Abstract
Since the unexpected discovery of the antipsychotic activity of chlorpromazine, a variety of therapeutic agents have been developed for the treatment of schizophrenia. Despite differences in their activities at various neurotransmitter systems, all clinically effective antipsychotics share the ability to interact with D2 class dopamine receptors (D2R). D2R mediate their physiological effects via both G protein-dependent and independent (β-arrestin 2-dependent) signaling, but the role of these D2R-mediated signaling events in the actions of antipsychotics remains unclear. We demonstrate here that while different classes of antipsychotics have complex pharmacological profiles at G protein-dependent D2R long isoform (D2LR) signaling, they share the common property of antagonizing dopamine-mediated interaction of D2LR with β-arrestin 2. Using two cellular assays based on a bioluminescence resonance energy transfer (BRET) approach, we demonstrate that a series of antipsychotics including haloperidol, clozapine, aripiprazole, chlorpromazine, quetiapine, olanzapine, risperidone, and ziprasidone all potently antagonize the β-arrestin 2 recruitment to D2LR induced by quinpirole. However, these antipsychotics have various effects on D2LR mediated Gi/o protein activation ranging from inverse to partial agonists and antagonists with highly variable efficacies and potencies at quinpirole-induced cAMP inhibition. These results suggest that the different classes of clinically effective antipsychotics share a common molecular mechanism involving inhibition of D2LR/β-arrestin 2 mediated signaling. Thus, selective targeting of D2LR/β-arrestin 2 interaction and related signaling pathways may provide new opportunities for antipsychotic development.
Keywords: BRET, schizophrenia, signaling, functional selectivity
While the etiology of schizophrenia, a devastating chronic mental illness affecting 1–2% of human population, remains unclear, many of its symptoms can be attenuated by treatment with various classes of antipsychotic drugs. In fact, since the discovery of chlorpromazine in the 1950s and its antipsychotic properties, a large number of antipsychotics have been developed but their clinical efficacy and side-effect profiles could still be improved significantly. Generally, these compounds can be divided in 3 different categories: conventional (typical) antipsychotics, atypical antipsychotics, and the recently-introduced dopamine receptor partial agonist antipsychotics (dopamine-serotonin system stabilizers) (1). The second generation of antipsychotics (atypical and compounds with partial agonist activity at dopaminergic receptors) affect other neurotransmitter systems in addition to dopamine and offer advantages over the typical antipsychotics by targeting multiple schizophrenia symptoms and having less potential to induce extrapyramidal side effects or increase serum prolactin secretion (2). Nevertheless, the potential for other side effects still exist with these compounds mostly due to their off-target actions (3, 4).
While having complex pharmacological actions at several neurotransmitter systems, all antipsychotics principally act, albeit with different potency, on the dopamine (DA) system. It has been demonstrated that clinical efficacy of essentially all antipsychotic drugs (including traditional and newer antipsychotics) is directly correlated with dopamine D2 receptor (D2R) binding affinity and their capacity to antagonize this receptor (5, 6). It is commonly believed that the D2R, which belongs to the G protein-coupled receptor (GPCR) family, mediates the major part of its signaling and functions by coupling to Gi/o proteins to negatively regulate cAMP production. Thus, studies aimed at assessing the efficacy of antipsychotics on D2R signaling have classically been mainly concerned with measuring Gi/o-mediated inhibition of cAMP. However, recent evidence suggests that GPCRs may mediate their physiological functions by engaging signaling pathways via G protein-independent mechanisms (7). Upon stimulation, D2R, like other GPCRs, are regulated by processes leading to phosphorylation of the receptor by G protein-coupled receptor kinases (GRKs), uncoupling of the receptor from G protein activation, recruitment of the multifunctional scaffolding molecules, β-arrestins, and receptor internalization. Recent evidences indicate that β-arrestins, despite their critical involvement in regulating the GPCR's desensitization and trafficking, can also be positive mediators for signaling, leading to formation of intracellular complexes of signaling molecules (7). In particular, D2R have been recently shown to engage the Akt/Glycogen Synthase Kinase 3 (GSK-3) signaling pathway by a G protein–independent mechanism that involves a previously unidentified signaling complex comprised of β-arrestin 2, Akt, and the multimeric protein phosphatase PP2A (8, 9). This signaling mode, which is involved in the expression of certain dopamine associated behaviors (8, 10) and can be disrupted by lithium salts (11) seems to be mostly regulated by postsynaptically expressed dopamine D2LRs (9, 12).
Several lines of evidence support an important role for Akt and its downstream target GSK-3 in schizophrenia (10, 11, 13–17). In fact, lower Akt protein levels concomitant with a higher level of active GSK-3 have been found in brains of schizophrenic patients (13) and a significant association of Akt1 haplotypes with schizophrenia has been reported (14). Furthermore, several antipsychotics can affect GSK-3 phosphorylation in various in vitro and in vivo experimental models (13, 15–17) and human postmortem studies (13). Altogether, these observations raise the possibility that the D2R-dependent β-arrestin 2/Akt/GSK-3 signaling pathway might be involved in the actions of antipsychotics.
Here, by using newly developed BRET assays, we compared the effects of several commonly used and putative antipsychotics on the D2LR activation-induced recruitment of β-arrestin 2 and the Gi/o-dependent cAMP inhibition. We observed that while different antipsychotics have various actions on Gi/o pathway, all of them potently block dopamine-dependent β-arrestin 2 recruitment to D2LR. These BRET assays could provide a simple in vitro system for predicting the potential antipsychotic activity of new compounds
Results
BRET Assay to Monitor D2LR-Mediated Activation of Gi/o.
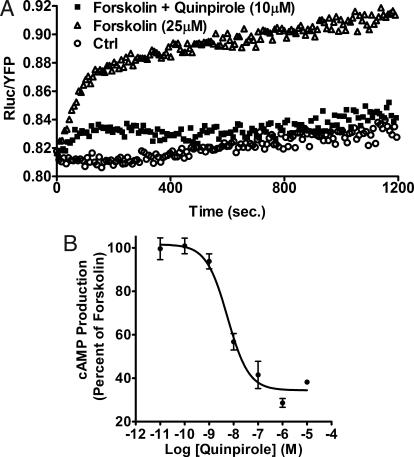
Gi/o protein-mediated cAMP inhibition is the best-characterized response to D2R activation. To measure and compare the agonist and antagonist activity of antipsychotics on this “classical” D2R signaling, we used a BRET adapted version of the ICUE 2 (indicator of cAMP using Epac) cAMP biosensor developed previously for FRET (18). For BRET experiments, the Exchange Protein Activated by cAMP (EPAC) was tagged at the N terminus with the Renilla luciferase and at the C terminus with citrine, an improved version of yellow fluorescent protein (YFP) (19). Sensitivity of this BRET cAMP sensor has already been validated (19) and was further confirmed by direct comparison of cAMP measurements performed using Enzyme Immuno Assay in response to D2LR activation (data not shown). Stimulation of HEK cells coexpressing both the biosensor and D2LR with the adenylylcyclase (AC) activator, forskolin (25 μM), induced a robust and sustained increase in cAMP production (Fig. 1A). Using this system, activation of Gi/o protein-coupled D2LR and inhibition of AC can be monitored as the D2R agonist quinpirole (1 μM) abolished cAMP formation induced by forskolin. The maximal AC inhibition occurred immediately after agonist stimulation and remained constant for at least 20 min in the presence of the agonist (Fig. 1A).
Fig. 1.
BRET measurements of cAMP levels in living cells to monitor dopamine D2L receptor activation. (A and B) Variations of cAMP levels in HEK293 cells stably expressing the EPAC biosensor and D2LR. (A) Kinetics of cAMP variations after treatment of the cells at time 0 with forskolin (25 μM) in the absence (▵) or presence (■) of quinpirole 1 μM as indicated and the emission ratios (Rluc/YFP) were measured. Cells incubated in PBS (○) were used as a negative control. (B) Dose-response curve of quinpirole for inhibiting forskolin-stimulated cAMP accumulation in HEK cells. cAMP production was normalized to the percentage of forskolin-stimulated cAMP accumulation (set at 100%).
As shown in Fig. 1B, quinpirole potently inhibited forskolin-stimulated cAMP accumulation in living cells with an EC50 of 5.7 nM ± 0.76. However, under the same conditions, dopamine generated a biphasic dose-response curve [supporting information (SI) Fig. S1]. In fact, at low concentrations (up to 0.1 μM), dopamine decreased cAMP levels, while at higher concentrations an increase in cAMP production was observed. This biphasic response of dopamine resulted from endogenously expressed dopamine D1-like receptors (D1R) in HEK cells as pretreatment of HEK cells with SCH23390, a selective D1R antagonist, generated a monophasic dopamine dose-response of cAMP inhibition with an EC50 of 18 nM ± 0.85 (Fig. S1). Also, SKF81297, a selective D1R agonist, showed a monophasic dose-dependent increase in cAMP production (data not shown). Thus, the use of the selective D2R agonist quinpirole, in the assay, avoids this confound.
BRET Assay to Monitor β-Arrestin 2 Recruitment to Activated D2LR.
Given the potential importance of the β-arrestin 2-mediated signaling pathway in the actions of dopamine in vivo (8), and since little is known about the effect of antipsychotics on this pathway, we adapted a BRET assay to monitor activity of the antipsychotics on D2LR mediated β-arrestin 2 recruitment as a readout of β-arrestin 2 mediated signaling. The use of β-arrestin recruitment to activated receptors has been developed to follow activation of multiple GPCRs and even used as a high-throughput screening tool to identify ligands (20, 21).
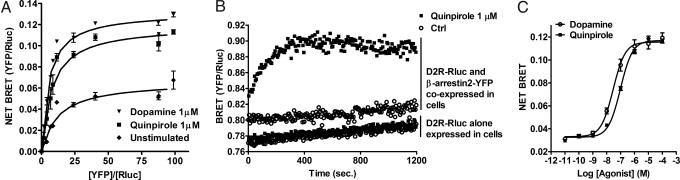
Murine dopamine D2LR and β-arrestin 2 were cloned from a striatum cDNA library and fused to their C terminus with Renilla Luciferase (Rluc), the BRET donor, or the yellow variant of EGFP (YFP), the BRET acceptor, respectively. As measured by competitive binding, addition of the Rluc to the C terminus of D2LR did not modify the affinity of tested antipsychotics for D2LR (data not shown). BRET titration assays between D2LR and β-arrestin 2 were carried out to assess whether agonists-promoted BRET changes reflected active recruitment of β-arrestin 2 to the receptor. In both unstimulated and stimulated HEK cells, a hyperbolic curve was obtained, demonstrating the progressive occupancy of the BRET donor with increasing quantity of the acceptor (Fig. 2A) (22). Stimulation of D2LR with either quinpirole or dopamine at 1 μM induced a reduction of the BRET50 (ratio of YFP/Rluc leading to 50% of the maximal BRET signal) (BRET50 = 12.38; 7.76, and 5.43 for unstimulated, quinpirole, and dopamine respectively) and an increase of the maximal BRET (BRETmax = 0.066; 0.12, and 0.13 for unstimulated, quinpirole, and dopamine respectively) compared to unstimulated cells, which is consistent with an active recruitment of the β-arrestin 2 to the receptor (Fig. 2A). The basal and agonist-mediated interaction of D2LR with β-arrestin 2 are specific since in control experiments, using free YFP as BRET acceptor, only much weaker and linear bystander BRET signals were observed with increasing YFP concentrations, indicating the nonspecific nature of the BRET signal arising from the interaction of soluble YFP with D2LR-Rluc (see Fig. S2). This bystander BRET signal observed between YFP and D2LR-Rluc was unaffected by dopamine or quinpirole treatment as can be seen by maximal BRET signal and BRET50 values that were similar to unstimulated cells.
Fig. 2.
BRET measurements of β-arrestin 2 recruitment to dopamine D2L receptor in living cells. (A) BRET titration curve for the β-arrestin 2 recruitment to D2LR. BRET was measured in cells expressing a fixed amount of Rluc-tagged D2LR and increasing amounts of YFP-tagged β-arrestin 2, treated (with dopamine 1 μM ▾ or quinpirole 1 μM (■) or not (♦). Specificity of the BRET signal between D2LR and the β-arrestin 2 was controlled by measuring BRET between Rluc-tagged D2LR and YFP alone, treated or not with dopamine and quinpirole (see Fig. S2). (B) Kinetic of β-arrestin 2 recruitment to D2LR after addition, at time 0, of quinpirole 1 μM (■) or PBS (○). As a negative control, similar experiments were carried out on cells transiently expressing only Rluc-tagged D2LR. (C) Dose-response curve of quinpirole for β-arrestin 2 recruitment to D2LR. Cells coexpressing Rluc-tagged D2LR and YFP-tagged β-arrestin 2 were stimulated with dopamine (○) or quinpirole (■). Results are expressed in Net BRET as described in the Methods section. Data represent the mean ± SEM of 3 to 7 independent experiments each performed in duplicate.
We next determined the kinetic of β-arrestin 2 recruitment to D2LR after agonist stimulation using “real-time” BRET measurements. In cells expressing only D2LR-Rluc, as expected, quinpirole at 1 μM did not induce BRET variations compared to untreated (PBS) cells. However, in cells coexpressing D2LR-Rluc and β-arrestin 2-YFP, quinpirole rapidly increased BRET levels, which were stable for at least 20 min (Fig. 2B). Similar results were obtained using dopamine as agonist (data not shown). By comparing the basal BRET levels obtained from cells expressing D2LR-Rluc alone and cells coexpressing both the receptor and the β-arrestin 2-YFP, we obtained a net BRET value of 0.03, reflecting the basal interaction of β-arrestin 2 with D2LR in unstimulated cells. Fig. 2C shows dose-response curves for dopamine and quinpirole for β-arrestin 2 recruitment to D2LR in living cells with EC50 values of 29.2 nM ± 0.81 and 97 nM ± 0.92, respectively.
Intrinsic Activity of Different Classes of Antipsychotics on D2R-Induced Signaling.
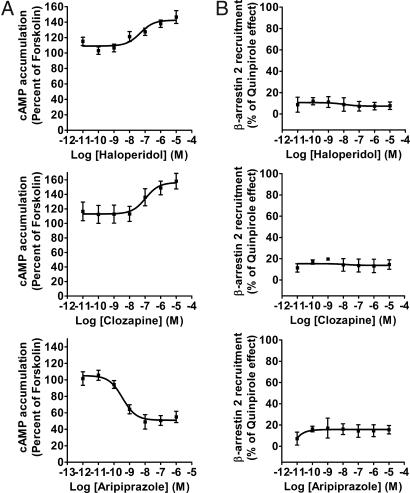
Using the BRET assays described above, we next evaluated the effects of different classes of antipsychotics on D2LR signaling. First, the intrinsic activity of several antipsychotic compounds (haloperidol, clozapine, aripiprazole, desmethylcozapine, chlorpromazine, quetiapine, olanzapine, risperidone, and ziprasidone) on D2LR-induced Gi/o activation was determined in HEK cells stably expressing D2LR and the EPAC biosensor (Fig. 3A and Fig. S3). In this assay, all of the antipsychotics acted as inverse agonists on D2LR, with exception of aripiprazole that induced a decrease of cAMP levels. This effect of aripiprazole is not surprising since this compound is known to be a partial agonist at D2LR with respect to modulation of the cAMP pathways (23). The efficacy and pEC50 values obtained for these compounds are summarized in Table 1. Next, the intrinsic activity of the same antipsychotics on β-arrestin 2 recruitment to D2LR was also assessed. HEK cells coexpressing D2LR-Rluc and β-arrestin2-YFP were stimulated with the different doses of antipsychotics. As shown in Fig. 3B (and Fig. S4), none of these compounds were able to induce β-arrestin 2 recruitment to D2LR. Importantly, aripiprazole, which acts as a partial agonist on the cAMP pathway, had no activity on D2LR in this assay and did not induce β-arrestin 2 translocation either (see below).
Fig. 3.
Intrinsic activity of haloperidol, clozapine, and aripiprazole on Gi/o activation and D2LR -mediated β-arrestin 2 recruitment. (A) Dose–response of haloperidol, clozapine, and aripiprazole on adenylylcyclase inhibition through D2R. As described in Methods section, HEK 293 cells stably expressing EPAC biosensor and D2R were stimulated with haloperidol (Upper Left), clozapine (Middle Left) or aripiprazole (Bottom Left) in presence of forskolin (25 μM). cAMP production was normalized to the percentage of forskolin-stimulated cAMP accumulation (set at 100%). (B) BRET measured in cells coexpressing Rluc-tagged D2LR and YFP-tagged β-arrestin 2 and stimulated with haloperidol (Upper Right), clozapine and aripiprazole (Middle and Bottom Right, respectively). Data are expressed as the percentage of quinpirole maximum effect (1 μM) and represent the mean ± SEM of 3–5 independent experiments each performed in duplicate.
Table 1.
Intrinsic and antagonist activity of different antipsychotics on dopamine D2L receptor on the Gi/o pathway and β-arrestin 2 recruitment
| Compounds | Intrinsic activity for Gi/o pathway |
Intrinsic activity for β-arrestin 2 pathway |
Antagonist activity for Gi/o pathway |
Antagonist activity for β-arrestin 2 pathway |
||||
|---|---|---|---|---|---|---|---|---|
| pEC50 | Emax (%) | pEC50 | Emax (%) | KB (nM) | Emax (%) | KB (nM) | Emax (%) | |
| Quinpirole | *8.25 ± 0.12 | 66.02 ± 1.05 | 7.01 ± 0.04 | 100 ± 1.01 | N.A. | N.A. | N.A. | N.A. |
| Haloperidol | 7.32 ± 0.39 | −42.6 ± 4.5 | N.A. | N.A. | 0.28 ± 0.16 | 104 ± 4.5 | 0.12 ± 0.03 | 83.7 ± 2.5 |
| Clozapine | 6.97 ± 0.52 | −56.3 ± 9.8 | N.A. | N.A. | >10,000 | N.A. | 67.7 ± 14.0 | 85.7 ± 8.2 |
| Aripiprazole | *9.45 ± 0.24 | 49.0 ± 3.9 | N.A. | N.A. | 0.12 ± 0.05 | 29.9 ± 5.6 | 1.07 ± 0.32 | 69.6 ± 2.8 |
| Desmethylclozapine | 6.53 ± 0.52 | −28.0 ± 11.7 | N.A. | N.A. | >10,000 | N.A. | 186 ± 107 | 98.2 ± 32.5 |
| Chlorpromazine | 8.81 ± 0.41 | −28.6 ± 4.0 | N.A. | N.A. | 0.72 ± 0.1 | 95.4 ±5.1 | 5.04 ± 1.21 | 95.6 ± 6.2 |
| Quetiapine | 7.40 ± 0.40 | −34.0 ± 6.0 | N.A. | N.A. | >10,000 | N.A. | 88.5 ± 8.8 | 84.6 ± 6.4 |
| Olanzapine | 8.60 ± 0.65 | −33.8 ± 5.6 | N.A. | N.A. | 2.10 ± 0.6 | 105 ± 7.4 | 1.95 ± 0.28 | 83.8 ± 3.5 |
| Risperidone | 9.25 ± 0.35 | −16.4 ± 2.8 | N.A. | N.A. | 1.08 ± 0.9 | 96.3 ± 7.0 | 0.02 ± 0.01 | 83.0 ± 3.0 |
| Ziprasidone | 10.0 ± 1.2 | −23.8 ± 6.1 | N.A. | N.A. | 0.13 ± 0.04 | 88.7 ± 7.3 | 0.03 ± 0.02 | 69.3 ± 3.6 |
The values of KB, representing the molecular affinity of the competitive antagonist, were determined using the equation: KB = IC50/ ((2+([A]/EC50)n)1/n−1), where the concentration of agonist is [A], the concentration of agonist producing 50% maximal response is EC50, and n is the Hill coefficient of the agonist dose-response curve (29). Abbreviation: N.A.: not applicable. For the intrinsic activity of the antipsychotics for Gi/o, efficacy is expressed in percent of forskolin effect. For the antagonistic activity of the antipsychotics on Gi/o pathway and β-arrestin 2 recruitment, efficacy is expressed in percent of quinpirole maximum effect (1 μM).
*pEC50 of agonist activity for Gi/o pathway.
Antagonist Activity of Different Classes of Antipsychotics on D2LR Signaling.
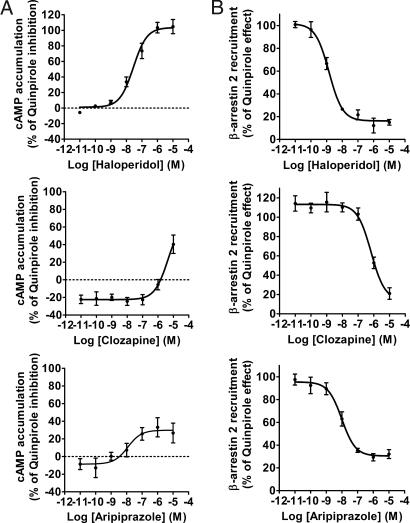
Using the same assays, we next addressed the antagonistic properties of the antipsychotics on both quinpirole induced Gi/o signaling and β-arrestin 2 recruitment to D2LR. Dose-response curves of the different antipsychotics on quinpirole-induced cAMP inhibition are shown in Fig. 4A and Fig. S5. The KB and efficacy values of the compounds tested are presented in Table 1. Almost all of the compounds were able to block dose-dependently quinpirole effects but with markedly different potencies and efficacies (Table 1). Thus, with exception of clozapine, desmethylclozapine, and quetiapine that had low efficacies against 1 μM quinpirole, all of the other antipsychotics blocked the effects of quinpirole almost completely. However, when quinpirole was used at a lower concentration, 100 nM, clozapine, desmethylclozapine, and quetiapine were able to completely abolish quinpirole effect dose-dependently (data not shown). Unlike the other antipsychotics, aripiprazole blocked quinpirole effect with maximum efficacy of only 30% likely due to its partial agonist activity on D2LR (see Fig. 3A and Table 1).
Fig. 4.
Antagonist activity of haloperidol, clozapine, and aripiprazole on Gi/o activation and β-arrestin 2 recruitment induced by quinpirole. (A) Dose-response curve for haloperidol, clozapine and aripiprazole (Upper, Middle, and Bottom Left) for inhibiting adenylylcyclase inhibition induced by quinpirole. HEK 293 cells stably coexpressing EPAC biosensor and D2LR were treated with the different antipsychotics and quinpirole (1 μM) in the presence of forskolin (25 μM). (B) Dose-response curve for haloperidol (Upper Right), clozapine and aripiprazole (Middle and Bottom Right, respectively) for inhibiting β-arrestin 2 recruitment induced by 1 μM of quinpirole. BRET was measured in cells coexpressing Rluc-tagged D2LR and YFP-tagged β-arrestin 2. Data represent the mean ± SEM of 3–5 independent experiments each performed in duplicate.
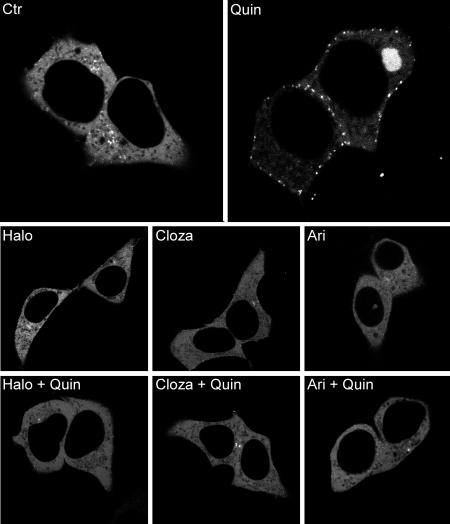
We next determined the antagonist activity of these antipsychotics on β-arrestin 2 recruitment induced by quinpirole. As typically shown in Fig. 4B (and Fig. S6), all antipsychotics were highly efficacious in blocking quinpirole induced β-arrestin 2 translocation to the D2LR. The different potencies of all drugs tested for inhibiting translocation are summarized in Table 1. Strikingly, aripiprazole, which acts as a partial agonist on the cAMP pathways but has no intrinsic agonistic activity on β-arrestin 2 recruitment, was able to fully antagonize quinpirole-induced β-arrestin 2 recruitment with a KB of 1.07 nM ± 0.32 (see Fig. 3B). Similarly, with the exception of chlorpromazine and olanzapine, all other antipsychotics displayed potencies toward the β-arrestin 2 recruitment that were 3–150-fold higher than at the G protein-mediated pathway. To further confirm the results obtained by BRET assay, confocal microscopy in living cells was used to visualize the effect of haloperidol, clozapine, and aripiprazole on the β-arrestin 2 recruitment to D2LR. HEK cells coexpressing human D2LR and YFP-tagged β-arrestin 2 were stimulated with quinpirole (Fig. 5 Upper Right) or the antipsychotics (Fig. 5, Lower Center) at 10 μM. After 30 min of treatment, only quinpirole induced formation of fluorescent puncta at the plasma membrane reflecting β-arrestin 2 translocation. In unstimulated cells, β-arrestin 2 tagged with YFP was distributed throughout the cytosol (Fig. 5, Upper Left). Pretreatment of cells for 30 min with the antipsychotics completely abolished β-arrestin 2 recruitment induced by quinpirole confirming the results obtained by BRET with the murine D2LR (Fig. 5, Lower Left, Center, and Right).
Fig. 5.
β-arrestin 2-YFP translocation to D2LR. Fluorescence microscopy was used to visualize β-arrestin 2-YFP recruitment to the human D2R long form in cells incubated for 30 min with: no drug (Ctr), haloperidol (10 μM) (Halo), clozapine (10 μM) (Cloza), aripiprazole (10 μM) (Ari), quinpirole (10 μM) (Quin). For antagonistic activity (Bottom), cells were pretreated with the different antipsychotics for 30 min and then stimulated for 30 min with quinpirole (10 μM). Representative pictures of each condition are shown (n = 3).
Discussion
Dysregulation of the dopamine system and its physiological actions through the D2-like receptors are believed to contribute to either the pathogenesis or manifestations of schizophrenia. Dopamine D2Rs are classical GPCRs and are coupled to Gi/o. However, D2R activation can also regulate the Akt-GSK3 pathway through a G protein independent mechanism involving the scaffolding protein β-arrestin 2 (8). Since the Akt-GSK3 signaling pathway has been suggested to be involved in schizophrenia (10, 11, 13–17, 24) and that all clinically effective antipsychotics act, at least in part, by interacting with D2R, we compared the potencies of antipsychotics with regards to blocking β-arrestin 2 recruitment to D2R versus their well studied effects on Gi/o pathway activation. At least in the striatum, the negative regulation of Akt by dopamine was shown to be mostly a postsynaptic phenomena regulated by D2LR (9). Since both the long and the short isoforms of D2R have different functions in vivo with D2LR acting mainly at postsynaptic sites (12), we investigated signaling pathways activated by D2LR. For this purpose, we adapted two BRET approaches that allowed us to monitor both the β-arrestin translocation to D2LR and the Gi/o protein activation.
Using these assays, we observed that all antipsychotics tested have no intrinsic activity with regards to D2LR induced β-arrestin 2 recruitment, while they all (with the exception of aripiprazole) act as inverse agonists at the Gi/o pathway. Assessment of the antagonist potencies of the antipsychotics on these two pathways showed a markedly lower KB for the majority of the compounds to antagonize β-arrestin 2 recruitment compared to AC inhibition induced by quinpirole (see Table 1). Thus, there seems to be a clear difference between the potencies of anti psychotics to antagonize these two signaling paradigms allowing them to discriminate one pathway versus the other. Ultimately, it would be interesting to explore whether the efficacies of these compounds as antipsychotics and their relative liability for extrapyramidal side effects, correlate with their profile for either of these pathways. However, the observed differences in the activity of antipsychotics raise important questions regarding the molecular mechanism underlying the preferential antagonism of the arrestin pathway. Moreover, antipsychotics can also interact with and affect β-arrestin 2 recruitment to the presynaptic D2R short isoform (data not shown). Whether antipsychotics can selectively modulate different putative signaling modes of D2SR and contribute to their in vivo effects may be interesting to examine.
While aripiprazole displays partial agonism at dopamine D2LR coupling to Gi/o, our results clearly show that aripiprazole has no effect by itself on β-arrestin 2 recruitment to D2LR while potently antagonizing β-arrestin 2 translocation induced by quinpirole or dopamine. Aripiprazole has been shown to lack the ability to induce internalization of the long form of D2R (25), a mechanism known to be β-arrestin dependent. The fact that aripiprazole at the same time exerts partial agonist activity with regards to cAMP production highlights the functional selectivity of aripiprazole at D2LR for the Gi/o pathway. Our data provide support for the hypothesis that aripiprazole is a functionally selective agent (biased agonist) for D2LR rather than simply a partial agonist (26). It is likely that D2LR can exist in an ensemble of distinct conformations that could engage different signaling modalities in response to the binding of different ligands. In fact, such functional selectivity has been shown recently for different GPCRs such as the β2-adrenergic receptor, angiotensin II type 1 receptor (AT1), CCR7, CXCR4, and the μ-opioid receptor (for review see ref. 27). Furthermore, one leading hypothesis to explain the unique clinical properties of atypical antipsychotics such as clozapine, postulates the importance of non-dopaminergic actions, particularly at serotonin receptors. While this study did not assess directly the effects of these compounds on serotonin receptor functions, we found that all clinically effective antipsychotics block with high potency D2LR/β-arrestin 2 interaction. Thus, antagonism at the D2LR/β-arrestin 2-dependent pathway rather than their actions on D2LR/Gi/o pathway or other neurotransmitter systems could have better predictive value for their therapeutic efficacy.
In summary, we investigated the activity of antipsychotics on the different modalities of D2LR signaling involving the Gi/o activation and β-arrestin 2 recruitment. By using a BRET approach, we clearly show that all classes of antipsychotics completely abolish β-arrestin 2 recruitment to D2LR in response to quinpirole, while the activity profile of these compounds on Gi/o-mediated cAMP production is highly variable. Evaluation of drug activity on β-arrestin 2 recruitment to D2LR may provide an effective approach for the development of future antipsychotics.
Methods
Reagents.
All cell culture reagents and buffers were from Gibco and Sigma, and FBS from JRH Biosciences. Quinpirole, dopamine, isoproterenol, haloperidol, clozapine, chlorpromazine, and forskolin were purchased from Sigma. Collagen was from Roche. Aripiprazole, desmethylclozapine, olanzapine, quetiapine, risperidone, and ziprasidone were provided by H. Lundbeck A/S. Coelenterazine h was purchased from Promega.
Cell Culture and Transfections.
Human embryonic kidney 293 cells (HEK293T; Gibco and Sigma) were maintained in Dulbecco's Modified Eagle's medium supplemented with 10% (vol/vol) of FBS, 2 mM L-glutamine and 0.05 mg/ml of Gentamicin at 37°C in a humidified atmosphere at 95% air and 5% CO2. Transient transfections were performed 24 h after cell seeding using calcium phosphate protocol.
Bioluminescence Resonance Energy Transfer Measurement.
For BRET assays phenol red free medium was removed from HEK293T cells and replaced by Phosphate Buffer Saline (PBS) containing calcium and magnesium and 0.003% (wt/vol) ascorbic acid.
For measurement of cAMP variation using the EPAC biosensor, the experiments and reads were performed at 37°C using Mithras LB 940 instrument (Berthold). The assay was started by adding 10 μl of the cell-permeant substrate specific for Renilla luciferase, coelenterazine h to the well to yield a final concentration of 5 μM. The agonist activity of the compounds was measured by adding those 5 min after the Rluc substrate, and forskolin was then added to the wells 5 min after agonists. Reads of the plates started 5 min after the addition of forskolin. When the assay was conducted to determine antagonist properties of compounds against quinpirole, the different compounds were added simultaneously 1 min after the coelenterazine h. To follow the β-arrestin 2 recruitment to the dopamine D2LR receptor, the same protocols as above were used except that experiments and reads were performed at room temperature. Reads started 10 min after the addition of Rluc substrate and no forskolin was used.
BRET readings were collected using a Mithras LB940 instrument that allows the sequential integration of the signals detected in the 465 to 505 nm and 515 to 555 nm windows using filters with the appropriate band pass and by using MicroWin 2000 software.
For titration experiments, the acceptor/donor ratio was calculated as previously described (28).
For time course analysis of cAMP and the D2LR-β-arrestin 2 interactions (Figs. 1A and 2B), coelenterazine h was added 10 min before addition of PBS, forskolin, isoproterenol, or quinpirole. Readings were then collected at 5 to 6 s intervals for the next 20 min.
Fluorescence Microscopy.
The β-arrestin 2 translocation assay in living cells was assessed as described in ref. 20 using a Zeiss LSM-510.
Statistical Analysis.
All data represent the mean ± SEM of a minimum three independent experiments in duplicate. All graphs, except time courses, are shown with standard error of mean, but for certain data points the error bars were smaller than the symbol used on the graph.
Additional Details.
See SI Text for a description of cDNA constructs, transfections, and SI Text for BRET experiments.
Supplementary Material
Acknowledgments.
We thank Dr. Terry Kenakin (Glaxo SmithKline Research and Development, NC) for fruitful discussions on analysis of antipsychotics activities. This work was supported in part by National Institutes of Health Grants NS-19576, MH-73853 and MH-82441 to MGC. An unrestricted gift from Lundbeck Research USA is also greatly appreciated. B.M. was recipient of a Fondation pour la Recherche Médicale postdoctoral fellowship and a European Marie-Curie Outgoing International Fellowship (FP6–2005-Mobility-6). A.S. held a fellowship from the Canadian Institutes of Health Research. V.G. was supported by the Graduate Program, Division of Pharmacology, Departments of Biomedical Sciences & Biotechnologies, University of Brescia, Italy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803522105/DCSupplemental.
References
- 1.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 2.Haro JM, Salvador-Carulla L. The SOHO (Schizophrenia Outpatient Health Outcome) study: Implications for the treatment of schizophrenia. CNS Drugs. 2006;20:293–301. doi: 10.2165/00023210-200620040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kroeze WK, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 4.Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 6.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 7.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu JM, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu JM, et al. A beta-arrestin 2 Signaling Complex Mediates Lithium Action on Behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Usiello A, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 13.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda M, et al. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700. doi: 10.1016/j.biopsych.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 17.Prickaerts J, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: A putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Violin JD, et al. {beta}2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 19.Barak LS, et al. Pharmacological characterization of membrane-expressed human trace amine associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer (BRET) cAMP biosensor. Mol Pharmacol. 2008 doi: 10.1124/mol.108.048884. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 21.Hamdan FF, Audet M, Garneau P, Pelletier J, Bouvier M. High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin 2 recruitment assay. J Biomol Screen. 2005;10:463–475. doi: 10.1177/1087057105275344. [DOI] [PubMed] [Google Scholar]
- 22.Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro DA, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 24.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32:67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- 26.Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Salahpour A, Masri B. Experimental challenge to a “rigorous” BRET analysis of GPCR oligomerization. Nat Methods. 2007;4:599–600. doi: 10.1038/nmeth0807-599. author reply 601. [DOI] [PubMed] [Google Scholar]
- 29.Kenakin T. A Pharmacology Primer, Second Edition: Theory, Applications, and Methods. Oxford: Academic; 2006. pp. 1–299. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.