Abstract
Human autoimmune (AI) diseases are difficult to treat, because immunosuppressive drugs are nonspecific, produce high levels of adverse effects, and are not based on mechanistic understanding of disease. Destroying the rare autoreactive T lymphocytes causing AI diseases would improve treatment. In animal models, TNF selectively kills autoreactive T cells, thereby hampering disease onset or progression. Here, we seek to determine, in fresh human blood, whether TNF or agonists of TNF selectively kill autoreactive T cells, while sparing normal T cells. We isolated highly pure CD4 or CD8 T cells from patients with type 1 diabetes (n = 675), other AI diseases, and healthy controls (n = 512). Using two cell death assays, we found that a subpopulation of CD8, but not CD4, T cells in patients' blood was vulnerable to TNF or TNF agonist-induced death. One agonist for the TNFR2 receptor exhibited a dose-response pattern of killing. In type 1 diabetes, the subpopulation of T cells susceptible to TNF or TNFR2 agonist-induced death was traced specifically to autoreactive T cells to insulin, a known autoantigen. Other activated and memory T cell populations were resistant to TNF-triggered death. This study shows that autoreactive T cells, although rare, can be selectively destroyed in isolated human blood. TNF and a TNFR2 agonist may offer highly targeted therapies, with the latter likely to be less systemically toxic.
Keywords: agonist antibodies, autoimmunity, apoptosis, TNFR2, Crohn's
In animal models, the immunoregulatory cytokine TNF has been successfully use directly or indirectly as treatment for several autoimmune (AI) diseases (1–4). TNF augmentation also might be beneficial for humans with autoimmunity, based on indirect evidence from genetic and functional studies that suggests possible deficiencies in patients' TNF levels or signaling pathways (4). Using human blood specimens, we test addition of TNF, or more selective agonists of TNF, for their capacity to kill only autoreactive T cells in several AI diseases, while sparing normal T cells.
TNF is a vital cytokine in diverse immune responses, including enhanced cell survival or apoptosis (5, 6). TNF achieves these effects via distinct signaling pathways through two membrane receptors, TNFR1 (CD120a; p55/60) and TNFR2 (CD120b; p75/80). TNFR1 is ubiquitously expressed on all T cell populations, the entire lymphoid system, and most other cells. This explains TNF's systemic toxicity when used at high doses in oncology treatment and in diseases with normal TNF basal levels (7, 8). TNFR2, in contrast, is more restrictively expressed, found only on select subpopulations of T cells, endothelial cells, neurons, and other occasional cells (9). Here, we take advantage of the apoptotic role of TNF and the differential patterns of TNFR expression to test a targeted method of administering TNF and several agonists to selectively kill autoreactive T cells. This approach opens the way for a novel and more targeted strategy to treat autoimmunity.
Research over recent decades has been devoted to understanding the TNF receptor pathway and its normal role in T cell survival or in apoptosis to curb growth of activated T cell subpopulations once the immune response has succeeded. Upon TNF exposure, normal T cells rapidly activate NFκB, a transcription factor that leads to their survival. NFκB in the cytoplasm is part of a larger protein complex that requires both direct and indirect proteasome processing and ubiquination of inhibitory subunits for successful transport to the nucleus to promote transcription of cell survival genes. When chemicals or mutations block NFκB activation in vitro, TNF signaling selectively triggers cell death via apoptosis, especially in activated T cells, which depend on this induced pathway (10, 11).
Defects in TNF signaling, most likely in lymphocyte subpopulations, are common across several AI diseases in both animals and humans (12–24). The defects, whether genetic and/or functional in origin, affect activation of NFκB. The first described defect was in the AI-prone NOD mouse, a model of spontaneous diabetes and Sjogren's syndrome. In this model, the LMP2 proteasome subunit protein expression decreases as mice progress toward disease. The missing proteasome LMP2 subunit blocks the activation of the NFκB signaling complex, preventing it from entering the nucleus to activate prosurvival genes (16). Proteasome blockage also leads to TNF mRNA instability, thereby lowering levels of TNF translation (17). Remarkably, this same proteasome subunit protein is missing in one form of human AI disease, Sjogren's syndrome (18). Genetic and protein processing defects in the NFκB pathway are also found in humans with lupus, Crohn's, rheumatoid arthritis, and ulcerative colitis (13, 19–21). These findings, taken together, suggest that exogenous TNF prevents entry of unprocessed NFκB into the nucleus, thereby leading to TNF-induced apoptosis of autoreactive T cells.
To explore TNF's role as a selective killer of autoreactive CD8 T cells in humans, we studied blood specimens from AI patients. We exposed TNF, and agonists that mimicked TNF's function, to T cells isolated from the specimens. One TNF agonist was selective for TNFR1, whereas others were agonists for TNFR2. We sought to answer several questions. Can TNF or its agonists induce cell death in the rare subpopulation of T cells that are autoreactive? Is TNF-induced death restricted only to autoreactive cells, but not to other subpopulations of activated T cells? Can TNF be shown in culture to kill those autoreactive CD8 T cells specific to a given autoantigen? Finally, in pursuit of a less toxic therapeutic approach, we ask whether autoreactive T cells also can be killed by an agonist for a single TNF receptor, such as TNFR2, which is more limited in its cellular distribution than is TNFR1.
Results
Quality Blood Preparations Improve Ability to Track the Death of Diabetic T Cells with TNF Exposures.
For >40 years, human blood lymphocytes for various studies have been obtained with Ficoll separated peripheral blood lymphocytes (PBLs). These methods were used in this study. We show that, in 387 diabetic and matched control samples prepared by Ficoll, TNF killed by apoptosis a significantly greater subpopulation of T cells (P = 0.003) in diabetic but not control samples. If fewer numbers of paired patient and control samples were studied, the trends were not detectable [see Results in supporting information (SI) Text and Table S1]. As frequently reported in the literature, Ficoll separated cells have poor viability, yield, and purity (Fig. S1).
To standardize T cell preparations from freshly drawn blood, we developed and applied nongradient separation methods. Direct positive selection of magnetically tagged CD4 or CD8 T cells yielded more viable cells that were purer and more representative of the original numbers of starting cells (Fig. S1C). T cell separation methods were further automated by using robotic platforms to allow high yields and consistently viable preparations. Fresh samples using solely magnetic separation preparations were >95% viable, >95% pure, and achieved >85% yield of the starting cells in blood. This contrasted with typical preparations of Ficoll-isolated PBLs with viability of 30–60%, poor purity, and yields of only 20–40% of starting samples. A series of 256 samples from both diabetics and controls showed reproducibility of separation methods (Fig. S1C). The magnetic separation methods were used thereafter, eliminating the need for large sample study sizes, because each sample was more representative and highly viable.
TNF Treatment Induces Death of a Subpopulation of CD8 but Not CD4 T Cells in AI Patients.
To determine whether freshly isolated preparations of human diabetic T cells were killed with TNF, we studied two types of purified and highly viable subpopulations of CD4 vs. CD8 T cells using two different cell death assays.
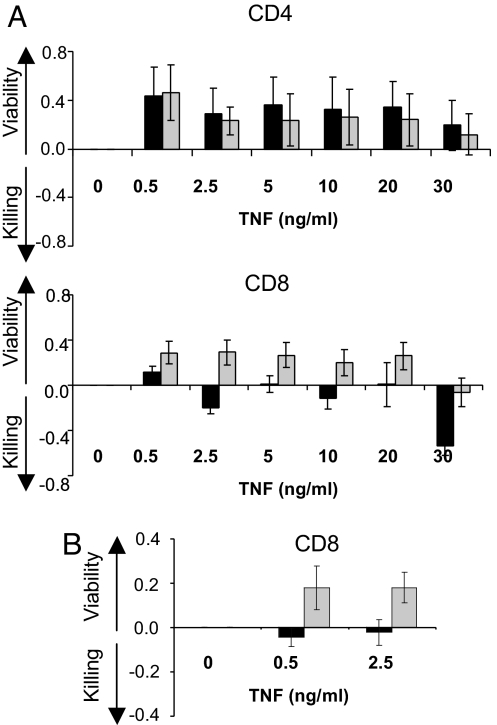
The first was the lactate dehydrogenase (LDH) assay, which measures cell death and cell proliferation via LDH, a product in the cytosol of damaged cells. In isolated CD4 T cells, no TNF-induced killing was observed in diabetic or control samples. TNF induced mild proliferation, but not cell death, in both patient and control cells (Fig. 1A). No significant differences were observed at all TNF doses, ranging from 0.5 to 30 ng/ml. Using the same assay, TNFs effect on CD8 cells was strikingly different. TNF killed diabetic CD8 T cells but not control CD8 T cells at all TNF doses (P = 0.08, 0.002, 0.02, 0.01, 0.018, and 0.001). Only 12 pairs of diabetic and control samples were needed to obtain significance (Fig. 1A). As reported in the literature, we too found TNF induced mild T cell proliferation, but only in control samples of purified CD8 T cells, not in CD8 diabetic cells at higher doses.
Fig. 1.
TNF treatment of purified human CD4 or CD8 T cells from type 1 diabetics (black bar) compared with controls (shaded bar) for viability vs. killing (A) TNF treatment of CD4 T cells (n = 9 pairs, Upper) or TNF treatment of CD8 T cells (n = 12 pairs, Lower), using the LDH assay. (B) TNF treatment of purified human CD8 T cells from larger samples (n = 23 pairs) of type 1 diabetics and controls, using the WST-1 assay.
As confirmation that TNF kills a subpopulation of diabetic CD8 T cells, an expanded study of 23 pairs of samples from diabetics and controls was examined by using the WST-1 assay, which measures cell proliferation directly but death indirectly. TNF at doses of 0.5 or 2.5 ng/ml induced mild proliferation of control CD8 T cells but death of diabetic CD8 T cells (P = 0.0029, 0.009) (Fig. 1B).
Although the target organs of AI diseases vary, diverse genetic errors in the TNF-signaling pathway are a unifying feature that may contribute to each disease (12–15, 22–24). Purified CD8 T cells from a range of AI patients were studied with the LDH assay. Because other AI patients have normal blood sugars, this study ruled out the impact of high blood sugars, a feature of diabetes that also influences TNF sensitivity. For all AI diseases examined, TNF-sensitive CD8 T cells were detected. TNF killed a subpopulation of CD8 T cells in patients with lupus, psoriasis, Crohn's disease, hypothyroidism, and multiple sclerosis (Fig. S2). TNF-induced killing was sufficiently robust that a single AI vs. control sample pair could be shown to exhibit a dose-response effect. These findings suggest that TNF-induced killing of a subpopulation of CD8 T cells extends to other AI diseases.
Some Crohn's and rheumatoid arthritis patients are treated with anti-TNF therapies such as Etanercept and Remicade, making it difficult to expand the blood to T cells unaltered by the standard of care. A side effect of anti-TNF therapies in 5–50% of Crohn's and rheumatoid arthritis patients is the development of new autoantibodies, which are sometimes followed by new onset AI diseases including systemic lupus, and type I diabetes, among others (4). We were fortunate to repeatedly study a Crohn's patient who, after 6 months of anti-TNF therapy, developed both new onset type 1 diabetes and systemic lupus. The T cells from this Crohn's patient, 1 year after cessation of anti-TNF therapy, showed TNF-induced death in a dose–response manner in the LDH assay (Fig. S2G). We were also fortunate to study a type 1 diabetic who, at the age of 40, developed rheumatoid arthritis but was not treated with anti-TNF therapy. As Fig. S2H shows, this AI patient who coexpressed both diseases similarly exhibited a dose–response in TNF-induced death of CD8 T cells.
TNFR2 Agonist Alone Kills a Subpopulation of CD8 T Cells from AI Patients.
TNF acts by binding to two cell surface receptors, TNFR1 and TNFR2, although the intracellular machinery associated with these receptors is dissimilar. One TNFR1 and three TNFR2 agonist antibodies were tested on purified CD8 T cells to determine whether stimulating each receptor alone could kill AI CD8 T cells with greater specificity.
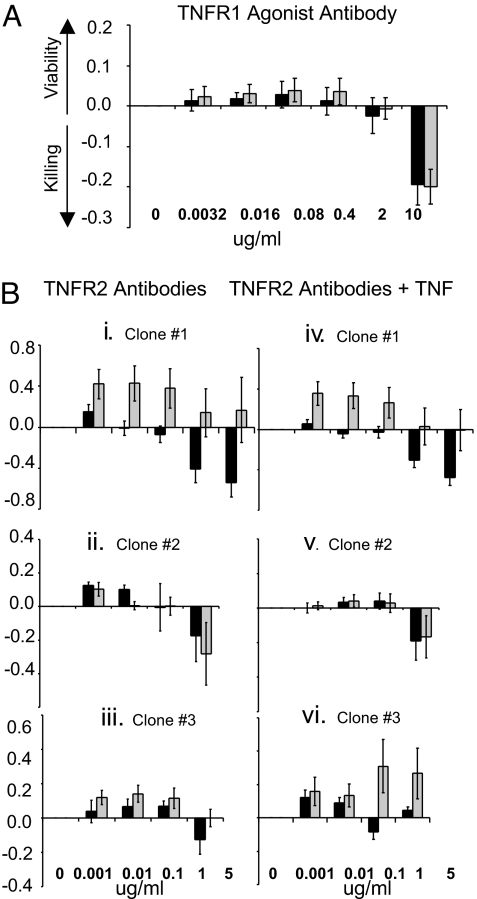
We first examined TNFR1 agonism on purified CD8 T cells from type 1 diabetics compared with controls. Using the LDH assay, TNFR1 agonism caused equally mild CD8 T cell proliferation in diabetic and control CD8 T cells. No significant differences in CD8 T cell responses were found over a range of agonist concentrations. The TNFR1 agonist was given to 11 matched pairs at doses of 0.0032, 0.016, 0.08, 0.40, and 2 μg/ml. P values were all >0.60 (Fig. 2A).
Fig. 2.
Effect of TNFR1 vs. TNFR2 agonist antibodies on death of Type 1 diabetic (dark bar) compared with control (shaded bar) CD8 T cells (A) TNFR1 agonist antibody treatment of purified human CD8 T cells from 11 pairs of type 1 diabetics and controls. (B) TNFR2 agonist treatment of purified human CD8 T cells without TNF (left column) or with TNF (right column) in type 1 diabetics compared with controls. (i) iv TNFR2 agonist clone #1 (n = 8 paired samples, Left; n = 8 paired samples, Right), (ii, v) TNFR2 agonist clone #2 (n = 5 sets of paired samples, Left; n = 5 paired samples, Right), (iii, vi) TNFR2 agonist clone #3 (n = 5 paired samples on Left; n = 5 paired samples on Right). Except for TNFR2 clone #1 (and high doses in Fig 2vi, as explained in the text), all P values were >0.05 with or without TNF.
Next, we examined three TNFR2 agonists and their ability to induce death in CD8 T cells from diabetic patients, using the LDH assay. TNFR2 agonist clone #1, at all concentrations, and with a sample size of only eight paired samples, induced CD8 cell death exclusively in diabetic samples (Fig. 2Bi). For the dose range tested, the P values were all significant, at 0.04, 0.02, 0.05, 0.04, and 0.03. Because some TNFR2 antibody agonists are known to be potentiated by addition of TNF, we also incubated the cells with TNF to observe the impact of bireceptor stimulation after applying the TNFR2 agonist (25). Fig. 2Biv shows that TNF neither potentiated nor inhibited the capacity of this TNFR2 agonist. The killing remained significantly greater in diabetic cells, with P values of 0.01, 0.01, 0.05, 0.04, and 0.01 over the dose range.
Two other TNFR2 agonists were screened with the LDH assay for their capacity to induce CD8 T death in diabetic cells. TNFR2 agonist clones #2 and #3 were ineffective at killing significantly more diabetic CD8 T cells. They showed no overall differences between diabetics and controls (Fig. 2B ii and iii) or differences with addition of TNF (Fig. 2B v and vi). The only exception was that agonist clone #3 stimulated proliferation of control CD8 T cells at the highest TNF concentrations more than the diabetic (Fig. 2Bvi).
Because TNFR2 agonist clone #1 effectively and specifically killed a subpopulation of diabetic CD8 T cells, at least as effectively as TNF, we used the WST-1 assay to replicate the finding on a larger sample (n = 51 matched pairs). As with the LDH assay, we found a gradual dose-related increase in killing of a subpopulation of diabetic CD8 cells. At agonist doses of 0.1, 0.5, and 1 μg/ml, P values were 0.99, 0.17, and 0.01 (Fig. S3). Agonist clone #1 also induced slight proliferation of control CD8 cells. Specific assays of cell death by apoptosis were also studied with TNFR2 agonism. The Caspase 3/7 assay, a luminescent assay of apoptosis in mammalian cells, was used with diabetic and control CD8 T cells. This assay, like the previously described data with AnnexinV apoptotic death of CD8 T cells with TNF, confirmed TNFR2 agonism clone #1 killed diabetic CD8 T cells by apoptosis. The Caspase 3/7 product is a transient noncumulative apoptotic product peaking at different times between 4 and 6 h in fresh T cells. The data show CD8 T cells from type 1 diabetics have a dose-related increase in TNFR2 agonist killing of CD8 T cells (Fig. S4)
Last, TNFR2 agonist clone #1 also killed CD8 T cells from patients with other AI diseases. Not only did this TNFR2 agonist kill CD8 T cells from diabetics, but it also killed CD8 T cells from patients with lupus, Graves', psoriasis, and multiple sclerosis (Fig. S5).
Autoreactive Diabetic CD8 T Cells to an Insulin Fragment Die with TNFR2 Agonism.
Autoreactive T cells in diabetics show cytotoxicity against self-peptides correctly presented through HLA class I alleles (26). For example, peptide-specific autoreactive T cells against the insulin B chain 10–18 have been identified in islet allograft recipients. During recurrent autoreactivity, T cells from blood, when coincubated with an insulin peptide loaded to a class I proteins, show reactivity (27). With our improved capacity to isolate pure, viable, and representative CD8 T cells from fresh blood, we investigated whether long-term diabetics harbor CD8 T cells with reactivity to the insulin peptide fragment in the HLA-A2 (*0201) allele. Those CD8 T cells would be indicative of autoreactive T cells.
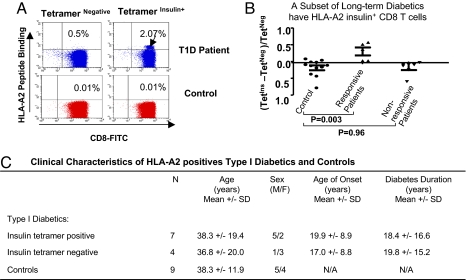
A long-term diabetic had detectable autoreactive CD8 T cells with binding to HLA-A2 insulin B10–18 compared with a HLA-A2 nondiabetic control (Fig. 3A). Neither nondiabetic nor diabetic CD8 T cells bound to the irrelevant antigen presented in the control tetramer reagent (tetramer-negative). Analyzing more patients and controls by the same methods, somewhat <50% of our HLA-A2+ long-term diabetics statitically showed insulin B chain 10–18 autoreactive CD8+ T cells (Fig. 3B) when large numbers of isolated and pure CD8 T cells were studied. An analysis of long-term diabetics with or without insulin tetramer reactive CD8 T cells compared with controls revealed near-perfect matching of the samples. Clinical characteristics, such as difference in age of onset or duration of diabetes, did not predict whether long-term diabetics possessed insulin tetramer reactive CD8 T cells. Indeed, long-term diabetics >10 years of disease could have the detectable presence of this subclone of autoreactive CD8 T cells (Fig. 3C). On average, long-term diabetics with insulin tetramer positive cells were 38.3 +/− 19.4 years and had diabetes for 18.4 +/− 16.6 years. All CD8 cells used for these studies were 95% viable and 95% pure and were representative, with a yield of at least 85% of the starting T cell population.
Fig. 3.
A subset of insulin autoreactive CD8 T cells can be identified in some long-term diabetics (A) Insulin B10–18 tetramer positive CD8 frequency in a highly pure preparation of CD8 T cells from a long-term diabetic (age of onset 13 years, duration of diabetes 8 years) and a paired control. Both patient and control were HLA-A2.1+. Representative two-color dot plots are presented. (B) Insulin B10–18 tetramer positive CD8 frequency in long-term type 1 diabetics (n = 11) both positive and negative patients and controls. (C) Clinical characteristics of HLA-A2 positive type 1 diabetics with and without insulin B10–18 tetramer staining compared with matched controls.
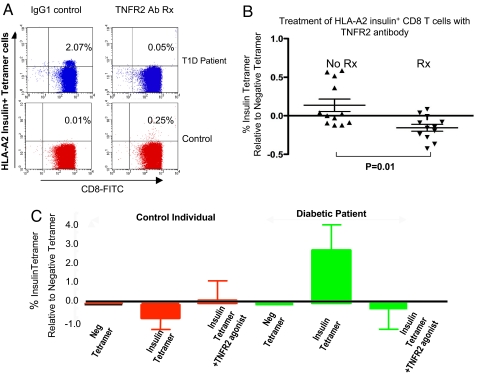
CD8 autoreactive T cells are thought to represent the cells sensitive to TNF-induced death in the earlier WST-1 and LDH assays. To further confirm and refine the ability of TNFR2 agonists or TNF to selectively kill autoreactive T cells, insulin tetramer T cell death was studied in vitro within the highly pure CD8 isolated cells from fresh blood. Fig. 4A shows that 6-hour treatment with the TNFR2 agonist in one diabetic specifically eliminated insulin autoreactive T cells. The study was expanded to more diabetics with varying numbers of autoreactive T cells displaying reactivity to insulin to the B chain 10–18 amino acids. Insulin-reactive CD8 T cells treated with brief exposure to the R2 agonist antibody consistently died (Fig. 4B). One insulin tetramer positive patient was studied on four separate occasions over a 3-year time span (Fig. 4C). This patient on each visit repeatedly showed the presence of autoreactive CD8 T cells to the insulin plus tetramer reagent. The patient's autoreactive cells repeatedly died in culture after low-dose exposure to the TNFR2 agonist. In contrast, cells from a control exposed to a TNFR2 agonist demonstrated mild proliferation, no false-positive insulin tetramer reactivity, and no death upon exposure to TNFR2. Further studies confirmed that TNF, like the TNFR2 agonist, could also killed insulin autoreactive T cells (Fig. S6).
Fig. 4.
Treatment of insulin autoreactive CD8 T cells with TNFR2 agonist clone #1 kills the pathogenic cells. (A) Targeted elimination of insulin B10–18 tetramer+ T cells with a 6-h treatment with TNFR2 agonist in a long-term diabetic compared with matched control T cells (B) Insulin B10–18 specific CD8 T cells from type 1 diabetics consistently decrease in culture when treated with TNFR2 agonist in culture for 6 h. (C) Repeat analysis of the same tetramer insulin positive long-term diabetic over a 3-year period repeatedly reveals the insulin autoreactive T cells. Autoreactive CD8 T cells can be repeatedly eliminated with a brief TNFR2 agonist exposure to diabetic cells.
Activated, but Not AI, CD8 T Cells from Diabetics Are Resistant to TNFR2 Agonism.
Because antigen-specific autoreactive T cells from diabetics die with TNF or TNFR2 agonism, it was important to establish whether other subpopulations of activated or memory T cells from diabetic patients were also susceptible to TNF-triggered death. As Fig. S7 shows in two different diabetics, neither EBV nor CMV-specific T cells from Type 1 diabetics die when exposed to TNFR2 agonism. In these flow cytotetric studies direct disappearance of tetramer positive CD8 T cells were monitored (Fig. S7A) and direct staining for dead cells with the death detection reagent called Sytox (Fig. S7B). These data confirmed non-AI but antigen-specific CD8 T cells are not susceptible to TNF agonism death. TNFR2 agonism therefore is targeted to death of only pathogenic T cells related to the diabetics.
Discussion
Destruction of rare autoreactive T cells in AI disease has been an elusive therapeutic goal designed to produce marked benefit over current treatments that are nonspecific and plagued by adverse effects. Here, we report that TNF exposure kills a subset of human CD8 T cells from type 1 diabetes and other AI diseases. In contrast, CD4 T cells from type 1 diabetics are resistant to TNF-triggered death. Death of this subpopulation of CD8 T cells is also triggered with a specific agonist for TNFR2 that mimics TNF's actions. TNFR1 agonists do not trigger cell death of diabetic autoreactive T cells. We also show that, in type 1 diabetes, a subpopulation of insulin-autoreactive CD8 T cells specific for the HLA class I insulin fragment die upon exposure to a TNFR2 agonist, confirming that the TNF pathway could be a target for new types of treatments. Activated CD8 T cells in diabetics to nonautoreactive peptides such as directed to CMV or EBV viral fragments, are resistant to TNF agonism, confirming the specificity of the TNF pathway as a targeted method of killing only autoreactive CD8 T cells.
An abundance of murine models establish the therapeutic benefit for administering TNF or TNF induction to animals with late-stage AI disease (1–4). Although many models have been proposed to account for TNF's therapeutic benefit, one candidate mechanism in mouse models is direct autoreactive T cell death (28). This current article broadly confirms that blood samples taken from humans with diverse AI diseases have sensitive autoreactive CD8 cells populations that selectively undergo apoptosis induced by TNF or a TNFR2 agonist.
The capacity of a TNFR2 agonist to kill autoreactive diabetic CD8 T cells has therapeutic implications for drug safety. Systemic TNF therapy for cancer patients, especially at high doses, can cause toxicity (7, 8). Although cancer patients may already have high TNF levels at the onset of therapy, perhaps accounting in part for the added toxicity, the fact remains the therapeutic window for TNF may be narrow. In contrast, systemic administration of a TNFR2 agonist to baboons, even at high doses, is associated with minimal toxicity (29). This most likely reflects more restricted distribution of the TNFR2 receptor, unlike the body-wide expression of TNFR1. In general, TNFR2 is expressed only on minor populations of lymphocytes like highly activated T cells, endothelial cells, and some subpopulations of neurons (9). This suggests that the use of TNFR2 agonism for selective T cell death might be further targeted by ensuring blood–brain barrier penetrance does not occur. Because we found that autoreactive CD8 T cells die only with TNFR2 stimulation, therapeutic use of TNFR2 agonist without TNFR1 agonism is likely to be less toxic than TNF.
Refinements in our isolation of human T cells made it feasible to study specific subpopulations of T cells with reliability. Traditional methods of separating lymphocytes with Ficoll gradients were labor-intensive and required very large sample sizes to verify pathogenic T cells in long-standing autoimmunity. Gradient separation methods yield only a small fraction of representative T cells of poor viability (30). For the majority of our studies, isolated CD8 T cells were obtained by magnetic cell separation methods, which eliminated traditional gradient separation processes. Our blood separation standardization procedures to retrieve representative T cell populations allowed fewer paired patient and control samples to be obtained and expedited the identification of T cells sensitive to TNF-triggered cell death. Indeed these refined isolation methods allowed insulin fragment autoreactive T cells to be detected routinely in patients with diabetes of >10 years duration. This last accomplishment was important to establish that the T cells vulnerable to TNF cell death were the rare autoreactive T cells.
Because our findings showing potential benefits of TNF or TNF agonism for treating AI, it seems paradoxical that anti-TNF therapies are a major therapeutic class of drugs currently marketed for AI. TNF antagonists have provided clinical benefit to about half of AI patients, those with rheumatoid arthritis and Crohn's disease. Yet an expanding body of research in animal models on spontaneous autoimmunity suggests the opposite strategy may be warranted (4). Furthermore, in humans, several clinical observations deserve mention. First, many Crohn's and rheumatoid arthritis patients never respond to TNF antagonists. Second, long-term treatment with anti-TNF drugs can be accompanied by onset of new or aggravated forms of autoimmunity, sometimes new autoantibodies, suggesting that, for some AIs, anti-TNF therapy may not be the drug of choice (4). Last, some AI diseases like multiple sclerosis worsen when treated with anti-TNF (31–34). Therefore, the new analysis here, combined with previously published animal and human studies, argue for the opposite therapeutic strategy for some AI diseases, namely to boost or restore TNF. Activation defects in the transcription factor NFκB, which is part of the TNF signaling pathway, may leave autoreactive T cells more vulnerable to TNF-induced apoptosis. Genetic findings indirectly support polymorphic diversity in this signaling pathway in human AI diseases. Overall, certainly type 1 diabetic patients and perhaps subsets of patients with other AI diseases could benefit from the targeted removal of autoreactive cells with TNF or TNFR2 agonism.
With chronic diseases such as diabetes and other forms of autoimmunity, most therapies have traditionally used nonspecific immunosuppression, because it was thought that the rare autoreactive T cells could not be identified, much less selectively killed. A defective TNF signaling pathway, which leads to cell death, now provides, at least in vitro, a unique opportunity in human AI diseases to kill only autoreactive T cells.
Materials and Methods
Human Subjects.
Patients with type 1 diabetes or other AI diseases ranging from lupus, multiple sclerosis, hypothyroidism, celiac disease, Crohn's disease, Graves' disease, Sjogren's syndrome, and psoriasis, were recruited over a 5-year period from Massachusetts General Hospital with full institutional approval and with informed consent. Further information is available as Materials and Methods in SI Text.
Blood Preparation.
PBLs were isolated by two major methods, using Ficoll (Table 1, Fig. S1) or nongradient methods with only magnetic beads (all other Figs. 1–4 and Figs. S2–S7). Further information is available as SI Text.
Flow Cytometry Studies.
For most flow cytometry studies, gates were set “open” for inclusion of all cells. The “open gate” included cells of all sizes but excluded cell debris, RBCs, fragmented cells, and apoptotic bodies. The open gate was chosen because cells undergoing cell death, especially by apoptosis, can display changes in light-scattering properties. This method does not necessarily produce a “pretty” picture, but, with an event of 100,000 per sample, the trends are statistically reliable. A BD FACS Calibur flow cytometer machine (Becton Dickinson) was used for the analysis.
TNF Receptor Antibodies.
TNF (Leinco Technologies) and several TNFR1 and TNFR2 antibodies were purchased from the following sources for use on the magnetically separated T cells. TNFR antibodies were clone MR2-1 (TNFR2) and MR1-2 (TNFR1) (Cell Sciences), 80M2 (TNFR2) (Cell Sciences HM2022) (35, 36) and Sigma T1815 (clone 22221.311; Sigma) also specific to the TNFR2 receptor. Isotype-specific control antibodies, matched to the antibody agonists, were performed at times to rule out nonspecific effects of added Ig. TNF antibodies also were sometimes tested with the addition of TNF (2.5 ng/ml) to observe whether the agonist effect was either potentiated or inhibited.
Further information is available online as Materials and Methods in SI Text.
Supplementary Material
Acknowledgments.
We thank The Iacocca Foundation. We also appreciate Dr. M. Davis and L. Murphy, who provided written and administrative assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803429105/DCSupplemental.
References
- 1.Satoh J, Seino H, Abo T. Recombinant human tumor necrosis factor α suppresses autoimmune diabetes in nonobese diabetic mice. J Clin Invest. 1989;84:1345–1348. doi: 10.1172/JCI114304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grewal IS, et al. Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in non-obese diabetic (NOD) mice by preventing the development of autoreactive islet specific T cells. J Exp Med. 1996;184:1963–1974. doi: 10.1084/jem.184.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadelain MW, et al. Prevention of diabetes in the BB rat by early immunotherapy using Freund's adjuvant. J Autoimmun. 1990;3:671–680. doi: 10.1016/s0896-8411(05)80034-4. [DOI] [PubMed] [Google Scholar]
- 4.Kodama S, Davis M, Faustman DL. The therapeutic potential of tumor necrosis factor for autoimmune disease: A mechanistically based hypothesis. Cell Mol Life Sci. 2005;62:1850–1862. doi: 10.1007/s00018-005-5022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 6.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 7.Sidhu RS, Bollon AP. Tumor necrosis factor activities and cancer therapy–a perspective. Pharmacol Ther. 1993;57:79–128. doi: 10.1016/0163-7258(93)90037-e. [DOI] [PubMed] [Google Scholar]
- 8.Hieber U, Heim ME. Tumor necrosis factor for the treatment of malignancies. Oncology. 1994;51:142–153. doi: 10.1159/000227329. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor type 2. Curr Med Chem. 2004;11:2205–2212. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- 10.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: An apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 11.Baetu TM, et al. Disruption of NF-kappaB signaling reveals a novel role for NF-kappaB in the regulation of TNF-related apoptosis-inducing ligand expression. J Immunol. 2001;167:3164–3173. doi: 10.4049/jimmunol.167.6.3164. [DOI] [PubMed] [Google Scholar]
- 12.Abbott DW, Polk DB. NODing off and Ramping up. Inflamm Bowel Dis. 2005;11:860–861. doi: 10.1097/01.mib.0000171284.39894.18. [DOI] [PubMed] [Google Scholar]
- 13.Karban AS, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 14.Hegazy DM, et al. NFkappaB polymorphisms and susceptibility to type 1 diabetes. Genes Immun. 2001;2:304–308. doi: 10.1038/sj.gene.6363776. [DOI] [PubMed] [Google Scholar]
- 15.Deng GY, et al. Association of LMP2 and LMP7 genes within the major histocompatibility complex with insulin-dependent diabetes mellitus: Population and family studies. Am J Hum Genet. 1995;56:528–534. [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Faustman D. NOD mice are defective in proteasome production and activation of NF- kappaB. Mol Cell Biol. 1999;19:8646–8659. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol. 2008;45:13–24. doi: 10.1016/j.molimm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Krause S, et al. Immunoproteasome subunit LMP2 expression is deregulated in Sjogren's syndrome but not in other autoimmune disorders. Ann Rheum Dis. 2006;65:1021–1027. doi: 10.1136/ard.2005.045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammer GM, Tsokos GC. Abnormal T lymphocyte signal transduction in systemic lupus erythematosus. Curr Dir Autoimmun. 2002;5:131–150. doi: 10.1159/000060555. [DOI] [PubMed] [Google Scholar]
- 20.Abbott DW, et al. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Plenge RM, et al. TRAF1–C5 as a risk locus for rheumatoid arthritis–A genomewide study. N Engl J Med. 2007;357:1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Faustman D. Essential role of HLA-encoded proteasome subunits in NF-κB activation and prevention of TNF-α induced apoptosis. J Biol Chem. 2000;275:5238–5247. doi: 10.1074/jbc.275.7.5238. [DOI] [PubMed] [Google Scholar]
- 24.Yan G, Fu Y, Faustman DL. Reduced expression of. Tap1 and Lmp2 antigen processing genes in the nonobese diabetic (NOD) mouse due to a mutation in their shared bidirectional promoter. J Immunol. 1997;159:3068–3080. [PubMed] [Google Scholar]
- 25.Leeuwenberg JF, et al. Evidence for exclusive role in signalling of tumour necrosis factor p55 receptor and a potentiating function of p75 receptor on human endothelial cells. Cytokine. 1995;7:457–462. doi: 10.1006/cyto.1995.0062. [DOI] [PubMed] [Google Scholar]
- 26.Faustman D, et al. Linkage of faulty major histocompatibility complex class I to autoimmune diabetes. Science. 1991;254:1756–1761. doi: 10.1126/science.1763324. [DOI] [PubMed] [Google Scholar]
- 27.Pinkse GG, et al. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci USA. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi T, Kodama S, Faustman DL. Reply to ‘LMP2 expression and proteasome activity in NOD mice’. Nat Med. 2000;6:1065–1066. doi: 10.1038/80353. [DOI] [PubMed] [Google Scholar]
- 29.Welborn MB, III, et al. A human tumor necrosis factor p75 receptor agonist stimulates in vitro T cell proliferation but does not produce inflammation or shock in the baboon. J Exp Med. 1996;184:165–171. doi: 10.1084/jem.184.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhtreiber WM, et al. Methods to characterize lymphoid apoptosis in a murine model of autoreactivity. J Immunol Methods. 2005;306:137–150. doi: 10.1016/j.jim.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 31.van Oosten BW, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 32.Enayati PJ, Papadakis KA. Association of anti-tumor necrosis factor therapy with the development of multiple sclerosis. J Clin Gastroenterol. 2005;39:303–306. doi: 10.1097/01.mcg.0000155126.82171.32. [DOI] [PubMed] [Google Scholar]
- 33.The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–65. Anonymous. [PubMed] [Google Scholar]
- 34.Thomas CW, Jr, Weinshenker BG, Sandborn WJ. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn's disease. Inflamm Bowel Dis. 2004;10:28–31. doi: 10.1097/00054725-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Leeuwenberg JF, Jeunhomme TM, Buurman WA. Slow release of soluble TNF receptors by monocytes in vitro. J Immunol. 1994;152:4036–4043. [PubMed] [Google Scholar]
- 36.Grell M, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.