Abstract
Cortical GABAergic dysfunction, a hallmark of both schizophrenia (SZ) and bipolar (BP) disorder pathophysiologies may relate to the hypermethylation of GABAergic gene promoters (i.e., reelin and GAD67). Benefits elicited by a combination of atypical antipsychotics with valproate (VPA) (a histone deacetylase inhibitor that may also activate brain DNA demethylation) in SZ or BP disorder treatment prompted us to investigate whether the beneficial action of this association depends on induction of a putative DNA demethylase activity. To monitor this activity, we measured the ratio of 5-methyl cytosine to unmethylated cytosine in reelin and GAD67 promoters in the mouse frontal cortex and striatum. We compared normal mice with mice pretreated with l-methionine (5.2 mmol/kg s.c. twice a day for 7 days) to hypermethylate promoters, including reelin and GAD67. Clinically relevant doses of clozapine (CLZ) (3.8 to 15 μmol/kg twice a day s.c. for 3 days) and sulpiride (SULP) (12.5 to 50 μmol/kg twice a day for 3 days) but not clinically relevant doses of haloperidol (HAL) (1.3 to 4 μmol/kg twice a day s.c. for 3 days) or olanzapine (OLZ) (4 to 15 μmol/kg twice a day for 3 days) exhibited dose-related increases in the cortical and striatal demethylation of hypermethylated reelin and GAD67 promoters. These effects of CLZ and SULP were dramatically potentiated by a clinically relevant VPA dose (0.5 mmol/kg twice a day for 3 days). By activating a DNA demethylase, the association of CLZ or SULP with VPA may facilitate a chromatin remodeling that normalizes the GABAergic gene expression down-regulation detected in the telencephalic regions of SZ and BP patients.
Keywords: antipsychotics, chromatin remodeling, valproate, GAD67, reelin
In specific populations of telencephalic GABAergic neurons of patients with schizophrenia (SZ) and bipolar (BP) disorder, down-regulation of the expression of several vulnerability genes, including GAD67 (GAD1), reelin (RELN), NR2A (GRIN2A), and GAT1 (SLC6A1) (1–4), is probably related to an hypermethylation of the corresponding promoters (5, 6), which could be sustained by an hyperactivity of DNA methyltransferases (DNMTs) including DNMT1 and DNMT3a (7, 8, Veldic, personal communication). This concept is supported by clinical studies performed several decades ago with l-methionine (MET) administration to SZ patients. Such a treatment for 2–3 weeks in doses of 10–20 g/day, exacerbates psychotic symptoms in 40–50% of SZ patients (9), likely by increasing the brain levels of the methyl-donor S-adenosyl-methionine (SAM), which is the natural cofactor for the catalytic activity of DNMTs.
In the mouse frontal cortex (FC), the increase of SAM content elicited by protracted MET treatment induces a covalent methylation in the 5′ position of the cytosine ring at dinucleotide CG or tri-nucleotide CNG sequences of specific promoters†, including those corresponding to reelin and GAD67 (10, 11). The hypermethylation of reelin and GAD67 promoters induced by MET is likely responsible for the transcriptional down-regulation of reelin and GAD67 expression in telencephalic GABAergic neurons of animals receiving this amino acid (10, 11). These considerations allow speculation that in the treatment of SZ and BP disorders, one might also include inhibitors of DNMT catalytic activity to increase reelin and GAD67 expression (12).
Recent studies suggest that an alternative approach that may increase the expression of reelin, GAD67, or other gene promoters that are decreased in SZ is to administer drugs such as VPA and other histone deacetylase inhibitors (i.e., MS–275, trichostatin A) that reduce DNA-methylation by inducing brain DNA demethylation (13–15). The possibility that promoter methylation could remain stable in GABAergic neurons because they lack DNA demethylase activity has been challenged recently (13). Evidence that the MET-induced hypermethylation of the reelin, GAD67, and other GABAergic promoters can be effectively reversed by VPA and other HDAC inhibitors (13–15) supports the concept that a putative brain DNA demethylase activity may be induced by drugs and that this activity could play a pivotal role in changing the promoter methylation patterns in GABAergic neurons.
Published clinical studies (16–18) suggest that drugs such as VPA are efficacious in the treatment of SZ and BP disorder when used in combination with typical or atypical antipsychotics. The symptomatic benefits elicited by a combination of VPA and antipsychotics in the treatment of SZ prompted us to study whether the targets of these drugs combinations could lead to specific chromatin remodeling changes via the activation of a DNA demethylase operative at selected (i.e., reelin and GAD67) promoters.
These experiments were carried out with typical and atypical antipsychotics including HAL, CLZ, SULP, and OLZ. HAL (butyrophenone), a typical antipsychotic, is active on the positive but not on the negative symptoms of SZ and is a potent D2 receptor blocker that causes a high liability of extrapyramidal side effects. CLZ (pyperazinyl-dibenzo-[1–4]-diazepine) and OLZ (pyperazinyl-[1–5]-benzodiazepine), two atypical antipsychotics with low efficacy on negative symptoms, at antipsychotic doses have weak antagonistic action on D2 receptors and therefore express low extrapyramidal side effect liability. SULP (aminosulfonyl-methoxybenzamide), an atypical antipsychotic that also reduces negative symptoms, at high doses may act as a D3/D2 receptor antagonist and may induce extrapyramidal side effects. In the present study, we report that fronto-cortical (FC) and striatal reelin and GAD67 promoter hypermethylation induced in mice by protracted (7 days) MET administration can be reversed by atypical (CLZ, SULP) antipsychotics given either alone or coadministered with VPA but not by HAL and OLZ given either alone or with VPA.
Taken together, these data suggest that coadministration of VPA and CLZ or VPA and SULP induce reelin and GAD67 promoter demethylation by activating a DNA demethylase expressed in selected populations of cortical or striatal GABAergic neurons but not in the liver.
Results
VPA Induces Reelin Promoter Demethylation.
We quantified the ratio of 5′ methylated cytosines (5mC) to the unmethylated cytosines of murine reelin (from −520 to −198 bp) or GAD67(−760 to −446 bp) CpG-enriched-promoter sequences. Using competitive PCR with genomic internal standards (14), we measured the fraction of reelin or GAD67 promoters immunoprecipitated by specific anti-MeCP2 or anti-5mC antibodies. The results reported in Table 1 show that in the FC of mice treated for 7 days with MET (5.2 mmol/kg s.c. twice a day, followed by 3 days of vehicle)—to induce reelin promoter hypermethylation—the ratios of methylated/unmethylated promoter, measured with the two above described methods, are virtually identical.
Table 1.
Comparison of cytosine methylation in a CpG island-enriched promoter region of reelin measured after immunoprecipitation with 5m-cytosine or MeCP2 antibodies
| Treatment |
Cytosine methylation of Reelin promoter, % of total |
|
|---|---|---|
| Antibody | 5m cytosine | MeCP2 |
| Control | 58 ± 3.0 | 62 ± 4.3 |
| VPA (0.5 mmol/kg s.c. twice a day for 3 days) | 35 ± 4.0* | 38 ± 2.6* |
| Clozapine (15 μmol/kg s.c. twice a day for 3 days) | 38 ± 5.2* | 37 ± 7.9* |
| Clozapine + VPA | 9.2 ≥ 2.5** | 8.2 ± 1.2** |
The ratio of methylated to total reelin promoter cytosines was determined in nucleosomes by measuring with PCR the fraction of reelin promoter immunoprecipitated with 5-methyl cytosine antibodies (left column) or MeCP2 antibodies (right column) vs the total reelin promoter (input) before immunoprecipitation. Control mice were treated for 7 days with MET (5.2 mmol/kg twice a day) followed by 3 days of treatment with vehicle. VPA and Clozapine were administered for 3 days after MET withdrawal. Each value is the mean ± SE of three mice. *, P < 0.05 when compared with control; **, P < 0.01 when compared with control. ANOVA followed by Bonferroni comparison.
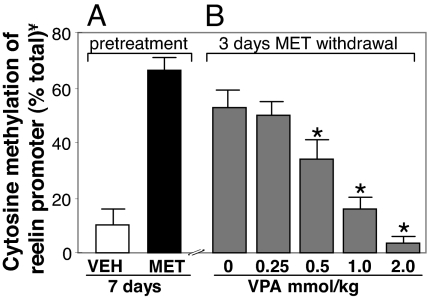
Fig. 1A shows that the ratio of methylated/unmethylated reelin promoter measured after MeCP2-ChIP in the FC of mice pretreated for 7 days with vehicle or MET is approximately 10% of total in vehicle-treated mice and rises to approximately 70% with MET treatment. The levels of reelin promoter methylation induced by 7 days of MET treatment decline slowly to reach 50% after 6 days of MET withdrawal (14) but, as shown in Fig. 1B, the reelin promoter remains elevated after 3 days of MET withdrawal. In contrast, if VPA is administered for 3 days after MET withdrawal, there is a dose-related decrease in reelin promoter hypermethylation. In the FC of mice, which received 0.5 mmol/kg of VPA (which is a dose expected to increase mouse brain levels of drug to an extent similar to that measured in the brains of patients that receive 0.1–0.3 mmol/kg of VPA, 10), the fraction of methylated reelin promoter is decreased by 30–35%. As shown in Table 1, these results were replicated using nucleosomal chromatin immuno-precipitation with either MeCP2 or 5mC antibodies. Hence we have used this dose of VPA to test whether the antipsychotics potentiate or reduce brain DNA demethylation induced by VPA. We previously reported that in mice treated with VPA, the magnitude of MeCP2 binding to the hypermethylated reelin promoter was approximately proportional to the number of methylated CpG dinucleotides measured with sodium bisulfite mapping (compare references 10 and 11).
Fig. 1.
VPA facilitates reelin promoter demethylation. (A) Mice were pretreated for 7 days with vehicle (open bar) or MET (5.2 mmol/kg s. c. twice a day) (filled bar) to induce hypermethylation of the reelin promoter. (B) After MET treatment termination, VPA (0 to 2.0 mmol/kg s. c. twice a day for 3 days) induces a dose-related decrease of reelin promoter methylation (shaded bars). Reelin promoter methylation was measured 2 h after the last injection of VPA. ¥, depicted are the ratios between the amount of reelin promoter fragment immunoprecipitated with MeCP2 antibodies (MeCP2-ChIP) and the amount of the corresponding promoter fragment in the initial non immunoprecipitated extract (input). The data represent the mean ± SE of 3 to 5 mice. *, P < 0.01 when the effect of VPA is compared with the group with no VPA. (One-way ANOVA followed by Bonferroni comparison).
Association of Antipsychotics with VPA Accelerates Reelin and GAD67 Promoter Demethylation.
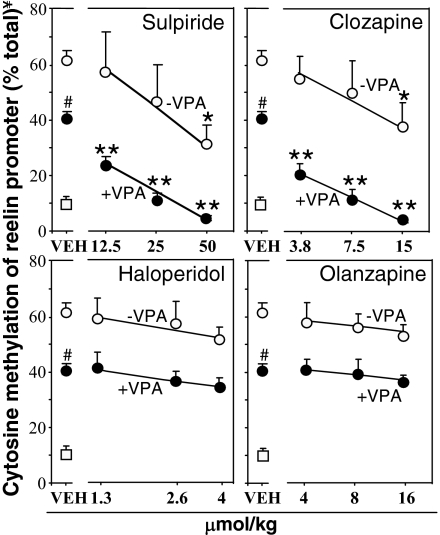
We next tested whether CLZ, SULP, OLZ, and HAL induce DNA demethylation per se or only when associated with VPA. As shown in Fig. 2, CLZ (3.8–15 μmol/kg s.c.) or SULP (12.5–50 μmol/kg s.c.) given twice a day for 3 days elicits a dose related increase of FC reelin promoter demethylation. Furthermore, at every dose studied, CLZ or SULP synergistically enhance reelin promoter demethylation elicited by a dose of VPA that per se only partially (30–35%) increases promoter demethylation (Fig. 2 and Table 1). Of note is that in mice that received VPA with CLZ (15 μmol/kg) or VPA with SULP (50 μmol/kg), the extent of methylated reelin promoter is below that measured in the FC of mice that never received a MET treatment (Fig. 2). In these experiments, reelin promoter methylation was measured 2 h after the last drug injection.
Fig. 2.
Clozapine and sulpiride alone or in combination with valproate (VPA) but not haloperidol or olanzapine induce reelin promoter demethylation in mouse FC. Mice were pretreated for 7 days with MET (5.2 mmol/kg s. c. twice a day for 7 days). After termination of MET treatment, various doses of clozapine, sulpiride, haloperidol, olanzapine, or vehicle alone or combined with VPA (0.5 mmol/kg s.c.) were administered twice a day for 3 days. Reelin promoter methylation was measured 2 h after the last injection. Open circles denote MET pretreated mice that did not receive VPA. Filled circles denote MET pretreated mice that received VPA. Open squares denote mice never treated with MET. In these mice, VPA (0.5 mmol/kg) failed to elicit a significant decrease of reelin promoter methylation. The data represent the mean ± SE of three animals per group. *, P < 0.05 when CLZ and SULP in absence of VPA were compared with the respective VEH-treated mice. **, P < 0.01 when sulpiride + VPA- and clozapine + VPA-treated mice were compared with VEH + VPA-treated mice. #, P < 0.05 when VEH +VPA-treated mice were compared with the respective VEH-treated mice. One-way ANOVA followed by Bonferroni comparison. ¥, Cytosine methylation at reelin promoter was measured as described in Fig. 1.
As opposed to CLZ and SULP, HAL (1.5–4 μmol/kg s. c.) and OLZ (4–16 μmol/kg s.c.) failed to induce statistically significant changes even when administered in combination with VPA (Fig. 2).
To elicit a marked reelin promoter demethylation, CLZ or a combination of CLZ and VPA must be given repeatedly (multiple injections for three days); in fact, reelin promoter demethylation was not increased 2 h after a single injection of CLZ (15 μmol/kg s.c.) or a single coadministration of VPA (0.5 mmol/kg s.c.) plus CLZ (15 μmol/kg s.c.). For example, after a single injection, the percentage of reelin promoter methylation is 62 ± 5 in vehicle, 59 ± 8 in CLZ alone, and 47 ± 13 in VPA+CLZ treated mice (n = 3).
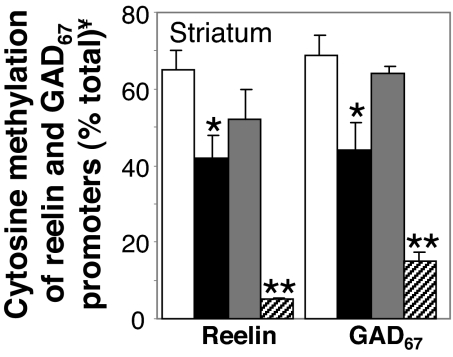
The association of VPA (0.5 mmol/kg s.c.) with CLZ (15 μmol/kg s.c. twice a day) at a dose schedule that induces reelin promoter demethylation in FC, also exerts a similar effect in the striatum (Fig. 3). We used this brain area because its GABAergic neurons are a possible target for the beneficial action of typical neuroleptics in psychosis and striatum expresses an almost homogeneous population (≈90%) of GABAergic medium spiny neurons that include reelin. A demethylation of GAD67 promoter similar to that expressed by the reelin promoter also occurs in these cells (Fig. 3).
Fig. 3.
Clozapine (CLZ) in combination with valproate (VPA) induces reelin and GAD67 promoter demethylation in mouse striatum. Mice were pretreated for 7 days with MET (5.2 mmol/kg s.c. twice a day for 7 days). After termination of MET treatment, vehicle (open bars), VPA (0.5 mmol/kg; filled bars), CLZ (15 μmol/kg, shaded bars), or a combination of CLZ and VPA (striped bars) were administered s.c. twice a day for 3 days. Reelin or GAD67 promoter methylation was measured 2 h after the last injection. The data represent the mean ± SE of 3 animals per group. *, P < 0.05 when VPA treated mice were compared with VEH treated mice; **, P < 0.01 when CLZ + VPA treataed mice were compared with VEH + VPA treated mice. One-way ANOVA followed by Bonferroni comparison. ¥, cytosine methylation of reelin and GAD67 promoters was determined as described in Fig. 1.
Reelin Promoter Demethylation Induced by Coadministration of VPA and CLZ Is Brain Selective.
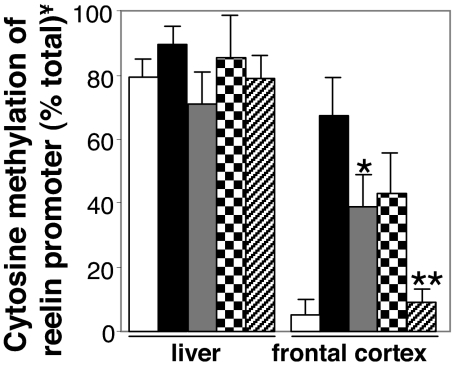
In the liver, the reelin promoter is constitutively hypermethylated (>80% of the cytosine sites are methylated), and in fact, its degree of methylation is not significantly increased by MET administration (Fig. 4). Moreover, in the liver, the methylation of reelin promoter cytosines failed to be lowered by the coadministration of VPA (0.5 mmol/kg twice a day for 3 days) or CLZ (15 μmol/kg twice a day for 3 days) at doses that dramatically decrease reelin promoter methylation in the FC of the same mice (Fig. 4).
Fig. 4.
Clozapine alone or in combination with valproate (VPA) induces reelin promoter demethylation in mouse FC but not in liver. Mice were pretreated for 7 days with vehicle (open bars) or MET (5.2 mmol/kg twice a day) (filled bars) followed by 3 days with vehicle. After termination of MET treatment, VPA (0.5 mmol/kg, shaded bar), CLZ (15 μmol/kg, checkered bars), or a combination of CLZ with VPA (strip bars) were administered s.c. twice a day for 3 days. Reelin promoter methylation was measured 2 h after the last injection. The data represent the mean ± SE of three animals per group. *, P < 0.05 when CLZ treated mice were compared with the respective VEH-treated mice. **, P < 0.01 when CLZ + VPA-treated mice were compared with either VEH- or VEH+ VPA-treated mice. One-way ANOVA followed by Bonferroni comparison. ¥, Cytosine methylation of reelin promoter was determined as described in Fig. 1.
SAM Levels in FC Are not Modified by Coadministration of VPA and CLZ.
To establish whether coadministration of VPA and CLZ in mice may indirectly reduce reelin or GAD67 promoter methylation by reducing SAM brain levels, we measured the FC levels of this methyl donor. MET treatment produces a significant increase in FC levels of SAM 2 h after the last MET injection (Table 2). Three days after MET withdrawal, mice pretreated for seven days with MET, express levels of SAM similar to those of the vehicle group (Table 2). Moreover, SAM levels remained unchanged after VPA and CLZ coadministration (Table 2).
Table 2.
Coadministration of VPA and Clozapine (CLZ) fail to alter the levels of SAM (S-adenosyl methionine) and SAH (S-adenosyl homocysteine) in FC of mice receiving MET
| Pretreatment(7 days) | Posttreatment(3 days) | SAM, pmol per mg of protein | SAH, pmol per mg of protein |
|---|---|---|---|
| Vehicle* | Vehicle† | 309 ± 21 | 45 ± 2.0 |
| MET‡ | – | 550§ ± 50 | 69§ ± 7.0 |
| MET‡ | Vehicle† | 375 ± 35 | 48 ± 3.5 |
| MET‡ | VPA + CLZ¶ | 387 ± 27 | 50 ± 4.0 |
Each value is the mean ± SE of three mice.
*Vehicle, twice a day s.c. for 7 days.
†Vehicle (twice a day s.c. for three days following MET treatment termination.
‡MET, 5.2 mmol/kg s.c. twice a day.
§P < 0.05 when compared with vehicle treated mice.
¶VPA (0.5 mmol/kg s.c.) + CLZ (15 μ mol/ kg s.c.) twice a day for three days following MET treatment termination.
Histone-3 Lysine (H3-K) Acetylation at Reelin Promoter in FC Is Selectively Increased After Treatment with VPA Associated with Either CLZ or SULP.
We reported that in the FC of VPA-treated mice, the increase of reelin and GAD67 promoter demethylation occurs with a parallel increase of acetylated H3-K9,14 (10, 14).
To test whether levels of acetylated H3-K9,14 increase in parallel with the enhancement of reelin promoter demethylation activity elicited by CLZ and SULP, acetylation of H3-K9,14 at reelin promoter was measured 2 h after the last injection of a 3-day treatment with CLZ, SULP, HAL, or OLZ, alone or in combination with VPA. As expected, Fig. 5 shows that VPA (0.5 mmol/kg s.c. twice a day for 3 days) elicits a 30–40% increase of acetylated H3-K9,14 levels associated with FC reelin promoter. CLZ and SULP injected alone at the highest doses also produce a small increase of acetylated H3-K9,14 levels. However, when CLZ and SULP are associated with VPA there is a synergistic effect with a twofold increase in the reelin associated with the acetylated H3-K9,14. This increase parallels the increase of reelin promoter demethylation (compare Figs. 2 and 5). Notably, HAL or OLZ, even at relatively large doses, were without an effect similar to that described for CLZ and SULP.
Fig. 5.
Clozapine and sulpiride increase acetylated H3K9,14 FC levels at reelin promoter in VPA treated mice. The FCs of the same animals used in Fig. 2 were analyzed in this experiment. Open circles denote mice that did not receive VPA. Filled circles denote mice that received VPA. The data represent the mean ± SE of three animals per group. *, P < 0.05 when SULP and CLZ without VPA were compared with the respective VEH-treated mice. **, P < 0.0 2 when SULP + VPA- and CLZ + VPA-treated mice were compared with VEH + VPA-treated mice. #, P < 0.05 when VEH +VPA-treated mice were compared with VEH treated-mice that did not receive VPA. Filled circles, one-way ANOVA followed by Bonferroni comparison.
Reelin Promoter Demethylation.
To examine the nuclear mechanisms operative in the induction of reelin promoter demethylation elicited by the coadministration of VPA and CLZ, we measured reelin promoter demethylation in FC nuclear extracts of mice receiving VPA (0.5 mmol/kg twice a day for 3 days), CLZ alone (15 μmol/kg twice a day for 3 days), or a combination of the treatment of VPA with CLZ.
As shown in Table 3, nuclear extracts obtained from the FC of mice receiving VPA have a significantly higher intensity of reelin promoter demethylation than nuclear extracts from mice receiving only vehicle. Moreover CLZ, in a dose that per se has a modest effect, elicits a synergistic increase of DNA demethylation when associated with VPA. In the assay reported in Table 3, demethylase activity was measured with subsaturating concentrations (0.1 nM) of methylated reelin promoter.
Table 3.
Clozapine in combination with VPA† increases nuclear DNA-demethylase activity in the mouse frontal cortex
| Treatment | 3H3C-CG-reelin promoter demethylation, pmol per milligram of protein per h |
|---|---|
| Vehicle | 0.93 ± 0.063 |
| VPA (0.5 mmol/kg, twice a day for 3 days) | 2.3 ± 0.29* |
| CLZ(15 μmol/kg, twice a day for 3 days) | 1.6 ± 0.074 |
| VPA + CLZ | 5.3 ± 0.17** |
Mouse FC nuclear extracts were incubated with 0.1nM of 3H3C-CG-reelin promoter (-693 to +141 bp) for 20 min. Each value is the mean ± SE of three mice. * P < 0.05 vs. vehicle; **, P < 0.01 vs. control (Student's t test).
†The last injection of VPA+CLZ was administered 2 h before measurement.
Kinetic studies using from 0.05 to 20 nM of a methylated reelin promoter fragment show that the apparent increase in promoter demethylation elicited by coadministration of VPA and CLZ is due to a twofold affinity shift of the enzyme for the methylated CpG reelin promoter (from 8.7 ± 0.55 in vehicle-treated to 3.9 ± 0.23 nM in VPA+CLZ-treated mice; P = 0.001, n = 3) whereas the maximal velocity is unchanged. These data suggest that the increase of nuclear DNA demethylation induced by coadministration of antipsychotics and VPA is due to an activation of preexisting enzyme molecules. This supports the conclusion that the therapeutic action of antipsychotics may have a nuclear target. Because CLZ added in vitro to the nuclear extract up to a concentration of 10 μM or VPA add up to a concentration of 0.05 mM or the combination of 10 μM CLZ + 0.05 mM VPA failed to change DNA demethylation, one may infer that CLZ action requires the preservation of the nuclear structure.
Discussion
The present study shows that CLZ and SULP, but not HAL and OLZ, administered in mice alone or in association with VPA, in clinically relevant doses for 3 days, down-regulate reelin and GAD67 promoter methylation in FC and striatum but not in liver. Moreover, in the mouse FC, the combination of clinically relevant doses of VPA and CLZ or that of VPA and SULP produces a synergistic rather than an additive increase of reelin and GAD67 promoter demethylation.
Furthermore, direct measurements of reelin promoter demethylation in nuclear extracts obtained from the FC of mice treated with VPA, CLZ, and VPA with CLZ suggest that the down-regulation of reelin promoter methylation elicited by these drugs can be attributed to an increase of a nuclear DNA demethylase activity.
The methylated domain DNA binding protein 2 (MBD2) has been associated with demethylase activity in replicating cancer cells (19), however, unveiling the biochemical nature of promoter demethylation in terminally differentiated neurons requires further investigation.
To establish whether coadministration of VPA and antipsychotics elicit their effects on reelin and GAD67 promoter methylation in GABAergic neurons, where these two genes are selectively expressed (7, 8), we used the striatum because in this brain area >90% of the expressed neurons are GABAergic. In the striatum, like in FC, VPA and antipsychotics synergistically activate reelin and GAD67 promoter demethylation. The similarities between FC and striatum and the differences of both tissues from liver further suggest that DNA demethylation induced by the coadministration of VPA and CLZ or VPA and SULP is a process that occurs in selected populations of telencephalic GABAergic neurons. Hence, it may provide a functional modulation of GABAergic tone that is decreased in psychosis (20).
In interpreting the molecular mechanisms operative in the increase of DNA demethylation activity by CLZ and SULP a primary action on D2 receptors can be excluded because HAL, which is a potent D2 receptor antagonist, fails to induce DNA demethylation activity whereas CLZ that at the doses used in clinical practice virtually fails to act as D2 receptor antagonist, may act on DNA demethylation particularly in association with VPA.
The present studies suggest that a combination of VPA with specific antipsychotics may induce DNA demethylation in GABAergic neurons by increasing the affinity of this enzyme for the methylated reelin and perhaps also for the GAD67 promoters.
Interestingly, CLZ and SULP in doses that per se increase reelin or GAD67 promoter demethylation also increase H3-K acetylation at the reelin promoter. Coadministration of VPA and CLZ or VPA and SULP synergistically up-regulates DNA demethylation activity (Fig. 2) and in FC also induces a synergistic nuclear H3-K hyperacetylation at the reelin promoter (Fig. 5). The synergistic interaction of VPA and CLZ or SULP suggests that the mechanism by which VPA (an HDAC inhibitor) increases H3-K acetylation may be different from the mechanisms used by CLZ or SULP. For SULP one must consider that we are dealing with a benzamide and some benzamides (i.e., MS-275) are potent inhibitors of selective classes of brain HDACs (21). However, it is difficult to relate the structure of CLZ to that of any known HDAC inhibitor. Perhaps, the reduction of MeCP2 binding elicited by CLZ may explain the increase in histone acetylation at reelin promoter because MeCP2 recruits HDACs while the reduced MeCP2 occupancy results in reduced HDAC binding. Alternatively it may be possible to consider whether a nuclear action of CLZ via H3-K covalent modification is mediated by a facilitation of a histone acetyl transferase or an activation of a mixed-lineage leukemia-1 (MLL-1) histone methyltransferase (22).
A limitation of the present interpretation of the synergistic effects of VPA with CLZ or VPA with SULP is the lack of a measure of the brain levels of these antipsychotics when they are coadministered with VPA. However, we do not believe that the results reported above are due to pharmacokinetic interactions with elevated CLZ or SULP levels. In fact, clinical studies in patients treated with VPA show that the levels of CLZ or other antipsychotics in plasma are similar to those measured in patients receiving an antipsychotic monotherapy (18, 23).
Conclusions
VPA has been associated for over a decade with atypical antipsychotics in the treatment of cognitive deficits and anxiety that are two frequent symptoms expressed by SZ and BP disorder patients (16–18). It has been suggested that when VPA is used in combination with atypical antipsychotics, this cotreatment leads to an earlier and more pronounced improvement of psychotic symptoms as compared with an antipsychotic monotherapy (16–18). The data presented here suggest that the coadministration of VPA with atypical antipsychotics may correct an altered nuclear epigenetic function operative in selected populations of cortical GABAergic neurons of SZ and BP disorder patients. In fact, both VPA and CLZ acting at histone tail lysines through acetylation or methylation have been reported to increase GAD67 mRNA and protein expression in the frontal cortex of rodents (10, 11, 22).
Our working hypothesis is that the epigenetic mechanisms operative in the pathophysiology of SZ and BP disorder may include a dysfunction of DNA demethylation in GABAergic neurons of these patients. Moreover, the induction of DNA demethylation by a specific class of antipsychotics may mediate the promoter hypomethylation of vulnerable GABAergic genes that are down-regulated in psychosis up-regulating their expression and thereby relieving the characteristic GABAergic pathologies of SZ and BP disorder.
Materials and Methods
Animals and Drug Administration.
Adult male Swiss Albino mice (Harlan, Indianapolis, IN), of 20–22 g of body weight, were treated with vehicle (VEH), MET (Sigma), VPA (Sigma), CLZ (Sandoz Pharmaceuticals), SULP (Sigma), HAL (Sigma), and OLZ (Lilly), alone or in combination as described in the Results and figure legends. MET and VPA were dissolved in H2O (0.1 ml/20 g of body weight). CLZ, SULP, HAL, and OLZ were dissolved with a drop of glacial acetic acid brought to pH 6 with the addition of NaOH.
ChIP Assays.
MeCP2 and acetyl H-3 ChIP assay.
Approximately 10 mg of FC, striatum, or liver tissue were used for this procedure. Details of the method have been reported (11, 14). Immunoprecipitation was performed overnight at 4 °C by the addition of 10 μg of MeCP2 or acetyl H3 antibody (Upstate Cell Signaling) to the sonicated solution. We previously reported (14) that on the western blot the MeCP2 antibody recognizes only a major band of protein corresponding to a molecular mass of approximately 75 kDa.
5-methyl CpG ChIP assays.
Genomic DNA from 10 mg of FC tissue was extracted using the Qiagen DNeasy Kit. After sonication to fragment DNA into nucleosomes, 4 μg of genomic DNA was incubated with 5-methyl cytosine monoclonal mouse antibody (Diagenode) overnight at 4°C. The DNA-antibody complex was then harvested using protein A/G agarose beads (BioVision). The DNA precipitated by the antibody was purified with proteinase K, followed by phenol/chloroform extraction and ethanol precipitation. The DNA pellet was resuspended in 10 mM Tris·HCl (pH 8.5) and used for PCR.
Measurements of Reelin and GAD67 Promoter Fragments by Quantitative Competitive PCR.
To quantify reelin and GAD67 promoters by PCR analyses, we used previously designed primers and colinear internal standards with deleted sequences (11, 14).
DNA Demethylase Activity.
Nuclear Extracts.
All procedures were performed at 4°C. Approximately 30–40 mg of tissue was homogenized in 200 μl of buffer containing 0.32 M sucrose, 0.05 M Tris·HCl, 0.1 mM EDTA, and 1 mM PMSF, pH 7.4. After centrifugation at 1000 × g for 10 min, the supernatant was removed and the pellet was resuspended in a buffer containing 0.15 M NaCl, 0.05 M Tris·HCl, 0.1 mM EDTA, and 0.1% Triton X-100 (Sigma), pH 7.4. The mixture was kept on ice for 30 min before centrifugation at 20,000 × g for 20 min. The supernatant was transferred into a new tube for demethylating activity assay.
Preparation of 3H3C-CG-reelin Promoter.
Recombinant mouse reelin promoter fragment (−692 bp to +141 bp) was subcloned into plasmid pCpGL (25). This reelin promoter sequence is functionally active in driving reporter expression (unpublished results). The promoter was subjected to 5-cytosine methylation using S-adenosyl [methyl-H3] methionine (3H-SAM) (Amersham) and SssI DNA methyltransferase (BioLabs). The pCpGL plasmid (3,872 bp) is a CpG-free luciferase reporter vector and the reporter portion is not methylated by SssI DNA methyltransferase (25). Hence, we are using it as a carrier for the methylated reelin promoter fragment. The methylation reaction mixture contains 35 pmol reelin promoter fragment, 100 μCi 3H-SAM (SA: 81 Ci/mmol), and 200 units SssI, in 200 μl of buffer (50 mM NaCl, 10 mM Tris, 10 mM MgCl, and 1 mM DTT, pH 7.9). Incubation was carried out for 2 h at 37°C. The pCpGL plasmid containing the methylated reelin promoter fragment was extracted with phenol/chloroform followed by ethanol precipitation. The precipitate was washed with 70% ethanol and resuspended in 200 μl of Tris-EDTA buffer, pH 7.5. Approximately 1 μCi 3H3C (12.5 pmol) were incorporated into 1 pmol of the CpG reelin promoter. The extent of reelin promoter methylation was evaluated using HpaII (New England BioLabs) digestion. No major digested bands were observed on 3% agarose gel electrophoresis in the methylated reelin promoter, indicating that almost complete methylation occurs [supporting information (SI) Fig. S1, lane 2].
Demethylation reaction.
The conditions for the demethylation reaction such as pH, temperature, and incubation time were optimized. The reaction was carried out as described before (26) with minor modifications: 37°C for 20 min in 200 μl of buffer (pH 7.5) containing 0.1 M imidazole, 0.5 mM DTT, 20 mM EDTA, 100 nM aurintricarboxylic acid (ATA, DNase inhibitor), different concentrations (0.1 to 20 nM) of 3H-methylated reelin promoter, and 0.1 to 0.25 mg of nuclear extract proteins. At the end of the incubation period, the 3H-methylated reelin promoter was extracted using phenol/chloroform and precipitated with ethanol. The pellet was dried and the radioactivity incorporated in reelin promoter was counted in a scintillation counter. To test whether demethylation occurs as a consequence of an enzymatic reaction, the effect of heat (100°C for 5 min) and proteinase K treatment on nuclear extracts were performed. Both preheated or proteinase K-treated nuclear extracts failed to show demethylating activity on the methylated substrate. Finally, to test whether a contaminating DNase activity in the nuclear extracts might contribute to the degradation of the reelin promoter fragment, 20 pmol of the methylated reelin promoter/plasmid DNA complex were incubated for 4 h in a volume of 0.2 ml with or without 0.25 mg of nuclear extract protein in the presence or absence of 100 nM the DNase inhibitor ATA. Despite an almost complete demethylation, no signs of DNA degradation were observed using a) 3% agarose gel electrophoresis (Fig. S1), b) PCR assay of the amount of residual reelin promoter fragment, c) measurement by HPLC of 2,-deoxyribonucleotide 5, monophosphates (27) remaining in the reelin promoter fragment after incubation with nuclear extracts, and d) digestion by HpaII after incubation of the hypermethylated reelin promoter/plasmid with nuclear extract (Fig. S1).
Statistical Analysis.
All results were expressed as mean ± SE. Student's t test and one-way ANOVA followed by the Bonferroni multiple comparison test were used to assess the significance of the differences between groups. The criterion for significance was P < 0.05 or 0.01. The criterion for significance was adjusted for multiple tests on the same dataset.
Supplementary Material
Acknowledgments.
We thank Dr. S. Akbarian (Department of Psychiatry, University of Massachusetts, Worcester, MA), and Dr. M. Szyf (Department of Pharmacology and Therapeutics, McGill University, Montreal, Canada) for constructive criticism and suggestions in the preparation of the manuscript. This work was supported in part by National Institute of Mental Health Grants MH071667 (to E.C.), MH070855 (to A.G.), and MH62682 (to D.R.G.).
Footnotes
The authors declare no conflict of interest.
We found that, in MET-treated mice (5.2 mmol/kg twice a day for 7 days), ≈5% of the total gene promoters are hypermethylated when measured by DNA methylation microarrays (NimbleGen, 5mC -ChIP-chip) (unpublished data).
This article contains supporting information online at www.pnas.org/cgi/content/full/0805493105/DCSupplemental.
References
- 1.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;562:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 3.Guidotti A, et al. Decreased reelin and glutamic acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorders: A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 4.Woo T-U W, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-d-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 5.Mill J, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayson DR, et al. The human reelin gene: Transcription factors (+), repressors (-) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther. 2006;111:272–286. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Veldic M, et al. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruzicka WB, et al. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt RJ, Benedict A, Davis J. Biochemical and sleep studies of schizophrenia: A review of the literature 1960–1970. Schiz Bull. 1971;4:10–44. [Google Scholar]
- 10.Tremolizzo L, et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 11.Dong E, et al. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci USA. 2005;102:12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa E, et al. Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics. 2007;2:29–36. doi: 10.4161/epi.2.1.4063. [DOI] [PubMed] [Google Scholar]
- 13.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 14.Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci USA. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassef AA, et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J Clin Psychopharmacol. 2000;20:357–361. doi: 10.1097/00004714-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Citrome L, et al. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatr Serv. 2004;55:290–294. doi: 10.1176/appi.ps.55.3.290. [DOI] [PubMed] [Google Scholar]
- 18.Kelly DL, et al. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatr Q. 2006;77:81–95. doi: 10.1007/s11126-006-7963-9. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M. A mammalian protein with specific demethylase activity for mCpG DNA. Nature. 1999;397:579–583. doi: 10.1038/17533. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti A, et al. GABAergic dysfunction in schizophrenia: New treatment strategies on the horizon. Psychopharmacology (Berlin) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 21.Simonini MV, et al. The benzamide MS-275 is a potent, long-lasting brain region-selective inhibitor of histone deacetylases. Proc Natl Acad Sci USA. 2006;103:1587–1592. doi: 10.1073/pnas.0510341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang HS, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer MC, Baldessarini RJ, Goff DC, Centorrino F. Clinically significant interactions of psychotropic agents with antipsychotic drugs. Drug Saf. 1996;15:333–346. doi: 10.2165/00002018-199615050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Chertkow Y, Weinreb O, Youdim MB, Silver The effect of chronic co-administration of fluvoxamine and haloperidol compared to clozapine on the GABA system in the rat frontal cortex. Int J Neuropsychopharmacol. 2006;9:287–296. doi: 10.1017/S1461145705005626. [DOI] [PubMed] [Google Scholar]
- 25.Klug M, Rehli M. Functional analysis of promoter CpG methylation using a CpG-free luciferase reporter vector. Epigenetics. 2006;1:127–130. doi: 10.4161/epi.1.3.3327. [DOI] [PubMed] [Google Scholar]
- 26.Gjerset RA, Martin DW., Jr Presence of a DNA demethylating activity in the nucleus of murine erythroleukemic cells. J Biol Chem. 1982;257:8581–8583. [PubMed] [Google Scholar]
- 27.Ramsahoye BH. Measurement of genome wide DNA methylation by reversed-phase high-performance liquid chromatography. Methods. 2002;27:156–161. doi: 10.1016/s1046-2023(02)00069-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.