Abstract
Ca2+ can stimulate cyclic nucleotide synthesis, but it is not known whether this signaling occurs in nerve terminals in response to activity. Here, in vivo imaging of Drosophila motoneuron terminals shows that activity rapidly induces a long-lasting signal from a transgenically expressed optical indicator based on the epac1 (exchange protein directly activated by cAMP 1) cAMP-binding domain. The epac1-cAMP sensor (camps) response in synaptic boutons depends on extracellular Ca2+ and ryanodine receptor-mediated Ca2+-induced Ca2+ release from the endoplasmic reticulum. However, mutations that inhibit rutabaga Ca2+-stimulated adenylyl cyclase and dunce cAMP-specific phosphodiesterase (PDE) have no effect. Instead, the activity-dependent presynaptic epac1-camps signal reflects elevation of cGMP in response to nitric oxide-activated guanylyl cyclase. Posttetanic presynaptic cGMP is long-lived because of limited PDE activity. Thus, nerve terminal biochemical signaling induced by brief bouts of activity temporally summates on a time scale orders of magnitude longer than fast transmission.
Keywords: cyclases, cyclic nucleotides, nerve terminal, plasticity, neuromuscular junction
The mechanisms that control cyclic nucleotide levels in the nerve terminal are not known. Activity-induced presynaptic Ca2+ influx could be involved because some cyclases are directly activated by Ca2+. For example, the rutabaga gene, which affects synaptic plasticity and development in Drosophila, encodes a neuronal Ca2+-stimulated adenylyl cyclase (1–3). However, this isoform is also stimulated by the Gsα G protein (4). Thus, it is not clear whether the presynaptic effects of the rutabaga adenylyl cyclase reflect a permissive background effect or activation by Gsα or Ca2+. Determining the mechanisms responsible for activating presynaptic cyclases has been difficult because it has not been possible to directly measure cyclic nucleotides in living nerve terminals.
Recently, a ratiometric FRET-based cAMP sensor (camps) that uses the cAMP binding domain of epac (exchange protein directly activated by cAMP) was generated to report activation of adenylyl cylase by forskolin and receptors (5). Here, in vivo imaging shows that activity rapidly induces a Ca2+-dependent epac1-camps response in Drosophila motoneuron synaptic boutons. Surprisingly, this response is long-lasting and is unaffected by mutants that disrupt rutabaga, which lowers neuronal cAMP (3), or the dunce cAMP-specific phosphodiesterase (PDE), which elevates neuronal cAMP (3, 6). Pharmacological and genetic experiments are presented that identify the presynaptic biochemical signaling detected by epac1-camps and explain how it temporally summates on a time scale of minutes.
Results and Discussion
FRET imaging of epac1-camps was performed in third-instar larval neuromuscular junction synaptic boutons. Seconds of 70-Hz nerve stimulation induced a drop in the emission from the FRET acceptor YFP and an increase in the emission from the FRET donor CFP (cyan fluorescent protein), resulting in a decrease in the YFP/CFP FRET ratio in type III peptidergic boutons (Fig. 1 A–C). Consistent with a FRET response, fluorescence induced by direct excitation of YFP was insensitive to activity (Fig. 1C Inset). A similar response was also produced in glutamatergic type Ib boutons (Fig. 1D). Thus, epac1-camps imaging establishes that tetanic activity increases cyclic nucleotide levels in diverse Drosophila motoneuron nerve terminals.
Fig. 1.
Activity-induced prolonged epac1-camps response in synaptic boutons. (A) Pseudocolor YFP/CFP FRET ratio images of muscle 12 type III bouton expressing Epac1-cAMPs before (Con) and 5 s after 70-Hz stimulation for 5 s (Stim). (Size bar: 1 μm.) (B) Rapid decrease in YFP and increase in CFP fluorescence from a single type III bouton upon 5 s of activity (arrow). (C) Normalized YFP/CFP ratio time course shows a loss in FRET for the experiment in B. (Inset) No response is seen with direct excitation of YFP (n = 4). Error bars, which indicate SEM, are comparable in size to symbols. (D) Time course of epac1-camps response in muscle 6/7 type Ib glutamatergic boutons stimulated for 5 s at 70 Hz (n = 5).
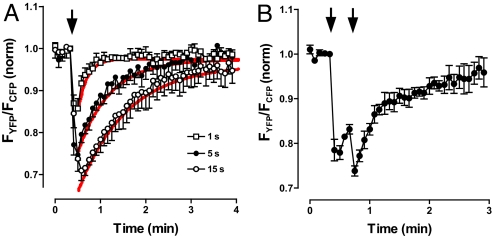
Presynaptic cyclic nucleotide responses depend on the duration of stimulation and are long-lasting. Specifically, FRET changes lasted at least 10-fold longer than initiating 1- to 15-s tetani (Fig. 2A). Furthermore, the time course of responses was dominated by first-order decay kinetics with time constants that increased with the duration of activity (Fig. 2A). Typically, neurotransmission electrical responses temporally summate on the time scale of seconds. However, the slow decay of activity-dependent cyclic nucleotide signals allows biochemical signaling induced by brief tetani to temporally summate on the time scale of minutes (Fig. 2B).
Fig. 2.
Temporal summation of epac1-camps responses. (A) FRET responses to 1 s (□, n = 5), 5 s (●, n = 5), and 15 s (○, n = 4) of stimulation of type III boutons. Red curves show exponential fits with time constants of 11.5, 40, and 60 s for stimulation for 1, 5, and 15 s, respectively. (B) Temporal summation of epac1-camps responses induced by two volleys of 2-s, 70-Hz stimulation (indicated by arrows) (n = 5). Error bars indicate SEM.
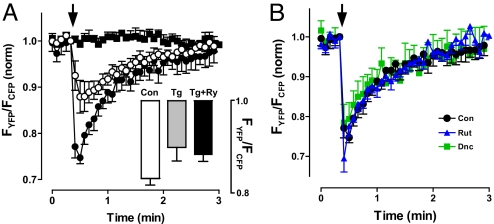
The presynaptic epac1-camps response depends on Ca2+ influx and Ca2+-induced Ca2+ release. First, removal of extracellular Ca2+ abolished the FRET change induced by a tetanus (Fig. 3A, compare filled circles and filled squares). Second, ryanodine (Ry) block of Ca2+-induced Ca2+ release by endoplasmic reticulum (ER) Ry receptors (RyRs), which eliminates activity-dependent vesicle mobilization and capture of transiting of transiting vesicles in Drosophila nerve terminals (7, 8), reduced the epac1-camps response (Fig. 3A, open circles). To verify that RyRs were responsible for this partial inhibition, ER Ca2+ was depleted with thapsigargin (Tg) (7). Under these conditions Ry had no effect (Fig. 3A Inset), verifyng that RyR-mediated Ca2+-induced Ca2+ release was involved. Furthermore, Tg and Ry effects were statistically indistinguishable, implying that RyRs fully account for the participation of ER Ca2+ stores (i.e., there was no evidence of autoreceptor-induced inositol trisphosphate-mediated ER Ca2+ release). Together, these results show that Ca2+-induced Ca2+ release from the ER amplifies cyclic nucleotide signaling induced by Ca2+ influx.
Fig. 3.
Epac1-camps responses to activity in type III boutons depend on Ca2+, but are unaffected by rutabaga Ca2+-stimulated adenylyl cyclase and dunce cAMP-specific PDE mutants. (A) Ca2+ dependence of FRET responses to a 5-s, 70-Hz tetanus. ●, control; ■, Ca2+-free medium; ○, inhibition of RyRs with 100 μM Ry (n = 5). (Inset) Tg (20 μM) mimics and occludes the Ry effect. Con, n = 8; Tg, n = 5; Tg + Ry, n = 5. (B) Type III bouton FRET responses to 5-s stimulations in control animals (black circles; n = 5), rutabaga mutant (rut1) animals (blue triangles; n = 5), and dunce mutant (dnc1) animals (green squares; n = 5). Error bars indicate SEM.
However, the identity of the generated cyclic nucleotide was not evident in initial genetic experiments. Specifically, mutants that eliminate native rutabaga Ca2+-stimulated adenylyl cyclase (rut1) (2) or the dunce cAMP-specific PDE (dnc1), which also affects synaptic development and plasticity in Drosophila (1, 3, 6), did not change the response to electrical activity (Fig. 3B). Thus, activity initiates signaling that does not involve two genes that influence cAMP levels in Drosophila neurons.
The Ca2+ dependence of the sensor response to activity coupled with the absence of rut1 and dnc1 effects led us to consider whether epac1-camps might be reporting cGMP instead of cAMP. Epac1-camps appears to be specific for cAMP because its low affinity for cGMP (Kd = 10.6 μM) ensures that guanylyl cyclase signaling in whole cells is not detected (5, 9). However, previous studies had not examined epac1-camps in small confined structures with NO-activated guanylyl cyclase such as Drosophila motoneuron boutons (10). Furthermore, the Ca2+ dependence of Drosophila NO synthase (NOS) (11) is consistent with a role for this pathway in the activity-induced epac1-camps response.
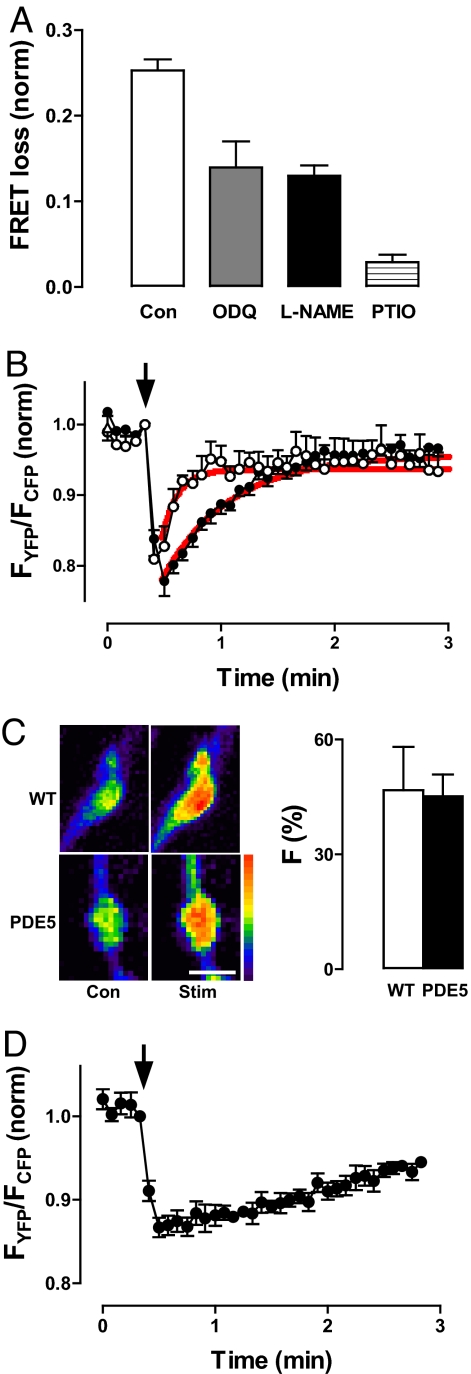
In fact, pharmacology and genetic experiments established that electrical activity activates NOS to stimulate guanylyl cyclase, resulting in presynaptic cGMP elevation. First, the epac1-camps response to electrical activity is reduced by the guanylyl cyclase inhibitor 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxalin-1-one (ODQ), the NOS inhibitor l-NG-nitroarginine methyl ester (l-NAME), and the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) (Fig. 4A; P < 0.05 for each treatment). Furthermore, transgenic presynaptic expression of cGMP-specific mammalian type 5 PDE (PDE5) (12, 13) shortened the epac1-camps response 4-fold and reduced its peak amplitude (Fig. 4B). Activity-evoked presynaptic Ca2+ responses measured with GCaMP, a genetically encoded Ca2+ indicator (14), were not affected by PDE5 (Fig. 4C), demonstrating that the efficacy of stimulation was not altered. Hence, because PDE5 does not hydrolyze cAMP, the prolonged epac1-camps response must be caused by elevation of cGMP. Therefore, four independent experimental criteria, along with results obtained with rutabaga and dunce mutants, establish that electrical activity induces NOS-guanylyl cyclase-mediated presynaptic cGMP synthesis.
Fig. 4.
Activity elicits presynaptic PDE-limited NO-dependent cGMP signaling. (A) FRET loss 5 s after cessation of stimulation (5 s, 70 Hz) in control boutons (n = 5), boutons treated with 100 μM ODQ (n = 6), 1 mM l-NAME (n = 5), or 1 mM PTIO (n = 6). Each of the drug effects are statistically significant (P < 0.05; one-way ANOVA, Dunnett's posttest). Error bars indicate SEM. (B) FRET time course in boutons expressing mammalian cGMP-specific PDE5 (○; n = 4) compared with controls also grown at 29°C (●; n = 5). Red lines show exponential fits with time constants of 8.1 and 34 s for PDE5-expressing and control boutons, respectively. Arrow indicates stimulation points. (C) PDE5 expression does not alter presynaptic Ca2+ responses to activity measured with GCaMP imaging. Stim, response to a 5-s, 70-Hz tetanus. PDE5, animals transgenically expressing mammalian cGMP-specific PDE5. (Left) Pseudocolor images of representative type III boutons. (Scale bar: 5 μm.) (Right) Increase in fluorescence from the Ca2+ indicator evoked by stimulation (n = 8). Error bars indicate SEM. (D) FRET response induced by a 5-s tetanus in boutons treated with 1 mM IBMX for 15 min before stimulation (n = 6). Arrow indicates stimulation points.
The slow decay in presynaptic cGMP after a brief tetanus could be caused by cGMP diffusion out of boutons or PDE-mediated hydrolysis. Specific inhibitors and mutants of the many potential Drosophila cGMP PDEs have not been described (12). Therefore, the role of native PDEs was examined with a wide-spectrum PDE inhibitor. Isobutylmethylxanthine (IBMX) dramatically slowed the posttetanic decay in presynaptic cGMP elevation (Fig. 4D). Coupled with the PDE5 result, this finding shows that the prolonged duration of the cGMP response is determined by limited PDE-mediated breakdown of cGMP.
In vivo imaging of epac1-camps in nerve terminals has yielded a number of striking results. First, activity-dependent cyclic nucleotide synthesis was demonstrated in synaptic boutons. This finding establishes a mechanism for coupling nerve terminal activity to cGMP signaling, which induces synaptic vesicle exocytosis at Drosophila motoneuron boutons (10) and supports plasticity at other synapses (15). Second, previous studies of NOS-cGMP at synapses have focused on transynaptic effects (e.g., NO as a retrograde signal) (15). However, because glutamatergic transmission was inhibited and postsynaptic muscle in this preparation does not produce cGMP in response to NO (10), NO generation and activation of guanylyl cyclase both occur presynaptically. This arrangement minimizes dilution of NO to produce robust activity-evoked cGMP responses in motoneuron terminals. Third, cGMP synthesized in response to brief bouts of activity is long-lived in synaptic boutons, because non-dunce PDE activity is limited in the nerve terminal. Hence, temporal summation of cGMP responses occurs on a time scale orders of magnitude longer than neurotransmission. Prolonged electrical activity-induced signaling may be important for a variety of NOS-dependent processes in insect nervous systems (16).
Materials and Methods
Cyclic nucleotide imaging experiments were performed on the third-instar larval neuromuscular junction from transgenic Drosophila expressing epac1-camps (UAS-epac1-camps) (17) driven by 386-GAL4. Female rutabaga (rut1) and dunce (dnc1) mutants were crossed with the males of the above line, and male offspring were used for experiments. For PDE5 expression, male 386-GAL4 UAS-epac1-camps were crossed with female flies with UAS-PDE5 (13) on chromosomes 1 and 3. Ca2+ was imaged in type III boutons after crossing OK6-GAL4;UAS-GCaMP males with female Canton S flies for WT controls or the above female UAS-PDE5 flies. Drosophila were grown at 25°C, except for PDE5 experiments and their matched controls, which were grown at 29°C to promote simultaneous GAL4-driven expression of UAS-epac1-camps and UAS-PDE5.
Imaging of filleted larvae was performed as described (7, 18). Briefly, the neuromuscular junction was prepared in HL3, which contained 70 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 20 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose, 115 mM sucrose, and 5 mM sodium Hepes, pH 7.2, supplemented with 10 mM l-glutamate to desensitize postsynaptic receptors. Depletion of ER Ca2+ stores with Tg used a protocol (7) that involved treatment for 20 min with Tg (or vehicle for controls) in Ca2+-free medium before continued treatment in HL3. In all experiments, nerve terminals were stimulated via intact segmental nerves with a suction electrode. Unless otherwise stated, type III peptidergic boutons on muscle 12 were imaged at room temperature with an upright epifluorescence Olympus microscope with a ×60 water-immersion 1.1 numerical aperture objective. Data were acquired with a cooled CCD ORCA-ER/AG camera (Hamamatsu Photonics). FRET imaging used a 440DF20 excitation filter, a 455DRLP dichroic mirror, and alternating 480DF30 (for CFP) and 535 DF25 (for YFP) emission filters on a filter wheel (Sutter Instruments). The ratio of emissions (YFP/CFP) was determined from background-subtracted YFP and CFP intensities. For each experiment the ratio was normalized to the initial FRET ratio before stimulation. Direct excitation of YFP was induced with a 500/20 filter. GCaMP was imaged by using standard fluorescein optics.
l-NAME was purchased from Sigma–Aldrich; ODQ, PTIO, and IBMX were from Calbiochem, and Tg was from Axxora. IBMX and ODQ were dissolved in DMSO to make stock solutions. The final concentration of DMSO in saline was no more than 0.1%, which had no effect on the neuromuscular junction.
Acknowledgments.
We thank Chandra Vignere for technical assistance; David Morton (Oregon Health and Science University, Portland), Shireen Davies (University of Glasgow, Glasgow, Scotland), David Deitcher (Cornell University, Ithaca, NY), Orie Shafer, and Paul Taghert (Washington University, St. Louis) for fly stocks; and Dr. Taghert for comments on a draft of this manuscript. This research was supported by National Institutes of Health Grant NS32385.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- 2.Levin LR, et al. The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 3.Cheung US, Shayan AJ, Boulianne GL, Atwood HL. Drosophila larval neuromuscular junction's responses to reduction of cAMP in the nervous system. J Neurobiol. 1999;40:1–13. doi: 10.1002/(sici)1097-4695(199907)40:1<1::aid-neu1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaev VO, et al. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 6.Davis RL, Kiger JA. Dunce mutants of Drosophila melanogaster: Mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981;90:101–107. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shakiryanova D, et al. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci. 2007;27:7799–7806. doi: 10.1523/JNEUROSCI.1879-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong MY, Shakiryanova D, Levitan ES. Presynaptic ryanodine receptor-CamKII signaling is required for activity-dependent capture of transiting vesicles. J Mol Neurosci. 2008 doi: 10.1007/s12031-008-9080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolaev VO, et al. Real-time monitoring of the PDE2 activity of live cells: Hormone-stimulated cAMP hydrolysis is faster than hormone-stimulated cAMP synthesis. J Biol Chem. 2005;280:1716–1719. doi: 10.1074/jbc.C400505200. [DOI] [PubMed] [Google Scholar]
- 10.Wildemann B, Bicker G. Nitric oxide and cyclic GMP induce vesicle release at Drosophila neuromuscular junction. J Neurobiol. 1999;39:337–346. doi: 10.1002/(sici)1097-4695(19990605)39:3<337::aid-neu1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Regulski M, Tully T. Molecular and biochemical characterization of dNOS: A Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proc Natl Acad Sci. 1995;92:9072–9076. doi: 10.1073/pnas.92.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies SA. Signaling via cGMP: Lessons from Drosophila. Cell Signal. 2006;18:409–421. doi: 10.1016/j.cellsig.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Broderick KE, et al. Ectopic expression of bovine type 5 phosphodiesterase confers a renal phenotype in Drosophila. J Biol Chem. 2004;279:8159–8168. doi: 10.1074/jbc.M304679200. [DOI] [PubMed] [Google Scholar]
- 14.Wang JW, et al. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 15.Feil R, Kleppisch T. NO/cGMP-dependent modulation of synaptic transmission. Handb Exp Pharmacol. 2008;184:529–560. doi: 10.1007/978-3-540-74805-2_16. [DOI] [PubMed] [Google Scholar]
- 16.Bicker G. NO news from insect brains. Trends Neurosci. 1998;21:349–355. doi: 10.1016/s0166-2236(98)01236-3. [DOI] [PubMed] [Google Scholar]
- 17.Shafer OT, et al. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitan ES, Lanni F, Shakiryanova D. In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat Protocols. 2007;2:1117–1125. doi: 10.1038/nprot.2007.142. [DOI] [PubMed] [Google Scholar]