 degradation and repression, we hypothesized that subtle amino acid changes in the coding region of Nrf2 might also affect proper Keap1-Nrf2 interaction and hence Nrf2 turnover in cancer.
Results
NRF2 Mutation in Human Cancer.
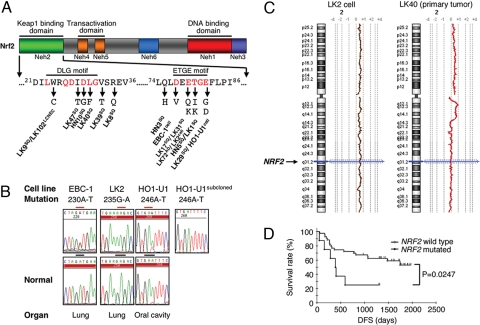
We sequenced the entire coding region of NRF2 in 85 cancer cell lines and found mutations in two lung cancer cell lines (LK2 and EBC1) and one oral cancer cell line (HO1-U1) [Fig. 1 A and B and supporting information (SI) Table S1]. We also examined 103 primary lung cancers of various histological subtypes and 12 primary head and neck (HN) cancers, and observed mutations in 11 lung tumors (10.7%) and 3 HN tumors (25%). All mutations (14/115, 12.2%) in primary tumors were missense amino acid substitutions and determined to be of somatic origin (Fig. 1A, Fig. S1 and Table S1), whereas no synonymous somatic alteration was detected. These mutations are conspicuously high in patients with a history of smoking (12/14) and occur frequently in squamous cell carcinoma (12/60, 20%). In lung cancer, no tumor harbored both NRF2 and KEAP1 mutations concurrently, and these alterations occur in tumors without EGFR mutation (Fig. 1A, Table 1, and Table S2). A homozygous Nrf2 mutant allele was observed in LK2 cells and one primary tumor (LK-40) and an increase in the gene copy number of NRF2 (3 copies) was also seen in LK2 cells (Fig. 1C). Prognostic analysis of lung squamous cell carcinomas carrying a mutation in NRF2 revealed a significant correlation with poor survival (P = 0.0247 by Log-rank analysis; Fig. 1D). Strikingly, the mutations were clustered in exon 2 and coded for amino acid changes in either the DLG or ETGE motifs of the regulatory Neh2 (Nrf2-ECH homology 2) domain (Fig. 1A) (19).
Fig. 1.
NRF2 mutations in human cancer. (A) Functional domains of Nrf2 protein showing distribution and types of NRF2 mutations. SQ, lung squamous cell carcinoma; AD, adenocarcinoma; LCNEC, large cell neuroendocrine carcinoma; HN, head and neck cancer; cell, lung cancer cell lines. (B) Representative DNA chromatograph of NRF2 mutations in human cancer cell lines with corresponding normal sequences in the bottom panel. (C) Copy number alteration map [shown by log2 ratio (tumor/normal)] of chromosome 2 in the tumors with mutated NRF2 and having lost the wild-type allele, detected by array-based comparative genomic hybridization. The arrow indicates the chromosomal location of NRF2. (D) Kaplan–Meier plot showing disease-free survival (DFS) of squamous cell lung carcinoma patients segregated according to the presence or absence of NRF2 mutations.
Table 1.
NRF2 mutation in cancer cell lines and cancer patients
| Sample name | Nucleotide mutation | Amino acid change | Tumor histology | Gender | Smoking status | Age |
|---|---|---|---|---|---|---|
| LK-1 | 239 C–G (heterozygous) | T80K | SQ | Male | Current | 68 |
| LK-8 | 101 G–A (heterozygous) | R34Q | SQ | Male | Current | 57 |
| LK-9 | 72 G–C (heterozygous) | W24C | SQ | Female | Former | 73 |
| LK-17 | 235 G–A (heterozygous) | E79K | SQ | Male | Former | 73 |
| LK-29 | 245 A–G (heterozygous) | E82G | SQ | Male | Former | 70 |
| LK-31 | 235 G–A (heterozygous) | E79K | SQ | Male | Current | 71 |
| LK-39 | 95 T–G (heterozygous) | V32T | SQ | Male | Former | 74 |
| LK-40 | 88 C–T (homozygous) | L30F | SQ | Male | Current | 70 |
| LK-47 | 83 T–C (heterozygous) | I28T | SQ | Female | Never | 59 |
| LK-72 | 235 G–C (heterozygous) | E79Q | AD | Male | Former | 75 |
| LK-102 | 72 G–C (heterozygous) | W24C | LCNEC | Male | Current | 77 |
| HN-3 | 225 A-C (heterozygous) | Q75H | HN | Female | Former | 51 |
| HN-5 | 239 C-T (heterozygous) | T80I | HN | Male | Never | 58 |
| HN-10 | 86 A-G (heterozygous) | D29G | HN | Male | Current | 74 |
| EBC-1 | 230 A–T (heterozygous) | D77V | NSCLC | NA | NA | NA |
| LK2 | 235 G–A (homozygous) | E79K | NSCLC | NA | NA | NA |
| HO1-U1 | 246 A–T (heterozygous) | E82D | OC | NA | NA | NA |
NRF2 Mutations Impair the Two-site Substrate Recognition Mechanism of Keap1.
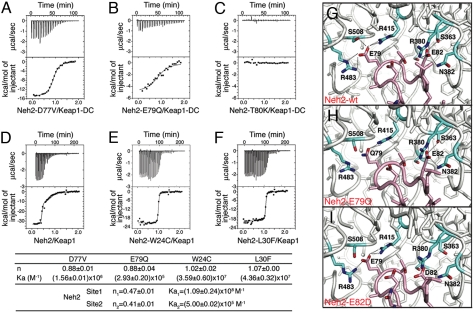
The double glycine repeat and the C-terminal region of Keap1 (hereafter refer as to Keap1-DC) recognize both the DLG and ETGE motifs of Nrf2 (20, 21). To evaluate the effect that mutations in the Nrf2 protein may have on its physical association with Keap1, we used isothermal calorimetry (ITC) to probe the interaction between purified recombinant Neh2 protein mutants (either Δ1–33Neh2/ETGE mutants or full length Neh2 DLG mutants), and the Keap1-DC or the full-length Keap1 protein. All of the mutations that occur in the ETGE motif (77DxETGE82), such as D77V, E79Q or T80K (Fig. 2 A–C), compromised the association with Keap1-DC. In particular, the T80K mutation displayed no observable affinity. The wild-type Neh2 protein exhibited a biphasic-binding curve with Keap1 (Fig. 2D), which has been shown to be a dimer in solution (20), indicating that two binding sites (DLG and ETGE) in the Neh2 domain are capable of interacting with Keap1. However, mutants bearing the missense alterations W24C or L30F within the DLG motif (23LxxQDxDLG31) regress to monophasic binding patterns (Fig. 2 E and F). The residual binding affinity most likely is contributed by interaction with the ETGE motif alone. These data unequivocally demonstrate that Nrf2 mutations occurring in either the DLG or ETGE motif can affect binding to the Keap1 dimer.
Fig. 2.
NRF2 mutations impair the two-site substrate recognition mechanism of Keap1. ITC titration profiles of Keap1-DC with Neh2[Δ1–33,D77V] (A), Neh2[Δ1–33,E79Q] (B), Neh2[Δ1–33,T80K] (C), and of Keap1 with Neh2 (D), Neh2[W24C] (E), Neh2[L30F] (F), raw thermograms (upper graph) and fitted binding isotherms (lower graph). Stoichiometry (n) and association constant (Ka) are as indicated. (G–I), binding site of the human Keap1-DC and wild-type ETGE peptide structure (G) and modeled structures of Keap1-DC with E79Q (H) or E82D (I) mutant ETGE peptide. Stick representation showing side chains of interacting residues from Keap1 (aqua) and ETGE peptide (pink). Dotted lines indicate predicted hydrogen bonds. Figures were drawn with PyMOL (www.pymol.org).
Using the x-ray crystal structure of human Keap1-DC in association with the ETGE peptide as template (PDB code 2FLU) (22) and by means of the MODELLER 9v1 program (23), we modeled some of the amino acid substitutions found in cancer patients or cell lines to access their impact on the structure-function relationship of Keap1 with Nrf2. The model detailing the structure of the E79Q mutation (Figs. 1A and 2H) suggests that compared with wild-type protein, there is a loss of electrostatic interaction with R483 and S508 of Keap1 (Fig. 2G), which likely accounts for the 1,000-fold reduction in binding affinity with Keap1 seen earlier (Fig. 2B). Similarly, the E82D mutation (Fig. 1A and Fig. 2I), despite both being acidic residues, seems to eliminate the contact with S363, R380, and S508 of Keap1, possibly because of the shorter side chain of Asp.
NRF2 Mutations Disturb Proper Nrf2-Keap1 Binding and Inhibit Keap1-Mediated Degradation of Nrf2 In Vivo.
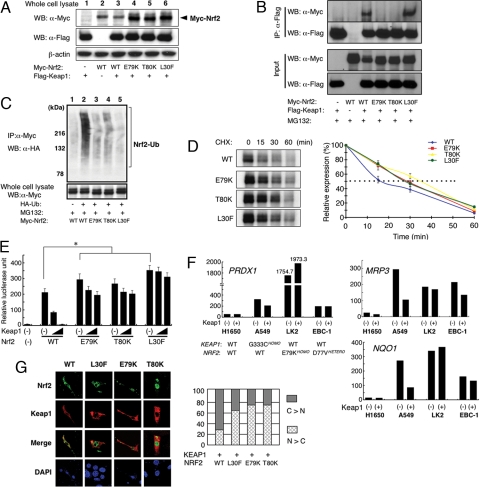
We next examined the interaction between mutant Nrf2 and Keap1 in vivo. Three of the Nrf2 mutations that were detected in cancer biopsies were selected (E79K, T80K, and L30F) and the proteins expressed in 293T cells, together with Keap1. All three mutant proteins showed enhanced doublet bands (Fig. 3A, lanes 4–6). Immunoprecipitation analysis revealed that the E79K and T80K mutations both had substantially reduced ability to interact with Keap1 (Fig. 3B). Protein harboring the L30F mutation retained the ability to associate with Keap1 to a similar degree as wild type, presumably because of the presence of an intact ETGE motif, whereas the weaker binding DLG motif alone could not sufficiently support a stable Nrf2/Keap1 interaction.
Fig. 3.
NRF2 mutations disturb proper Nrf2-Keap1 binding, inhibit Keap1-mediated degradation, promote transcriptional activity, and enhance nuclear localization of Nrf2 in vivo. (A) Expression level of the transfected Flag-tagged Keap1 and Myc-tagged wild-type or mutant Nrf2 (E79K, T80K, and L30F) in 293T cells. (B) Keap1-Nrf2 association was evaluated by immunoprecipitation of transfected 293T cells using anti-Flag antibody and immunoblotted by anti-Myc antibody. (C) Ubiquitination efficiency of wild-type or mutant Myc-tagged Nrf2 in vivo. (D) Immunoblot (left) showing degradation rate of Nrf2 protein from 15 to 60 min after cycloheximide chase. Intensities (%) relative to time 0 are plotted (right). Data represent mean ± SD of three independent runs. Dotted line indicates 50% expression of control. (E) Transactivation activities of Nrf2 mutants. Data represent mean ± SD. of three independent runs (*, P < 0.001). (F) Rescue analysis in KEAP1- or NRF2-mutated cancer cells. H1650, A549, LK2, and EBC-1 cells bearing both wild-type KEAP1 and NRF2 or mutations in one of the genes are indicated. Relative expressions of Nrf2 target genes peroxiredoxin1 (PRDX1), multidrug resistance protein 3 (MRP3), and NAD(P)H quinine oxidoreductase-1 (NQO1) to β-ACTIN with or without cotransfected KEAP1 plasmid are shown. (G) Nuclear accumulation of mutant Nrf2 proteins when coexpressed with Keap1 (left). Ratio of cytoplasmic/nuclear localization of Nrf2 proteins was shown as bar chart (right).
Because Keap1 plays a central role in ubiquitination and proteasomal degradation of Nrf2, we proceeded to analyze the ubiquitination status of mutant Nrf2. Wild-type and mutant Nrf2 were coexpressed with Keap1 and hemagglutinin (HA)-tagged ubiquitin, and the levels of Nrf2 ubiquitination were determined after treatment with MG132, a proteasomal inhibitor. Wild-type Nrf2 was efficiently polyubiquitinated whereas mutant Nrf2 proteins were only weakly polyubiquitinated (Fig. 3C). In particular, the L30F mutant was strongly resistant to ubiquitination. We also investigated whether mutant Nrf2 proteins are more stable than wild-type Nrf2. Wild-type and mutant Nrf2 were expressed in 293T cells and treated with cycloheximide to prevent new protein synthesis. Wild-type Nrf2 protein decreased rapidly (t½ = 16 min) as reported (7). By contrast, mutant Nrf2 proteins were degraded more slowly, having half-lives of approximately twice that of wild type (t½ = 28–35 min; Fig. 3D).
NRF2 Mutations Promote Transcriptional Activity and Nuclear Localization of Nrf2.
We next determined the transcriptional activation ability of mutant Nrf2 by analyzing luciferase activity from a reporter plasmid carrying a promoter region antioxidant-responsive element (ARE), the canonical Nrf2 recognition motif. Mutant Nrf2 proteins were significantly more active (P < 0.001 by unpaired t test) than wild-type Nrf2 (Fig. 3E). Coexpression of Keap1 markedly decreased luciferase activity induced by wild-type Nrf2 whereas the activity of mutant Nrf2 proteins was resistant to Keap1 inhibition (Fig. 3E). We also tested this reporter construct in cancer cell lines that carry genetic alterations in the Nrf2-Keap1 pathway (A549, LK2, and EBC1) and found that endogenous Nrf2 mutants also exhibited transactivation activity that was resistant to suppression by Keap1 coexpression, especially in LK2 cells, which carry a homozygous L79K mutation (Fig. S2). We further analyzed the endogenous expression of various Nrf2 target genes (NQO1, PRDX1 and MRP3) (15). The expression of these target genes was increased in cells with either altered KEAP1 (A549) or NRF2 (LK2 and EBC1), but remained at low basal levels in a cell line that had functional Nrf2 regulation (H1650). Introduction of Keap1 cDNA decreased expression of these genes in KEAP1 mutated A549 cells but failed to accomplish a similar down-regulation in cancer cells with mutated NRF2 (Fig. 3F). Immunofluorescent analysis also revealed that mutant Nrf2 proteins preferentially accumulated in the nucleus compared with wild type when coexpressed with Keap1 (Fig. 3G).
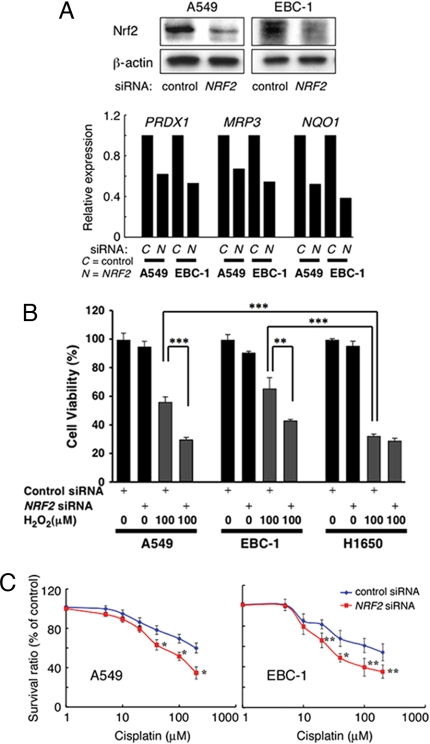
Down-regulation of NRF2 Restores Sensitivity to Oxidative Stress and Chemotherapeutic Agent.
Finally, we examined the effects of down-regulating Nrf2 in cancer cell lines where the Nrf2-pathway had become deregulated. Suppression of Nrf2 protein production by short interfering RNA (siRNA) reduced the expression of Nrf2-target genes (Fig. 4A). Because invasive cancer cells frequently encounter various oxidative stresses in their microenvironment (24), we examined whether activating mutations to Nrf2 affords these cells with resistance to oxidative stress. NRF2- or KEAP1-mutated cancer cells exhibited resistance to hydrogen peroxide treatment, and Nrf2 down-regulation in these cells significantly reduced cell viability (≈1.6-fold decrease in both cells) to a level similar to what was observed in Nrf2-pathway intact cancer cells (H1650) (Fig. 4B). Nrf2 downstream targets include enzymes for metabolizing (such as PRDX1) and exporting (MRP3) anti-cancer agents. Therefore, we also tested the effect of Nrf2-siRNA on drug sensitivity. We chose to examine the cytotoxic effect of cisplatin, a drug that is routinely used for treating lung cancers. Nrf2 down-regulation, in cancer cells harboring genetic alterations in the Nrf2-Keap1 pathway, significantly increased sensitivity to this compound (Fig. 4C).
Fig. 4.
Down-regulation of NRF2 restores sensitivity to oxidative stress and chemotherapeutic agent. (A) Down-regulation of Nrf2 expression by siRNA. Nonsilencing control or NRF2 siRNA was transfected into cancer cells with either KEAP1 (A549) or NRF2 (EBC-1) mutation. Cell lysates were electrophoresed and immunoblotted with anti-Nrf2 or anti-β-actin (loading control) antibody (top). Relative expressions of Nrf2 target genes (PRDX1, MRP3, and NQO1) to β-ACTIN are shown in the histogram (bottom). (B) Cell viability of siRNA treated A549, EBC-1, and H1650 cells exposed to oxidative stress. Data present percentage of cell viability after a 5-h treatment with H2O2 relative to nontreated control (mean ± SD of three independent runs) (C) A549 and EBC-1 cells were treated with control or NRF2 siRNA and various concentrations of cisplatin. Data present percentage of cell viability after 48 h relative to vehicle (DMSO)-treated control (mean ± SD of three independent runs). (*, P < 0.05; **, P < 0.01; ***, P < 0.001)
Discussion
Nrf2 degradation is governed by the Cullin3-Keap1 system, which is unique in terms of the adaptor-substrate recognition whereby the two Neh2 recognition motifs (DLG and ETGE) of a Nrf2 monomer bind to a Keap1 homodimer with different affinities; known as the two-site substrate recognition/hinge and latch model (Fig. S3). Unlike some other substrates of proteasomal degradation system, such as inhibitory kappa B (IKB) in inflammatory response and hypoxia-inducible transcription factor (HIF), which have to be post-translationally modified before recruiting to their respective E3 ligase complexes (25), Nrf2 is being constitutively recognized by Keap1 through negative-charged residues of two conserved degradation signal sequences in the substrate. Therefore, the structural integrity of both the Hinge (ETGE) and Latch (DLG) within the Neh2 domain is crucial for maintaining contemporaneous two-site binding for proper Nrf2 turnover and the narrow window of Nrf2-ARE mediated gene expression (21, 26). X-ray crystal structures of the Keap1-DC-DLG peptide and Keap1-DC-ETGE-peptide complexes have depicted that the side chains of Q26, D27, and D29 of the DLG motif (23LxxQDxDLG31) and E79 and E82 of the ETGE motif (77DxETGE82) have direct interactions with the Keap1-DC. Whereas T80 has both inter- and intramolecular interactions, Q75 has intramolecular contact (14, 21, 22). Cancer-related mutations within the ETGE motif are primarily substitutions of these essential residues for Keap1 recognition whereas mutations in the DLG motif are either changes in the conserved (D29 and L30) or nonconserved residues (W24, I28, V32, and R34). These findings suggest that the weaker Keap1-binding DLG region is more vulnerable to structural changes and fits into its anticipated role of the “latch” for the Nrf2-repression gate in the stress response. Collectively the NRF2 somatic mutations found in human cancers indubitably validate the necessity for simultaneous recognition of these two N-terminal degrons in Nrf2 for its timely proteolysis and regulation in vivo.
The correlation of mutation in the ubiquitin-proteasome pathway with cancer growth has long been recognized (27, 28). Altered degradation pattern of transcription factors and tumor suppressor proteins are found in cancers of different pathologies and origin, where oncogenic changes often arise from mutations in the subunits of E3 ligases. For example, von Hippel-Lindau tumor suppressor protein (pVHL) and BRAC1, together with BARD1 (BRAC1-associated RING domain 1), are components of E3 ubiquitin ligases (29–31). VHL ubiquitin ligase complex facilitates protein turnover of HIFα for angiogenesis or hypoxic response regulation, whereas BRAC1/BARD1 system targets a variety of substrates that involve in regulation of transcription, chromatin remodeling, DNA repair, and centrosome dynamics (32, 33). Mutations of pVHL or BRAC1 predispose tumorigenesis in patients probably by either enhancing adaptive capability of cancer cells to their microenvironment or by jeopardizing proper transcriptional regulations, DNA repair response, and genome stability, respectively (27, 28, 33). Similarly, recent studies have shown different somatic mutations of Keap1 (14–16), the substrate binding module of a Cullin3-based E3 ligase, in lung cancer patients that led to aberrant expression of ARE genes. Cancer-related somatic mutations in the degradation signals of Nrf2, presented in this study, for E3 ligase recognition again reinforce the importance of functional ubiquitin-proteasome system.
Additionally our results demonstrated that gain-of-function mutations of NRF2 were observed in approximately 11% of lung cancer. Together with previous reports (14–16), constitutive activation of Nrf2 (caused by KEAP1 or NRF2 mutations) occurs in >20% of lung cancer cases. Our analysis concurrently revealed frequent NRF2 mutation in head and neck cancers, suggesting perturbations of the Nrf2 pathway in a broad range of cancers, particularly those arising in tissues exposed to oxygen.
Intriguingly KEAP1-deficient mice, which show constitutive activation of Nrf2 in the whole body, develop postnatal hyperkeratosis without accompanying by increased cell proliferation in skin and upper digestive tract (34). Thus, preferential NRF2 mutations among patients diagnosed with squamous cell carcinoma and a history of smoking could be explained by stress-related Nrf2-dependent intervention to squamous cell lineage differentiation. However, malignant development induced by deviated Nrf2-mediated gene expression profile appears to be more complicated because a number of genes involved in cell proliferation were found to be transcriptionally modulated by Nrf2 through glutathione as the effector (35) and the growth rate of immortalized Keap1 null MEF cells also increased on culture dishes (16). Nevertheless, the existence of two tumors with both NRF2 alleles mutated and the association of poor prognosis suggest a direct role of Nrf2 mutation in tumor progression. In terms of stress cellular defense, temporary induction of cytoprotective enzymes by Nrf2 activation is beneficial to the repair of oxidative damage and cancer prevention before the accumulation of any genetic lesions at the early stage of carcinogenesis (36, 37). Although constitutive expression in a tumorigenic situation could be devastating because it could provide a survival advantage to invasive and metastatic cancer cells, such as the adaptation to microenvironment and the evolvement of chemoresistance in cancer cells that are known to occur in tumor hypoxia (38, 39). In addition, cross-talk between tumor hypoxia and induction of Nrf2 has also been suggested recently (40). It is also noteworthy that inhibition of the Nrf2 pathway in these cancer cells enhanced sensitivity to the anti-cancer reagent cisplatin. Taken together, our results set a new paradigm for cancer therapy in which the inhibition of Nrf2 could be used to enhance chemotherapeutic sensitivity. They further suggest that mutation in the Keap1-Nrf2 system may represent a novel oncogenic mechanism leading to malignancy (Fig. S3).
Materials and Methods
DNA Sample Collection.
DNA was extracted from cell lines using a DNA extraction kit (Qiagen). All tumor samples were subjected to laser-capture microdissection using LM200 (Arcuturus).
PCR Amplification and Genetic Analysis of NRF2.
All coding exons of NRF2 were amplified by PCR using High Fidelity Taq polymerase (Roche) and appropriate primers (Table S3). All PCR products were purified and analyzed by sequencing. A high-density oligonucleotide array (Human genomic CGH microarray 44B, Agilent Technologies) was used.
Isothermal Calorimetry.
ITC titration experiments were performed at 25°C on a VP-ITC system (MicroCal Inc.). All proteins were prepared as described in ref. 21. All runs were performed in triplicate. Binding data were analyzed using the computer program Origin version 5.0, supplied by MicroCal Inc. Data processing was carried out as reported (21).
Molecular and Cell Biology Analysis.
Anti-Myc (Cell signaling), anti-Flag (Sigma), and anti-HA (MBL) antibodies, and MG132 (Calbiochem), cycloheximide (Nacalai), and cisplatin (WAKO) were purchased, whereas anti-Nrf2 polyclonal antibody was produced by immunization with Nrf2 peptide. Immunoprecipitation, immunoblotting, and immunofluorescent analyses were performed as described (7, 8) with slight modifications.
Statistical Analysis.
The Kaplan–Meier method was used to estimate the probability of disease-free survival. Log-rank analysis was used to assess the significance of the differences between tumors with and without NRF2 mutation.
SI.
Further description of experimental procedures can be found in SI Materials and Methods, which is published as SI on the PNAS web site.
Supplementary Material
Acknowledgments.
We thank Dr. K. Nakayama (Kyushu University, Fukuoka, Japan) for HA-tagged ubiquitin vector and Dr. V.P. Kelly (Trinity College, Dublin) for critical reading of the manuscript. This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan; a grant for the program for promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio); and a grant for the program for promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research; the Japanese Ministry of Education, Culture, Sports, Science and Technology, and Exploratory Research for Advanced Technology-Japan Science and Technology Corporation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806268105/DCSupplemental.
References
- 1.Kasai H, Kawai K. Oxidative DNA damage: Mechanisms and significance in health and disease. Antioxid Redox Signal. 2006;8:981–983. doi: 10.1089/ars.2006.8.981. [DOI] [PubMed] [Google Scholar]
- 2.Motohashi H, Yamamoto M. Carcinogenesis and transcriptional regulation through Maf recognition elements. Cancer Sci. 2007;98:135–139. doi: 10.1111/j.1349-7006.2006.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahey JW, Kensler TW. Role of Dietary Supplements/Nutraceuticals in Chemoprevention through Induction of Cytoprotective Enzymes. Chem Res Toxicol. 2007;20:572–576. doi: 10.1021/tx7000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Gomez M, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iida K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi A, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devling TW, Lindsay CD, McLellan LI, McMahon M, Hayes JD. Utility of siRNA against Keap1 as a strategy to stimulate a cancer chemopreventive phenotype. Proc Natl Acad Sci USA. 2005;102:7280–7285. doi: 10.1073/pnas.0501475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern JT, Hannink M, Hess JF. Disruption of the keap1-containing ubiquitination complex as an antioxidant therapy. Curr Top Med Chem. 2007;7:977–983. doi: 10.2174/156802607780906825. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal AK. Jun and Fos regulation of NAD (P)H: Quinone oxidoreductase gene expression. Pharmacogenetics. 1994;4:1–10. doi: 10.1097/00008571-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Padmanabhan B, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohta T, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, et al. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem Biophys Res Commun. 2004;321:72–79. doi: 10.1016/j.bbrc.2004.06.112. [DOI] [PubMed] [Google Scholar]
- 18.Marzec JM, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 19.Itoh K, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong KI, et al. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong KI, et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;21:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1: Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25:3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marti-Renom MA, et al. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 24.Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004;64:7464–7672. doi: 10.1158/0008-5472.CAN-04-1725. [DOI] [PubMed] [Google Scholar]
- 25.Petroski MD, Desharies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–15. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 26.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: A hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 27.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 28.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–4789. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 29.Wu LC, et al. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 30.Pause A, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr., Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starita LM, Parvin JD. Substrates of the BRCA1-dependent ubiquitin ligase. Cancer Biol Ther. 2006;5:137–141. doi: 10.4161/cbt.5.2.2479. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi N, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 35.Reddy NM, et al. Genetic dissection of the Nrf2-dependent redox signaling regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics. 2007;32:74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 36.Bindra RS, Glazer PM. Genetic instability and the tumor microenvironment: Towards the concept of microenvironment-induced mutagenesis. Mutat Res. 2005;569:75–85. doi: 10.1016/j.mrfmmm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Adriana A, Sporn MC. The tumor microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Schmid T, Schnitzer S, Brüne B. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Kim YJ, et al. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.